-
PDF
- Split View
-
Views
-
Cite
Cite
Masatsugu Hamaji, Fumitsugu Kojima, Sho Koyasu, Tomomi Nobashi, Tatsuaki Tsuruyama, Hiroshi Date, Tatsuo Nakamura, A rigid and bioabsorbable material for anterior chest wall reconstruction in a canine model, Interactive CardioVascular and Thoracic Surgery, Volume 20, Issue 3, March 2015, Pages 322–328, https://doi.org/10.1093/icvts/ivu416
Close - Share Icon Share
Abstract
The optimal material for anterior chest wall reconstruction following chest wall resection remains controversial. The aim of this experimental study was to evaluate short-term, morphological and histological outcomes of anterior chest wall reconstruction with a rigid and bioabsorbable material in a canine model.
Twenty adult beagle dogs underwent anterior chest wall resection. In the experimental group (n = 10), the anterior chest wall was reconstructed with a rigid and bioabsorbable material composed of poly-L-lactide acid matrix (60 wt%) and uncalcined and unsintered hydroxyapatite particles (40 wt%), whereas in the control group it was (n = 10) reconstructed with dual polypropylene mesh sheets. Short-term complication rates were compared with a χ2 test. Postoperative sternal deviations were evaluated with sternal alignment angles using computed tomography and multiplanar reconstruction and were compared with Mann–Whitney U-test immediately after reconstruction, and at 1, 3, 6, 9 and 12 months postoperatively. Histological findings of the regenerated chest wall tissue were obtained after staining with haematoxylin and eosin and Elastica van Gieson (EVG) and compared at 3, 6, 9 and 12 months.
There was not a significant difference in the short-term postoperative complication rate (P = 0.53) and the complication rate was 20% (wound infection, n = 1 and lethal mediastinitis, n = 1) in the control group and 10% (wound infection, n = 1) in the experimental group. The postoperative sternal deviation was significantly less remarkable at 1 month (123.3 ± 32.2° vs 159.4 ± 19.7°, P = 0.027), 3 months (109.8 ± 34.7° vs 150.9 ± 34.2°, P = 0.039) and 12 months (61 ± 15.6° vs 170.3 ± 6.6°, P = 0.046) in the experimental group than in the control group, whereas no significant difference was noted immediately after reconstruction (165.7 ± 6.4° vs 168.4 ± 9.1°, P = 0.50). Histological findings showed dense connective tissue in the regenerated chest wall in both groups and showed chondroblasts in the regenerated chest wall tissue at 3 and 6 months only in the experimental group.
Our results suggest that anterior chest wall reconstruction with a rigid and bioabsorbable material is feasible and may be a valuable alternative to reconstruction with a non-rigid and non-absorbable material.
INTRODUCTION
Primary or metastatic malignant sternal tumours, which originate most often from the sternal body, are a rare entity. Surgical resection of malignant sternal tumours, followed by anterior chest wall reconstruction, is associated with favourable long-term overall survival and oncological outcomes [1–3].
The primary goals of anterior chest wall reconstruction are to postoperatively prevent ventilation problems (flail chest, respiratory failure and requirement of mechanical ventilation), protect underlying mediastinal structures and avoid chest wall deformity without excessive rigidity [1, 2, 4]. Anterior chest wall reconstruction has been most frequently performed with non-absorbable materials, which are non-rigid (bovine pericardium, polypropylene, polytetrafluoroethylene, cadaveric fascia lata, extracellular matrix, acellular dermal matrix or polyester) and/or rigid (titanium, acryl resin, cadaveric sternal allograft or polymethylmethacrylate), accompanied by soft tissue coverage [1–9].
The optimal material for anterior chest wall reconstruction remains controversial for several reasons. Overall postoperative complication rates of anterior chest wall reconstructions are not satisfactory [1–5, 9, 10], and appear to be higher than those of lateral chest wall reconstructions [4, 5, 11] frequently with respiratory or wound-related short-term complications [1, 3, 11]. Also, late complications are not negligible [1, 3, 4, 8]. Previous studies failed to show a significant advantage of rigid materials over non-rigid materials in preventing postoperative complications of anterior chest wall reconstructions [3–5, 10, 11], although Martini et al. [1] suggested that there was a tendency of rigid materials towards a lower postoperative complication rate. Specifically, although maintenance of chest wall stability and prevention of postoperative chest wall deformity are among the goals of anterior chest wall reconstruction, morphological evaluation of reconstructed chest walls has not been performed in previous studies, to compare rigid materials with non-rigid materials.
The aim of our experimental study is to investigate the feasibility of anterior chest wall reconstruction with a rigid and bioabsorbable material and to investigate whether it may be a valuable alternative to reconstruction with a non-rigid and non-absorbable material. To date, there has been no experimental study on anterior chest wall reconstruction with a rigid and bioabsorbable material.
MATERIALS AND METHODS
A rigid and bioabsorbable material
The Super-FIXSORB-MX® (Takiron Co Ltd, Osaka, Japan) is a rigid, bioabsorbable and radiopaque material, which has been indicated for clinical use as a bone fixation material with stability and osteoconductivity in orthopaedic, maxillofacial, thoracic and plastic procedures [12, 13]. It has been approved by Food and Drug Administration in 2007 for repair of bone fracture and fixation of bone fragments in maxillofacial regions. Its components are poly-L-lactide matrix (60 wt%) and uncalcined and unsintered hydroxyapatite particles (40 wt%) [14], which contribute to mechanical strength superior to that of the cortical bone for 6 months [14] and excellent bending strength, stiffness and torsional strength compared with that of titanium plates [15]. The bending strength decreases by 15% in 3 months and by 23% in 6 months in vitro, but there have been no data on the decrease in strength in vivo [14]. The 5 cm × 5 cm × 0.7 mm mesh type material was sterilized with ethylene oxide gas and packed in a sterile manner. Super-FIXSORB-MX® is degraded gradually by hydrolysis and finally absorbed by the human body in several years [16].
Surgical procedure
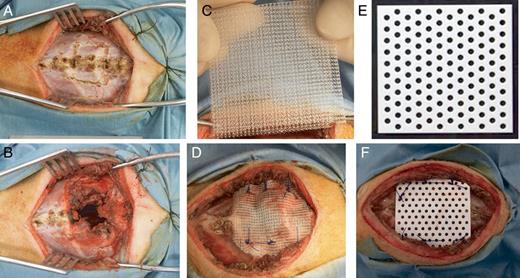
Adult beagle dogs (n = 20, 12 males and 8 females) weighing 8–14 kg were anaesthetized with an intramuscular administration of 15 mg/kg ketamine hydrochloride and 7 mg/kg xylazine and then intubated with an 8 or 8.5-mm endotracheal tube. Mechanical ventilation was maintained using inhalational sevoflurane. A 500-mg dose of ampicillin was injected intramuscularly prior to the skin incision. A mid-sternal incision was made in the supine position and bilateral pectoralis major muscle flaps were made. The flaps were retracted laterally (Fig. 1A). The 3-cm long sternal body along with a pair of rib cartilages was resected with full thickness (Fig. 1B).
A mid-sternal vertical incision was made in a supine position and bilateral pectoralis major muscle flaps were made and retracted laterally (A). The 3-cm long sternal body along with a pair of rib cartilages was resected with full thickness (B). Using 5× 5 cm dual polypropylene mesh (C), the bony defect was reconstructed with No. 1 interrupted polypropylene stitches in the control group (D). Using 5 cm × 5 cm × 0.7 mm, rigid and bioabsorbable mesh material composed of poly-L-lactide acid (60 wt%) and uncalcined and unsintered hydroxyapatite (40 wt%) (E), the defect was reconstructed with No. 1 interrupted polydioxanone stitches in the experimental group (F).
In the control group (n = 10, 6 males and 4 females), the bony defect was reconstructed with 5 × 5 cm dual polypropylene mesh (Marlex®, Bard, Inc., NJ, USA, Fig. 1C) using No. 1 interrupted polypropylene (Prolene®, Ethicon, Inc., NJ, USA) stitches (Fig. 1D). No drainage tube was placed, pectoralis major muscle flaps were sutured in the midline, and the wound was closed in layers with 3–0 polyglactin (Vicryl®, Ethicon, Inc., NJ, USA) stitches.
In the experimental group (n = 10, 6 males and 4 females), the defect was reconstructed with the above mentioned 5 cm × 5 cm × 0.7 mm Super-FIXSORB-MX® mesh material (Fig. 1E) using No. 1 interrupted polydioxanone (PDS II®, Ethicon, Somerville, NJ, USA) stitches (Fig. 1F). The wound was closed as described above.
All surgical procedures were performed by Japanese board-certified thoracic surgeons (M.H. and F.K.) in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1985). The experimental protocol was approved by the Animal Experimental Committee of Kyoto University.
Short-term and long-term postoperative complications of all dogs were followed up and documented until 12 months or until sacrifice.
Radiological analysis for morphological evaluation of postoperative chest wall deformity
All computed tomography (CT) images were obtained with a 16-row multidetector CT scanner (Alexion 16, Toshiba Medical Systems, Tochigi, Japan) at 0 month (n = 10, each), 1 month (n = 10, each), 3 months (n = 10, each), 6 months (n = 9, each), 9 months (n = 7, each) and 12 months (n = 6, each) postoperatively. All the dogs were given an intramuscular ketamine and xylazine for sedation. The images were obtained in the helical mode with 120 kV, 50 mA per section, a 512 × 512 matrix and 0.5-mm slice thickness. Three-dimensional images on all dogs were reconstructed in the control group and in the experimental group, using free computer software (OsiriX Medical Imaging Software). Morphological evaluation was performed with sternal deviation angles (sternal alignment angles) on the mid-sternal sagittal plane, which was obtained with multiplanar reconstruction using Aquarius NET Viewer (TeraRecon, San Mateo, CA, USA). All radiological images were interpreted by two board-certified radiologists (S.K. and T.N.), who were blinded to the characteristics of reconstruction materials.
Histological analysis for regenerated chest wall tissues and the bioabsorbable materials
The animals were randomly sacrificed with an intravenous injection of overdosed sodium pentobarbital. In the control group, the specimen including Marlex® mesh was harvested en bloc at 3 months (n = 1), 6 months (n = 2), 9 months (n = 1) and 12 months (n = 2). After macroscopic evaluation, specimens were preserved in 10% formalin solution for 7 days. Then specimens were dehydrated with a graded ethanol series, decalcified by immersion in 1% ethylenediaminetetraacetic acid (EDTA4Na) and 10% hydrochloric acid solution, embedded in paraffin wax, and sent for histological analysis by light microscopy after staining with haematoxylin and eosin (HE) and Elastica van Gieson (EVG). In the experimental group, the specimen was harvested, after the Super-FIXSORB-MX® mesh was removed. Then after macroscopic evaluation, specimens were sent in the same way at 3 months (n = 1), 6 months (n = 2), 9 months (n = 1) and 12 months (n = 2). All above specimens were examined and interpreted by a board-certified pathologist (T.T.).
The removed bioabsorbable materials (Super-FIXSORB-MX® meshes) were sent for scanning electron microscopy after macroscopically evaluated.
Statistical analysis
The short-term (within 30 days) postoperative complication rates were compared with a χ2 test between the control group and the experimental group. Descriptive statistics for continuous variables are reported as mean ± standard deviation. For the comparison of continuous variables between the groups, the Mann–Whitney U-test was used as appropriate. All statistic tests were two-sided, and a P-value of <0.05 was defined as statistically significant. The JMP version 10.0.1 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
RESULTS
Short-term and long-term postoperative complications in comparison with the control group and the experimental group
There was not a significant difference in the short-term postoperative complication rate between the groups (P = 0.53). The complication rate was 20% (superficial wound infection; n = 1, lethal mediastinitis; n = 1) in the control group and 10% (superficial wound infection; n = 1) in the experimental group. Both animals with wound infection were successfully treated with antibiotics and the reconstruction materials were not removed. However, the animal in the control group was noted for an abscess formation when sacrificed at 6 months postoperatively. No respiratory failure or flail chest was noted in either group.
Radiological findings for morphological evaluations of postoperative chest wall deformity
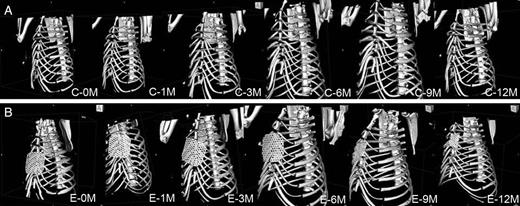
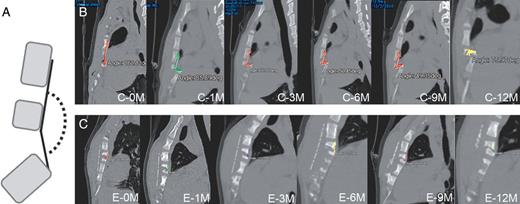
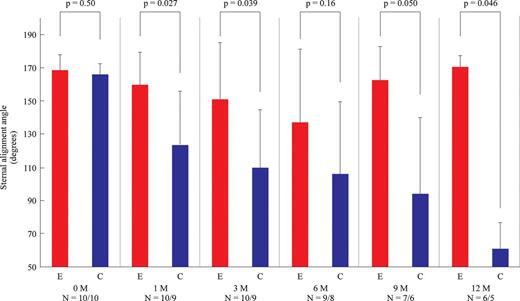
Representative three-dimensional images of the reconstructed chest walls in the control group and in the experimental group are shown in Fig. 2. In the experimental group, all the bioabsorbable materials were clearly visualized at each time point and appeared almost intact until 3 months postoperatively. Representative images of sternal deviations on the mid-sternal sagittal plane at each time point in the control group and in the experimental group are shown in Fig. 3. In the control group, the cephalad end of the lower remnant sternum tended to deviate dorsally, while the deviation was much less remarkable in the experimental group. Also, the sternal deviation appeared to aggravate between 0 month (immediately after reconstruction) and 3 months postoperatively. The measured angles for postoperative sternal deviations (sternal alignment angles) are summarized in Fig. 4. There was not a significant difference immediately after reconstruction between the control group and the experimental group (165.7 ± 6.4° vs 168.4 ± 9.1°, P = 0.50). There were significant differences between the groups at 1 month (123.3 ± 32.2° vs 159.4 ± 19.7°, P = 0.027), 3 months (109.8 ± 34.7° vs 150.9 ± 34.2°, P = 0.039) and 12 months (61 ± 15.6° vs 170.3 ± 6.6°, P = 0.046), whereas there were tendencies at 6 and 9 months (106.3 ± 42.9° vs 136.9 ± 44.1°, P = 0.16 and 94.3 ± 45.4° vs 162.4 ± 19.9°, P = 0.050, respectively) for less deviation in the experimental group than in the control group.
Representative three-dimensional images of the reconstructed anterior chest wall in the control group (A) and in the experimental group (B). The cephalad end of the lower remnant sternum tended to deviate dorsally in the control group. The rigid and bioabsorbable material in the experimental group appeared stable until 3 months, while it started to be degraded at 6 months. C: the control group; E: the experimental group; 0M: 0 month (immediately after reconstruction); 1–12M: 1–12 months postoperatively.
A schema for measurement of a sternal deviation angle (A), Representative the mid-sternal sagittal views on the sternal alignment at each point in the control group (B) and in the experimental group (C). The cephalad end of the lower remnant sternum tended to deviate dorsally more remarkably in the control group than in the experimental group. C: the control group; E: the experimental group; 0M: 0 month (immediately after reconstruction), 1–12M: 1–12 months postoperatively.
Chronological trends of measured sternal deviation angles in the control group and the experimental group. There was no significant difference in the angle between the groups immediately after reconstruction. The deviation angle showed a statistically significant difference between the groups at 1, 3 and 12 months. There was a tendency of the deviation to be less remarkable in the experimental group than in the control group at 6 and 9 months. C: the control group; E: the experimental group; 0M: 0 month (immediately after reconstruction), 1–12M: 1–12 months postoperatively.
Histological (macroscopic and microscopic) findings of the removed bioabsorbable materials and the regenerated chest wall tissue in the control group and in the experimental group
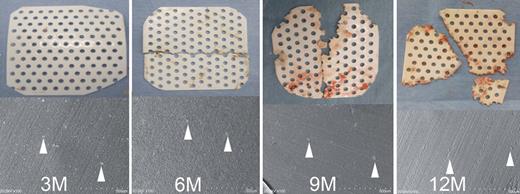
Macroscopic and microscopic images of the removed bioabsorbable materials are shown in Fig. 5. The bioabsorbable material was macroscopically intact at 3 months, and the progress in degradation was noted over time. The findings were compatible with the above radiological findings. The images on scanning electron microscopy showed calcium compounds derived from the animals.
Macroscopic and electron-microscopic findings of the removed bioabsorbable materials. The bioabsorbable material was macroscopically intact at 3 months, and thereafter the degradation process was noted over time. The images on scanning electron microscopy showed calcium compounds (shown by white arrowheads) derived from the animals. 3–12M: 3–12 months postoperatively.
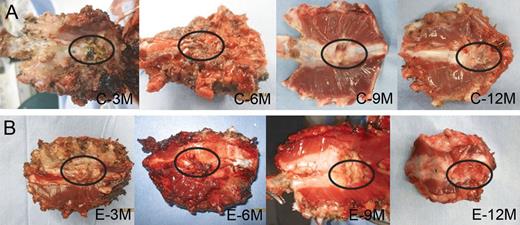
Macroscopic images of regenerated chest wall tissues in the control group and in the experimental group are shown in Fig. 6. In the control group, the polypropylene mesh was completely incorporated in the regenerated chest wall tissue. Dense fibrous tissue developed (encircled) in the bony defect. The regenerated chest wall tissue adhered moderately or severely to the mediastinal thymic tissue, which required sharp dissections at specimen harvesting. In the experimental group, the bioabsorbable material was not bound to the sternal cortex at 3 and 6 months following placement, but bound firmly to the sternal cortex at 9 and 12 months. Dense fibrous tissue developed (encircled) in the bony defect. The regenerated chest wall tissue adhered minimally or mildly to the mediastinal thymic tissue.
Macroscopic images (the mediastinal side) of the specimens (the regenerated chest wall tissue) in the control group (A) and in the experimental group (B). Dense connective tissue (encircled) filled the bony defect in all specimens of the control group and the experimental group. C: the control group; E: the experimental group; 3–12M: 3–12 months postoperatively.
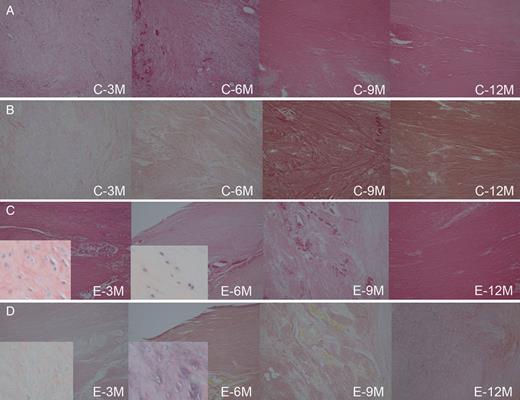
Microscopic images of regenerated chest wall tissues in the control group and in the experimental group are shown in Fig. 7. No granulation tissue was seen in either group. Dense collagenous fibres on HE and EVG stainings were noted to fill the bony defect in both groups. EVG stainings showed no apparent muscular fibres in either group. Chondroblasts were noted for infiltrating the regenerated chest wall tissue at 3 and 6 months in the experimental group, and they were not infiltrating any specimen in the control group. No regeneration of the sternum or osteoblast was noted in either group. In the experimental group, no particle of the bioabsorbable material was identified in any specimen of the chest wall tissue.
Microscopic images of the specimens (the regenerated chest wall tissues) in the control group ((A) haematoxylin and eosin staining, original magnification ×10) ((B) Elastica van Gieson staining, original magnification ×10) and in the experimental group ((C) haematoxylin and eosin staining, original magnification ×10) ((D) Elastica van Gieson staining, original magnification ×10). No granulation tissue but dense collagenous fibres filled the bony defect in the control group and in the experimental group. Chondroblasts (in the magnified parts, ×200) infiltrating the regenerated chest wall tissue at 3 and 6 months were noted in the experimental group, but not in the control group. C: the control group, E: the experimental group, 3–12M: 3–12 months postoperatively
DISCUSSION
Oncological anterior chest wall resection is associated with excellent long-term outcomes [1–3]. Complete resection of malignant chest wall tumours with wide margins is associated with a lower recurrence rate, and extensive chest wall resections require subsequent reconstructions with various materials (biological vs synthetic, bioabsorbable vs non-absorbable and rigid vs non-rigid) [1–11].
Most of the previous clinical or experimental studies on anterior chest wall reconstruction were non-comparative, therefore, it remains unclear which material is associated with more favourable outcomes following anterior chest wall reconstruction. In terms of preventing postoperative respiratory failure including flail chests, rigid materials may be preferred to non-rigid materials [1, 2, 10, 17]. Regarding postoperative wound-related complications including infection, biological or bioabsorbable materials rather than non-absorbable materials may be an advantage [1, 6, 18]. However, little is known about postoperative chest wall deformity.
Our hypotheses were that a bioabsorbable material may be associated with a lower rate of postoperative wound infection and that a rigid material may be advantageous in terms of postoperative chest wall deformity. Therefore, a rigid and bioabsorbable material composed of poly-L-lactide acid matrix (60 wt%) and uncalcined and unsintered hydroxyapatite particles (40 wt%), whose excellent biocompatibility was shown in previous studies [14, 19, 20], was compared with a non-rigid and non-absorbable material in this study.
Regarding short-term and long-term wound-related complications, our findings did not show a significant advantage of the experimental group over the control group. However, a lethal mediastinitis was noted only in the control group. A superficial wound infection was seen both in the control group and in the experimental group. Of interest, the infection recurred later in the control group, not in the experimental group. Our results showed that the short-term outcome of the rigid and bioabsorbable material was at least comparable with that of the non-rigid and non-absorbable material.
Our study suggests that the rigid and bioabsorbable material is associated with less postoperative chest wall deformity. All the reconstructed chest walls in both groups were successfully visualized in three-dimensional CT without artifacts that may complicate metal materials [20]. Postoperative chest wall deformity (the sternal deviation) appeared to progress until 6 months postoperatively, therefore the period may be of utmost importance in preventing postoperative chest wall deformity. The control group showed a dorsal deviation in the cephalad end of the lower remnant sternum, whereas the deviation was significantly less remarkable in the experimental group, which may be attributed to the rigidity of the reconstruction material. Although no bony regeneration was observed in either group, dense connective tissue (collagenous fibres) developed and filled the bony defect without granulation tissues, in contrast to a previous animal study on bioabsorbable materials [21]. We speculate that the dense connective tissue, chondroblasts in the regenerated chest wall tissue of the experimental group, and the animal-derived calcium compounds on the material may reflect a favourable biocompatibility of the material.
The main concern about bioabsorbable materials for anterior chest wall reconstruction is that those may be degraded or absorbed before reliably dense tissues develop in the bony defect. In fact, no group was successful in reconstructing a stable anterior chest wall with non-rigid bioabsorbable materials in adult patients [22, 23]. Because they were not successful due to the fact that their materials were degraded before dense connective tissue developed in the bony defect, we studied a bioabsorbable material that is degraded and absorbed over the years to offer a sufficient interval for maintaining chest wall stability until dense connective tissues fill the bony defect.
There are several limitations into this study. A limited extent of anterior chest wall resection was performed. No postoperative mediastinal or pleural drainage was performed with chest tubes. Our models do not reflect morbid or decompensated patients, neoadjuvant and adjuvant treatments, or infectious aetiologies. No evaluation was performed on protection of mediastinal organs or on dynamic movements of reconstructed chest walls.
In conclusion, anterior chest wall reconstruction with the rigid, and bioabsorbable material composed of poly-L-lactide acid matrix (60 wt%) and uncalcined and unsintered hydroxyapatite particles (40 wt%) appears feasible and may be a valuable alternative to anterior chest wall reconstruction with a non-rigid and non-absorbable material.
Conflict of interest: none declared.
REFERENCES
- postoperative complications
- adult
- dog, domestic
- durapatite
- eosine yellowish-(ys)
- hematoxylin
- hydroxyapatites
- mediastinitis
- muscle rigidity
- reconstructive surgical procedures
- sternum
- wound infections
- chest wall
- experimental study
- chondroblasts
- dense connective tissue
- polypropylene mesh
- anterior chest wall