-
PDF
- Split View
-
Views
-
Cite
Cite
Hongbin Xu, Takanori Sobue, Martinna Bertolini, Angela Thompson, Anna Dongari-Bagtzoglou, Streptococcus oralis and Candida albicans Synergistically Activate μ-Calpain to Degrade E-cadherin From Oral Epithelial Junctions, The Journal of Infectious Diseases, Volume 214, Issue 6, 15 September 2016, Pages 925–934, https://doi.org/10.1093/infdis/jiw201
Close - Share Icon Share
Abstract
Streptococcus oralis forms robust mucosal biofilms with Candida albicans that have increased pathogenic potential. In this study, using oral epithelial cultures, organotypic oral mucosal constructs, and a mouse model of oral infection, we demonstrated that S. oralis augmented C. albicans invasion through epithelial junctions. C. albicans and S. oralis decreased epithelial E-cadherin levels by synergistically increasing µ-calpain, a proteolytic enzyme that targets E-cadherin. In the mouse coinfection model this was accompanied by increased fungal kidney dissemination. Coinfection with a secreted aspartyl protease (sap) mutant sap2456 and S. oralis increased μ-calpain and triggered mucosal invasion and systemic dissemination, suggesting that fungal protease activity is not required for invasion during coinfection. We conclude that C. albicans and S. oralis synergize to activate host enzymes that cleave epithelial junction proteins and increase fungal invasion.
Candida albicans is a core component of the human oral mycobiome in health [1, 2]. Most oral fungal infections are superficial and caused by C. albicans under host-permissive conditions [3]. In severe immunosuppression C. albicans can invade epithelial barriers and reach the bloodstream, resulting in life-threatening systemic infections [4]. C. albicans uses 2 major mechanisms to compromise epithelial barriers: induced endocytosis and active penetration [5, 6]. Active penetration includes intracellular penetration and paracellular invasion through cell junctions [5, 7, 8]. Previous studies have shown that proteolytic degradation of E-cadherin in adherens junctions compromises epithelial barrier function and promotes C. albicans invasion [7, 9].
As most oral infections, the pathoecology of oropharyngeal candidiasis is complex and may involve bacteria such as streptococci, which are abundant colonizers in health and coexist with fungi in oral biofilms [3, 10]. C. albicans and oral streptococci interact via several molecular mechanisms, resulting in increased biofilm growth and pathogenic synergy [11, 12]. For example, using mucosal tissue constructs we showed that C. albicans promoted Streptococcus oralis biofilm growth, which in turn increased invasion of the oral and esophageal mucosa by C. albicans [13]. Furthermore, we demonstrated that coinfection of mice with S. oralis and C. albicans augmented oral thrush lesions and mucosal neutrophilic infiltration [14].
Similar to C. albicans paracellular invasion, increased neutrophil transmigration requires disassembly of epithelial cell junctions. In airway mucosa, disassembly of epithelial junctions for polymorphonuclear leukocyte migration is controlled by activation of μ-calpain (calpain 1) and m-calpain (calpain 2) [15]. These are calcium ion–dependent cysteine proteases involved in many cell processes [16], and their activities are strictly regulated in vivo. Activated calpain 1 and/or calpain 2 cleave E-cadherin and occludin in cell junctions to promote recruitment of polymorphonuclear leukocytes into the airways [15]. Because E-cadherin degradation is involved in neutrophil transmigration and C. albicans tissue invasion, and both processes are increased during coinfection, we hypothesized that C. albicans and S. oralis synergize to activate calpain and degrade E-cadherin. We provide evidence for the first time that this type of host modulation is mediated by synergy between 2 major representatives of the core bacterial and fungal oral microbiota.
MATERIALS AND METHODS
Strains and Growth Conditions
C. albicans SC5314 is a bloodstream isolate [17] that forms robust oral biofilms [10]. C. albicans Δsap2456 (M31) and reference strain CAI4-URA3 were described in reference [18]. C. albicans was maintained in yeast extract peptone dextrose agar and grown in yeast extract peptone dextrose broth, aerobically, at room temperature, on a shaker. S. oralis 34, S. oralis 108, Streptococcus gordonii Challis CH1, and Streptococcus mitis (American Type Culture Collection 49456) were grown in brain heart infusion medium (Oxoid), under aerobic, static conditions at 37°C and 5% carbon dioxide. To prepare nonviable cells, suspensions of yeast cells (108/mL) or bacterial cells (109/mL) were produced from overnight broth cultures or subcultures and heated at 95°C for 10 minutes or treated with 100 mmol/L thimerosal or 10% formalin for 1 hour, followed by extensive washing with phosphate-buffered saline [8, 19].
Mouse Oral Infection
The mice used were 6–12-week-old female C57BL/6 mice, purchased from Jackson Laboratories (animal protocol 100358-0215). The C. albicans–S. oralis mouse coinfection model has been described in detail elsewhere [14]. Briefly, mice were immunosuppressed with cortisone acetate (225 mg/kg, administered subcutaneously), on the first and third days after infection. A cotton pellet, saturated with 100 µL of microbial suspension (yeast cells [6 × 108/mL] and/or bacteria [2.5 × 109/mL]), was placed subglossally, in mice under anesthesia for 2 hours. Fresh suspensions of microorganisms were added daily in drinking water. Mice were killed on day 4 or 5 after inoculation.
RNA Extraction and Reverse-Transcription Quantitative Polymerase Chain Reaction
The mouse tongues were homogenized using a POLYTRON homogenizer, and the supernatants were beat by zirconia beads (Ambion). RNA was purified using the QIAgen RNeasy Mini Kit. RNA concentrations and quality were determined using a NanoDrop device (Thermo Scientific). Complementary DNA was synthesized with SuperScript III CellsDirect cDNA Synthesis kits (Invitrogen). Reverse-transcription quantitative polymerase chain reaction was performed with a Bio-Rad CFX96 cycler and the IQTM SYBR Green Supermix kit (Bio-Rad). Primer sequences for mouse genes were from PrimerBank [20, 21]. Sequences for fungal genes are shown in Supplementary Table 1.
Infection of Oral Mucosal Organotypic Constructs
Oral mucosal analogues that mimic keratinized (gingival) or nonkeratinized (floor of the mouth) human oral mucosa have been described elsewhere [22]. Briefly, the constructs consist of TERT-2–immortalized human oral keratinocytes (gingival, OKG4; floor of the mouth, OKF6) seeded on collagen type I–embedded fibroblasts (3T3). Tissues are airlifted for epithelial differentiation and stratification. Tissues were inoculated with 106 cells of C. albicans, 107 cells of streptococci, or a combination and incubated at 37°C in a 5% carbon dioxide incubator for 16 hours. Some tissues were pretreated overnight with calpeptin (40 µmmol/L; Millipore), a calpain inhibitor. In some experiments, recombinant human calpain 1 protein (Calbiochem; 40 µg/mL) was used with Candida.
Immunofluorescence Imaging
Immunofluorescence staining was described elsewhere [10, 13, 14]. Briefly, tissue sections or monolayers of epithelial cells (OKF6 or Madin-Darby canine kidney) were stained with anti-E-cadherin polyclonal antibody (BD Biosciences), rabbit anti–calpain 1 polyclonal antibody (Abcam), mouse anti–calpain 1 monoclonal antibody (Abcam), or the appropriate isotype control, followed by a fluorescein isothiocyanate– or tetramethylrhodamine isothiocyanate–labeled secondary antibody. Epithelial nuclei were visualized using Hoechst 33258 or Syto9 stain (Invitrogen). To visualize epithelial nuclei, cytoplasmic actin, and C. albicans simultaneously, Hoechst 33258 (or Syto9), tetramethylrhodamine isothiocyanate–Phaloidin (Sigma), and a fluorescein isothiocyanate–labeled anti-Candida antibody (Meridian Life Science) were used, respectively. S. oralis and S. mitis were visualized by means of fluorescence in situ hybridization with the Streptococcus-specific oligonucleotide probe STR405, conjugated to Alexa 546 [12]. Some tissues were visualized with a Zeiss LSM 780 confocal scanning laser microscope equipped with an argon (488- and 543-nm) laser, using a water immersion C-Apochromat ×40 objective. Other fluorescence images were captured using a Zeiss Axio Imager M1 microscope, using a ×20 objective. Candida cells penetrating into epithelial cells were quantified using the software BioImageXD [23]. The total fungal invasion area and surface biomass were quantified using the IMARIS 7.0 software package [24].
Western Blotting
Tongues were homogenized in lysis buffer [13]. To prepare epithelial monolayer lysates, 4 × 105 OKF6/TERT-2 cells per well were seeded in 6-well plates in complete keratinocyte serum-free medium (Invitrogen) and incubated overnight. The next day, cells were challenged with 4 × 105 heat-killed C. albicans, 4 × 106 heat-killed S. oralis, or a combination, in unsupplemented keratinocyte serum-free medium. Cell lysates were prepared after 48 hours. Total protein was quantified with Pierce BCA Protein Assay (Fisher Scientific), and 40 µg of protein was loaded per lane. After electrophoresis, transfer and blocking, membranes were incubated with goat anti-mouse (R&D) or anti-human E-cadherin (BD Biosciences) or rabbit anti–calpain 1 (Abcam). Glyceraldehyde-3-phosphate dehydrogenase or β-actin was used as an internal loading control. Proteins were detected with Western blot chemiluminescence reagents (Bio-Rad), according to the manufacturer's instructions. The signal density was measured with ImageJ software (National Institutes of Health; https://imagej.nih.gov/ij/).
Calpain Activity Assay
OKF6/TERT-2 cells were seeded on 24-well plates (105 cells per well) overnight, and the next day cells were challenged with 105 heat-killed, formalin-fixed or thimerosal-killed C. albicans, 106 heat-killed S. oralis, or their combination. T-butoxy-carbonyl-L-leucyl-L-methionine amide (20 µmol/L; Molecular Probes), a calpain-specific fluorogenic substrate, was added, and fluorescence was observed microscopically. To quantify fluorescence in a microplate reader, cells were lysed in 200-µL water/well. In some wells, calpeptin (40 µmol/L) or MDL-28179 (50 µmmol/L; Santa Cruz Biotech), a cell-permeable calpain-selective inhibitor, was added 30 minutes before microbial cells as a negative control. Under these conditions, inhibitors were not toxic to epithelial cells, as tested using a lactate dehydrogenase cytotoxicity assay (not shown).
Release of Soluble E-cadherin From Cell Junctions
OKF6/TERT-2 cells were incubated for 30 hours with heat-killed organisms as above, and supernatants were assayed for soluble E-cadherin with enzyme-linked immunosorbent assay, following the manufacturer's instructions (R&D). In some wells, cells were pretreated with calpain inhibitors for 30 minutes to test whether E-cadherin release was calpain mediated. E-cadherin release was also quantified in subnatants collected from mucosal constructs.
Statistical Analyses
Data were analyzed for statistically significant differences with Student t test, using GraphPad Prism software (version 6). Statistical significance for all tests was set at P < .05.
RESULTS
Oral Mucosal Invasion by C. albicans During Coinfection
Previous work showed that S. oralis augments invasion of C. albicans SC5314 into the submucosal compartment of stratified, nonkeratinized oral and esophageal mucosal constructs, formed by TERT-2–immortalized keratinocytes [13, 24]. To extend these findings, we tested whether constructs formed by OKG4/TERT-2 cells, representing the keratinized gingival mucosa, are also more permissive to C. albicans invasion in the presence of S. oralis. As seen in Figure 1A, when inoculated alone, C. albicans forms a biofilm on the mucosal surface with a portion of the biofilm organisms invading the multilayer construct. However, when coinoculated with S. oralis 34 (Figure 1A) or S. oralis 108 (not shown), the entire C. albicans biomass was observed invading through the mucosal layers into the submucosal compartment. When inoculated alone, neither S. oralis strain formed a biofilm on mucosal analogues, consistent with previous findings [13, 24].
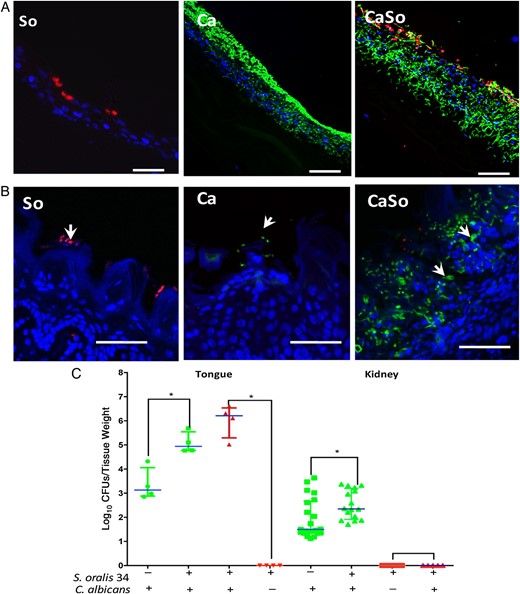
Streptococcus oralis increases Candida albicans oral mucosal invasion in vitro and in vivo. A, B, Mucosal analogues (A) or tongue tissues (B) infected with S. oralis 34 (So), C. albicans SC5314 (Ca) or both in combination (CaSo). Mucosal analogues were infected for 16 hours and tongues were excised 4 days' after infection. Representative tissue sections are shown where C. albicans (green) was visualized after staining with a fluorescein isothiocyanate–conjugated anti-Candida antibody and S. oralis (red) was visualized after fluorescence in situ hybridization with a Streptococcus-specific probe conjugated to Alexa 546; mucosal cell nuclei were counterstained with the nucleic acid stain Hoechst 33258 (blue). Overlay of 3-color images shows the position of organisms on the superficial or deeper layers of the oral mucosa (arrows). Bars represent 50 µm. C, Recovery of C. albicans and S. oralis from tongues and kidneys. Tongues and kidneys were homogenized, serially diluted and plated for C. albicans (green) or S. oralis (red) colony-forming unit (CFU) counts. Mice were killed 4 or 5 days after infection; tongues were collected on day 4, and kidneys on day 5. Log CFU counts are shown from up to 3 independent mouse experiments, with 4–8 mice per group; bars represent means with standard deviations, *P < .05 (comparison between Ca and CaSo groups). This figure is available in black and white in print and in color online.
To examine Candida invasion in vivo, we examined the mouse dorsal tongue surface. When each organism was inoculated alone, 4 days after infection Candida remained on the surface of the keratin layer (arrows in Figure 1B). However, when coinoculated with S. oralis, C. albicans formed robust biofilms, and hyphal organisms invaded more deeply into the mucosal layers. Interestingly, a large number of hyphae were observed within intercellular spaces (arrows). Compared with monoinfected mice, coinfected mice had higher C. albicans colony-forming unit counts in tongue tissues and greater systemic spread into the kidneys at 4 and 5 days, respectively (Figure 1C). Consistent with previous work [14], S. oralis was not recovered from tongues, unless the mice were coinoculated with C. albicans. Despite high recovery rates in coinfected mice, S. oralis did not invade into the mucosa or spread into the kidneys (Figure 1B and 1C).
Oral Mucosal E-cadherin Protein Levels in Coinfection
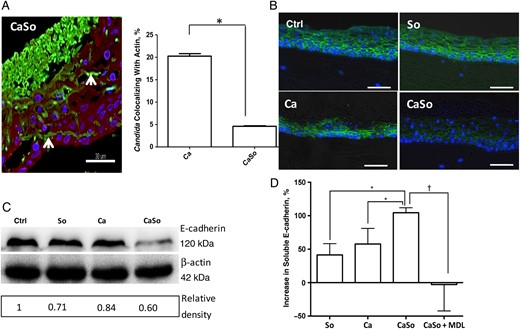
To begin to elucidate the mechanism of fungal invasion, we infected oral mucosal organotypic constructs with the 2 organisms and, using a triple immunofluorescence stain, we traced the position of invading hyphae within the stratified epithelium. Using confocal microscopy, we noticed that C. albicans hyphae invaded the mucosal compartment predominantly through intercellular spaces (Figure 2A). In coinfected tissues, only 5.3% of C. albicans cells that had invaded the mucosal compartment colocalized with actin with the remaining cells invading paracellularly, compared with 20.5% of the cells in monoinfection (Figure 2B). This suggested that coinfection with S. oralis promoted paracellular Candida invasion. Paracellular invasion by C. albicans requires disassembly of epithelial junction proteins [7, 25]. Thus, we asked whether E-cadherin was reduced in the presence of the 2 organisms. E-cadherin signal in mucosal constructs infected with S. oralis only was indistinguishable from uninfected tissues (Figure 2B). Tissues infected with C. albicans exhibited localized loss of E-cadherin, probably owing to proteolysis mediated by fungal aspartyl proteases [7]. Interestingly, when infected with both organisms, the E-cadherin signal was significantly reduced throughout the epithelial layers, suggesting a new mechanism of E-cadherin modulation (Figure 2B).
Oral mucosal E-cadherin is significantly reduced in vitro in coinfection. A, Confocal image of an oral mucosal construct infected with both organisms. Tissues were stained with a fluorescein isothiocyanate–labeled anti-Candida antibody (green), tetramethylrhodamine isothiocyanate–phalloidin (actin; red), and a nuclear stain (blue). Note the almost complete absence of red and green colocalization and the intercellular localization of hyphae (arrow). Bars represent 30 µm. Bar graph shows quantification of the percentage of cells that invade directly through epithelial cells, using the software BioImageXD to measure the percentage of colocalization of green (Candida albicans) and red (cytoplasmic actin) channels within the epithelial layers. *P < .001. B, E-cadherin immunofluorescence stain (green) in uninfected (Ctrl) or infected mucosal constructs. Epithelial constructs formed by OKF6/TERT-2 cells were infected with live Streptococcus oralis 34 (So), C. albicans SC5314 (Ca), or both in combination (CaSo) for 16 hours. Cell nuclei appear in blue. C, OKF6/TERT-2 cells were seeded in 6-well plates and challenged with heat-killed C. albicans SC5314 and/or S. oralis 34 in keratinocyte serum-free medium without supplements, and 48-hour cell lysates were analyzed with Western blotting to assess E-cadherin protein levels. β-Actin was used as an internal loading control. Numbers under each lane represent relative image density; the density of each band was measured with ImageJ software, and the relative image density was calculated by dividing calpain 1 band density by actin density and further normalized with the uninfected control. D, Supernatants were collected in parallel to assay release of soluble E-cadherin by enzyme-linked immunosorbent assay (bar graph). In some wells, the calpain-selective inhibitor MDL-28179 (MDL) or calpeptin was added. Results are means of triplicate experiments and are expressed as the percentage of change relative to unstimulated levels, with standard deviations, using the following formula: (stimulated levels − unstimulated levels)/unstimulated levels × 100%. *P < .05; †P < .005. Similar results were obtained with calpeptin (not shown). This figure is available in black and white in print and in color online.
We then asked whether costimulation with C. albicans and S. oralis can decrease cell-associated E-cadherin protein in a confluent cell monolayer. To prevent E-cadherin degradation mediated by fungal proteases, we used heat-killed organisms. As shown in Figure 2C, bacterial or fungal challenge alone did not significantly affect E-cadherin, whereas the combination of C. albicans and S. oralis triggered a significant reduction. To confirm that E-cadherin was released from cell junctions, soluble E-cadherin was quantified by enzyme-linked immunosorbent assay in culture supernatants. Results showed that S. oralis alone increased soluble E-cadherin release by 41%, C. albicans alone by 57.7% and the combination of the 2 organisms by 104.5% over basal, unstimulated levels (Figure 2D). Importantly, the calpain-selective inhibitor MDL-28179 completely inhibited the release of soluble E-cadherin triggered by these organisms.
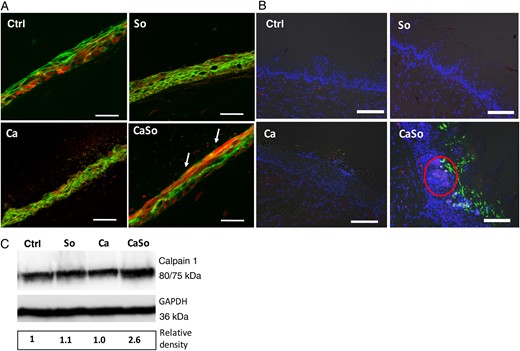
Similarly, in mice receiving combined oral inoculation with C. albicans and S. oralis E-cadherin was almost completely absent in intercellular junctions of the tongue mucosa (Figure 3A), and this was confirmed by Western blot analysis of tongue lysates (Figure 3B). To rule out the possibility that E-cadherin messenger RNA transcription was reduced owing to increased tissue destruction, we performed reverse-transcription quantitative polymerase chain reaction and found that E-cadherin messenger RNA expression was not significantly altered in any of the infection groups, compared with uninfected mice (Figure 3C). Together, these data suggested that C. albicans and S. oralis may manipulate the epithelial cell response to weaken the mucosal barrier via reduction of E-cadherin in intercellular junctions and that this process is calpain mediated.
Oral mucosal E-cadherin is significantly reduced in coinfected mice. A, Overlay images of tongue tissue sections stained for E-cadherin (green) and counterstained with nucleic acid stain Hoechst 33258 (blue). Mice were infected for 4 days. Bars represent 50 µm. B, On day 4 after infection, E-cadherin protein levels were assayed in tongue homogenates by means of Western blotting (upper panel); samples are shown from 2 mice in each of 4 groups. Numbers under each lane represent relative image density, calculated as in Figure 2. The density of each band was measured with ImageJ software, and the relative image density was calculated by dividing calpain 1 band density by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) density, and normalized with the uninfected control. C, E-cadherin messenger RNA levels in tongue tissues were also assayed by reverse-transcription quantitative polymerase chain reaction. Bars represent mean fold expression relative to the uninfected group (control [Ctrl]) in 4–5 mice per group. Abbreviations: Ca, Candida albicans only; CaSo, combination of C. albicans and Streptococcus oralis; So, S. oralis only. This figure is available in black and white in print and in color online.
Calpain Protein Activity in Oral Epithelium During Coinfection
Certain bacteria can reduce E-cadherin protein levels in intercellular junctions by activating calpain 1 and 2 proteins in airway or gastric epithelial cells [15, 26]. We thus reasoned that costimulation of oral epithelial cells with C. albicans and S. oralis may increase calpain protein and/or activity levels. To test this hypothesis, we first challenged epithelial (OKF6 and Madin-Darby canine kidney) monolayers with heat-killed C. albicans and S. oralis and observed cell fluorescence after adding a cell-permeable fluorogenic calpain substrate. A low basal level of calpain activity was present in oral epithelial cells, which increased somewhat with either organism alone (Figure 4A and Supplementary Figure 1). However, simultaneous challenge with both organisms triggered a significant increase in calpain fluorescence. To confirm microscopic observations, we quantified calpain fluorescence intensity using a fluorescence reader. The 2 organisms synergistically increased calpain activity, indicated by the significant increase in fluorescence intensity over the sum of fluorescence with each microorganism alone (Figure 4B). Calpain activity triggered by Candida and S. oralis did not differ significantly between thimerosal- or formalin-treated yeast and hyphae (Supplementary Figure 2). Interestingly, the combination of C. albicans with the phylogenetically related organisms S. gordonii or S. mitis did not synergistically increase calpain activity. Consistent with this observation, when tested in organotypic constructs, S. mitis did not significantly increase the invasive potential of Candida (Supplementary Figure 3).
Candida albicans and Streptococcus oralis synergistically increase calpain protein activity in oral epithelium. A, Fluorescence images of epithelial cells (OKF6/TERT-2) stained with a cell-permeable fluorogenic calpain substrate (blue), anti-E-cadherin antibody (red), and counterstained with nucleic acid stain Syto9 (green). OKF6/TERT-2 cells were incubated with heat-killed S. oralis 34 (So), C. albicans SC5314 (Ca) or their combination (CaSo) for 24 hours. Bars represent 50 µm. B, Calpain activity of OKF6/TERT-2 cells challenged with heat-killed S. oralis 34, Streptococcus gordonii CH1 (Sg), Streptococcus mitis (American Type Culture Collection 49456; Sm), C. albicans SC5314, or their combinations (CaSo, CaSg, CaSm) for 24 hours, as measured with a fluorescence reader. In some wells, cells were pretreated with the calpain inhibitor MDL-28179 (MDL) or calpeptin for 30 minutes before addition of microorganisms (open bars). Basal, unstimulated fluorescence has been subtracted from each value, resulting in negative mean values for some conditions. Results are means with standard deviations for each condition set up in triplicate, in 1 of 3 representative experiments. *P < .05 (for comparison with the sum of fluorescence with each microorganism [Ca or So] added alone). Similar results were obtained with calpeptin (not shown). C, OKF6/TERT-2 cells were seeded in 6-well plates and challenged with heat-killed C. albicans SC5314 and/or S. oralis 34 in keratinocyte serum-free medium without supplements, and 24-hour cell lysates were assayed with Western blotting to assess calpain 1 protein levels. β-Actin was used as an internal loading control. Numbers under each lane represent relative image density, calculated as described for Figures 2 and 3. This figure is available in black and white in print and in color online.
To explore whether calpain 1 was involved in this process we immunoblotted calpain 1 using an antibody that can detect both the full-length and the cleaved, active forms of the enzyme. As seen in Figure 4C, challenge of oral epithelial monolayers with the 2 organisms in combination mostly increased levels of the active 75-kDa form of calpain 1, whereas each organism alone had a lower effect.
To extend these observations in mucosal tissues, organotypic constructs and mouse tissues were stained for calpain 1 and E-cadherin protein expression using immunofluorescence. As shown in Figure 5A, when infected with both microorganisms, the calpain 1 signal was significantly intensified at the superficial mucosal layers where the E-cadherin signal was reduced, compared with monoinfection. In mice, monoinfection triggered weak calpain 1 fluorescence throughout the entire mucosa. In coinfection the intraepithelial calpain 1 fluorescence was stronger, especially in areas in contact with microbial biofilms (Figure 5B). These results were corroborated by Western blot analysis, which showed increased calpain 1 in coinfected mice (Figure 5C). The 75-kDa band, corresponding to the active protein, was not detectable in mouse tongue lysates because its concentration per cell is very low in vivo and varies according to tissue or organ type [27, 28]. Taken together, these data indicate that during oral infection C. albicans and S. oralis synergize to trigger a significant host epithelial modulation via increase in calpain 1.
Calpain 1 is increased in mucosal constructs and tongue tissues during coinfection. A, Immunofluorescence stain for calpain 1 (red) and E-cadherin (green) in OKF6/TERT-2 mucosal constructs after 16 hours of infection. Arrows indicate increased red and decreased green fluorescence intensity in the superficial layers of mucosal analogues infected with both organisms. Bars represent 50 µm. B, Overlay images of mouse tongue tissue sections stained for calpain 1 (red) and Candida albicans (green) and counterstained with nucleic acid stain Hoechst 33258 (blue). Tongues were excised on day 4 after infection. Red circle indicates area of increased calpain expression adjacent to invading fungal organisms. C, Calpain 1 protein levels were detected in tongue tissue homogenates of mice by means of Western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal loading control. Numbers under each lane represent relative image density, calculated as described for Figures 2 and 3. Bars represent 50 µm. Abbreviations: Ca, C. albicans SC5314 only; CaSo, C. albicans SC5314 and Streptococcus oralis 34 in combination; Ctrl, uninfected group; So, S. oralis 34 only. This figure is available in black and white in print and in color online.
Role of Secreted Aspartyl Proteases in Increased Fungal Invasion During Coinfection
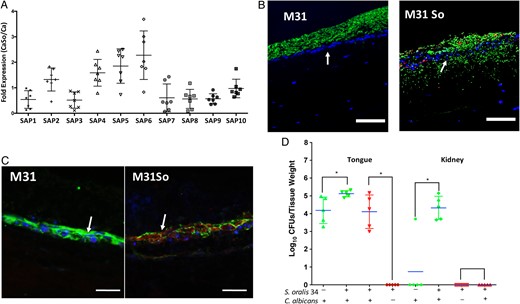
Previous work demonstrated that secreted aspartyl proteinases, particularly Sap5, are involved in the proteolytic degradation of E-cadherin during Candida paracellular invasion [7]. Because S. oralis increased C. albicans burden in mouse tongues, we reasoned that sap gene expression may also be elevated in coinfected mice, thus contributing to mucosal invasion. We screened expression of all Candida protease genes (sap1-10) and found that sap2 and sap4–6 gene expression was increased in coinfection relative to monoinfection (Figure 6A). To examine the role of these proteases in mucosal invasion we used a sap2,4,5,6 mutant, with or without S. oralis, to infect oral mucosal organotypic constructs and mice. As expected from previous findings [7], the mutant formed invasion-deficient biofilms on mucosal constructs. However, in the presence of S. oralis this mutant invaded past the basal epithelial layer into the submucosa (Figure 6B). To clarify the mechanism of invasion, we examined calpain 1 and E-cadherin expression in the mucosal constructs using double immunofluorescence staining. As expected, mucosal constructs coinfected with S. oralis and the Δsap2456 strain showed increased calpain 1 and decreased E-cadherin immunofluorescence, compared with the mutant alone (Figure 6C), suggesting that invasion is mediated by calpain 1.
Fungal secreted aspartyl proteases are not required for invasion during coinfection. A, Candida Sap1–10 gene messenger RNA levels were detected in mouse tongue tissues by reverse-transcription quantitative polymerase chain reaction. Results represent fold increase gene expression in coinfected (Candida albicans CAI4-URA3 combined with Streptococcus oralis 34) relative to single C. albicans–infected mice, on day 4 after infection. Means with standard deviations are shown from 5–6 mice in each of the 2 infection groups. B, Overlay images of mucosal tissue analogues infected with C. albicans strain Δsap2456 (M31) alone or in combination with S. oralis 34 (M31So). Tissues were stained with a fluorescein isothiocyanate–conjugated anti-Candida antibody (green) and an Alexa Fluor 568-labeled fluorescence in situ hybridization probe for S. oralis (red) and counterstained with nucleic acid stain Hoechst 33258 (blue), to visualize epithelial layers. Arrows indicate massive mucosal and submucosal invasion of strain M31 in the presence of S. oralis. C, Triple fluorescence staining for E-cadherin (green), calpain 1 (red), and epithelial nuclei (blue) of mucosal analogues infected with C. albicans strain M31 alone or in combination with S. oralis 34 (M31So). Arrows indicate increased calpain 1 and decreased E-cadherin signal in coinfected tissues. Bars represent 50 µm. D, Recovery of C. albicans and S. oralis from tongue and kidneys. Mice were infected with C. albicans strain M31, with or without S. oralis 34, for 4 or 5 days. Tongues and kidneys were homogenized, serially diluted and plated for C. albicans (green) or S. oralis (red) colony-forming unit (CFU) counts. Tongues were collected on day 4 and kidneys on day 5 after infection. Log CFU counts from 5–8 mice per group are shown. Bars indicate means with standard deviations, *P < .05 (comparison between M31 and M31So groups). This figure is available in black and white in print and in color online.
Oral fungal burdens in mice infected with the Δsap2456 mutant (with or without S. oralis) were attenuated at day 4 after infection compared with the reference strain (not shown), consistent with other reports [29]. However, high numbers of the Δsap2456 mutant were recovered from the tongues and kidneys of all mice coinfected with S. oralis, at days 4 and 5 after infection, respectively, demonstrating that proteases Sap 2,4–6 are not required for fungal colonization, invasion and systemic dissemination when mice are coinfected with streptococci (Figure 6D).
Role of Epithelial Calpain Activity in Increased Mucosal Fungal Invasion
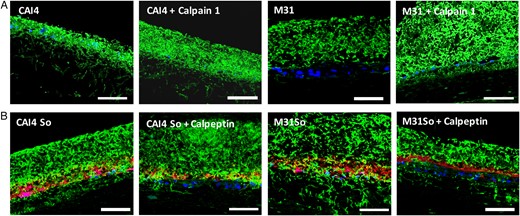
E-cadherin is cleaved by exogenously added calpain 1, and endogenous calpain activity can be inhibited by the tissue-permeable inhibitor calpeptin [30]. We therefore first tested whether, when added to C. albicans–infected mucosal constructs, calpain 1 could promote E-cadherin release and fungal invasion. In Candida-infected constructs calpain 1 triggered E-cadherin release similar to C. albicans–S. oralis–coinfected tissues (Supplementary Figure 4). Because strain CAI4-URA3 was highly invasive on its own, the additional effect of exogenous calpain 1 on invasion was small (Figure 7A and Supplementary Figure 5). However, calpain 1 triggered a massive invasion of the invasion-deficient Δsap2456 strain into the mucosal constructs. Next, we questioned whether the calpain inhibitor calpeptin can reduce fungal invasion in C. albicans–S. oralis–coinfected constructs. Calpeptin significantly reduced invasion of both reference and Δsap2456 strains in the presence of S. oralis, consistent with the significant reduction of E-cadherin release from coinfected tissues (Figure 7B and Supplementary Figure 4). Taken together, these results confirmed that calpain activity is involved in C. albicans mucosal invasion during coinfection.
Candida albicans mucosal invasion during coinfection is enhanced by calpain 1 and inhibited by calpeptin. A, Overlay images of mucosal analogue tissue sections stained with anti-Candida antibody (green), counterstained with nucleic acid stain Hoechst 33258 (blue). Tissues were infected with C. albicans strain M31 or reference strain CAI4-URA3 for 16 hours. Recombinant calpain 1 protein was added during infection. Bars represent 50 µm. B, Overlay images of mucosal analogue tissue sections stained with anti-Candida antibody (green) and Alexa Fluor 568–labeled probe for Streptococcus oralis (red) and counterstained with nucleic acid stain Hoechst 33258 (blue). Tissues were infected with C. albicans strain M31 or reference strain CAI4-URA3, with or without S. oralis 34 (So). Tissues were pretreated overnight with calpeptin, a calpain-selective inhibitor. Bars represent 50 µm. This figure is available in black and white in print and in color online.
DISCUSSION
Our results demonstrate that when C. albicans and S. oralis cocolonize the oral mucosa in high numbers they exploit epithelial calpain activity to compromise the integrity of the mucosal barrier. Although this is a common mechanism of epithelial barrier breach among certain pathogenic bacteria and parasites [31–34], this is the first report of interkingdom synergy between 2 commensal organisms, which can promote invasive, opportunistic infection.
C. albicans invades oral epithelial cells by 2 major mechanisms: induced endocytosis and active penetration [5, 8, 19]. Active penetration requires fungal viability and includes penetration into or between epithelial cells [5, 7, 19]. In an oral carcinoma culture, active penetration between cells was shown to be a rare event [8, 19]. In contrast, in stratified organotypic cultures, which more faithfully mimic the oral mucosa, both processes can occur: penetration into superficial, nonkeratinized epithelial cells and between cells in the deeper epithelial strata [7, 22, 24 and current study]. In monoinfected mucosal analogues approximately 80% of C. albicans cells invaded the deeper strata paracellularly, compared with 95% of cells forming biofilm communities with S. oralis. The latter is a result of E-cadherin dissolution mediated by both fungal proteases [7] and host calpain activity, as shown in this study. Although it is hard to quantify the relative contribution of host and fungal proteases in this process in vivo, based on our experimental evidence, both mechanisms are likely to play a role.
Calpains are cysteine proteases with activities strictly regulated by calcium under physiological conditions [27]. Thus, it is plausible that costimulation with C. albicans and S. oralis increases intracellular calcium flux [15], which enhances translocation of calpain to cell membranes [31]. Toll-like receptor 2 (TLR2) signaling can regulate calcium flux to control calpain activity [15, 26]. Our earlier studies showed that TLR2 expression is significantly up-regulated in the oral mucosa of mice coinfected with C. albicans and S. oralis [14]; thus, it is plausible that TLR2 signaling is involved in calpain activation during coinfection.
S. oralis coaggregation receptor polysaccharides (RPS), adhesin receptors for biofilm organisms, trigger TLR2 expression in endothelial cells. However high RPS concentrations are needed to trigger these responses [35]. S. oralis does not form robust oral mucosal biofilms unless C. albicans is present [13, 14, 24]. It is thus possible that by promoting S. oralis biofilm growth C. albicans increases the availability of RPS for epithelial TLR2 signaling that also requires Candida costimulation. RPS exist on most strains of S. oralis (including strain 34 used in most of our experiments [36]), but only on certain strains of S. gordonii and S. mitis [37]. In addition, there are structural differences among the 3 oral streptococcal species in the RPS disaccharide motifs responsible for biological activity [37], which may explain species-specific epithelial responses.
In conclusion we showed that a fungal and a bacterial member of the core human oral microbiota can synergize to compromise the integrity of the oral mucosal barrier by activating epithelial calpain 1. Candida can take advantage of the disassembly of intercellular junctions to invade deeper into the oroesophageal mucosa and disseminate systemically. Although this route of dissemination was shown to occur in immunocompromised mice, it is still unknown whether this process occurs in humans.
Notes
Acknowledgments. Thanks to the following for their kind gifts: D. Sanglard for Candida albicans Δsap2456 (M31) and reference strain CAI4-URA3, P. E. Kolenbrander for Streptococcus oralis 34, L. Tao for S. oralis 108, and J. Tanzer for Streptococcus gordonii Challis CH1.
Financial support. This work was supported by the US Public Health Service through the National Institute of Dental and Craniofacial Research (grant RO1 DE013986).
Potential conflicts of interest. All authors: No potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References




![Oral mucosal E-cadherin is significantly reduced in coinfected mice. A, Overlay images of tongue tissue sections stained for E-cadherin (green) and counterstained with nucleic acid stain Hoechst 33258 (blue). Mice were infected for 4 days. Bars represent 50 µm. B, On day 4 after infection, E-cadherin protein levels were assayed in tongue homogenates by means of Western blotting (upper panel); samples are shown from 2 mice in each of 4 groups. Numbers under each lane represent relative image density, calculated as in Figure 2. The density of each band was measured with ImageJ software, and the relative image density was calculated by dividing calpain 1 band density by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) density, and normalized with the uninfected control. C, E-cadherin messenger RNA levels in tongue tissues were also assayed by reverse-transcription quantitative polymerase chain reaction. Bars represent mean fold expression relative to the uninfected group (control [Ctrl]) in 4–5 mice per group. Abbreviations: Ca, Candida albicans only; CaSo, combination of C. albicans and Streptococcus oralis; So, S. oralis only. This figure is available in black and white in print and in color online.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/214/6/10.1093_infdis_jiw201/3/m_jiw20103.jpeg?Expires=1716329847&Signature=aMXwy8Dmze5YmDjo7lMzNacr~Z2fQwN7yXetUWqWjD5yPfJH4J780VD2dDzICJWhkPL2FiCs3uWrE6OZPsZ9dWZTpYRzp6vCEYNuSaKdiDh4nPUpQeYhYAo4XJm6IChUHTDWt64o57VmngfKZAYmUm7NnNCXt3u~cuRVrbbV7neEsHjNF5Gd-RP35eYgMpjY2FZCu8l4JuxnBrpquXmbVxQugWUz14wcFJ88ttHA1FrzdemcxxGY2HMt0sPzhijpMCBK7fcjNPrynh5rELvuEQ9cZDp4fB1Y554h9l1vAhWpF~XRtcL82eTIZIi1jkbRxVbRHKfWEdjntbrmUvi-MQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Candida albicans and Streptococcus oralis synergistically increase calpain protein activity in oral epithelium. A, Fluorescence images of epithelial cells (OKF6/TERT-2) stained with a cell-permeable fluorogenic calpain substrate (blue), anti-E-cadherin antibody (red), and counterstained with nucleic acid stain Syto9 (green). OKF6/TERT-2 cells were incubated with heat-killed S. oralis 34 (So), C. albicans SC5314 (Ca) or their combination (CaSo) for 24 hours. Bars represent 50 µm. B, Calpain activity of OKF6/TERT-2 cells challenged with heat-killed S. oralis 34, Streptococcus gordonii CH1 (Sg), Streptococcus mitis (American Type Culture Collection 49456; Sm), C. albicans SC5314, or their combinations (CaSo, CaSg, CaSm) for 24 hours, as measured with a fluorescence reader. In some wells, cells were pretreated with the calpain inhibitor MDL-28179 (MDL) or calpeptin for 30 minutes before addition of microorganisms (open bars). Basal, unstimulated fluorescence has been subtracted from each value, resulting in negative mean values for some conditions. Results are means with standard deviations for each condition set up in triplicate, in 1 of 3 representative experiments. *P < .05 (for comparison with the sum of fluorescence with each microorganism [Ca or So] added alone). Similar results were obtained with calpeptin (not shown). C, OKF6/TERT-2 cells were seeded in 6-well plates and challenged with heat-killed C. albicans SC5314 and/or S. oralis 34 in keratinocyte serum-free medium without supplements, and 24-hour cell lysates were assayed with Western blotting to assess calpain 1 protein levels. β-Actin was used as an internal loading control. Numbers under each lane represent relative image density, calculated as described for Figures 2 and 3. This figure is available in black and white in print and in color online.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jid/214/6/10.1093_infdis_jiw201/3/m_jiw20104.jpeg?Expires=1716329847&Signature=gDqMA4nZn3bcWi5JuSdivmkE9Iec46GEsw6l4AeeIHTy1QXbJPHl7LNdaBrCrvy5shs0emDvVXnVs4F7uPgbbC2c6PDDJugNPjjddFBwEixtcp7tYkmSfEK~FSBXw5guJMRxrF8T1VqoaVsW17ZYQekUfqc3HX22I~AP-5JmbyTel6FVf-FitnVSpAGYd55MpzLOecYxjTsQ8LkbR5rqcyRyxkYAVwXZ4jFRDNzREsCxm9-snQza-vf19oYHQlvsbFN51ONDk8zeG~KH~nbZkdKQqxqd4slSOBPUCiYwWDHpvGFAqwMv81iEjPZ0urAMb9rUzzq~y5dstEeV8eyK6w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)