-
PDF
- Split View
-
Views
-
Cite
Cite
Didier Dewailly, Sophie Catteau-Jonard, Anne-Céline Reyss, Catherine Maunoury-Lefebvre, Edouard Poncelet, Pascal Pigny, The excess in 2–5 mm follicles seen at ovarian ultrasonography is tightly associated to the follicular arrest of the polycystic ovary syndrome, Human Reproduction, Volume 22, Issue 6, June 2007, Pages 1562–1566, https://doi.org/10.1093/humrep/dem060
Close - Share Icon Share
Abstract
We previously hypothesized that the excess of 2–5 mm follicles seen at ovarian ultrasonography might be involved in the follicular arrest (FA) of polycystic ovary syndrome (PCOS), independently from the main putative contributors of FA, namely hyperandrogenism and hyperinsulinism.
A multivariate statistical analysis was applied retrospectively to clinical, biological and ultrasound data that were consecutively collected during 5 years in 457 patients with polycystic ovaries and in 188 age-matched non-hyperandrogenic and regularly cycling controls without PCO at ultrasound.
Stepwise discriminant analysis indicated that in PCOS the 2–5 mm follicle number (FN) gave the strongest correlation to severity of the FA, followed by age and then by fasting insulin level. The other variables [waist circumference (WC), 6–9 mm FN, serum testosterone, FSH, LH and ovarian area] were rejected by the analysis. Multiple linear regression indicated a significant and independent negative relationship between the 2–5 and 6–9 mm FN in the PCOS (r = − 0.186, P < 0.01) and control groups (r = − 0.281, P < 0.01). In PCOS only, the 6–9 mm FN was negatively and independently related to the WC (r = − 0.108, P < 0.05).
The size of the 2–5 mm follicle pool is an independent and important contributor to the FA of PCOS. This result could be explained by an exaggerated physiological inhibitory effect from this pool on the terminal follicle growth. The metabolic derangement of PCOS that also contributes to the FA would act through a different mechanism.
Introduction
Polycystic ovary syndrome (PCOS) is the main cause of anovulatory infertility in women (Franks, 2003). Oligo-anovulation (OA) results from ovarian follicle abnormalities that are thought to be 2-fold (Dewailly et al., 2003; Jonard and Dewailly, 2004). First, in all patients, early follicular growth is excessive, up to the step of follicles becoming sensitive to FSH (i.e. 2–5 mm in diameter). Secondly, the selection of one follicle from the increased pool of selectable follicles (i.e. 6–9 mm in diameter) and its further maturation to a dominant follicle under LH influence does not occur. This second abnormality in the folliculogenesis is named the follicular arrest (FA) and explains the ovulatory disorder of PCOS. Although the FA has not received yet a clear and unanimous explanation, hyperinsulinism (HI) and/or insulin resistance (IR) are considered as important, but not exclusive, contributors to this abnormality (Franks et al., 1998). Furthermore, FA does not always occur in patients with PCOS since some of them do ovulate monthly (Franks et al., 1998; Carmina and Lobo, 2001).
In contrast, the excess of small follicles appears as the salient and constant feature of PCO. Pathological studies have shown that the pool of growing primary and secondary follicles is 2–3‐fold that of normal ovaries while the pool of primordial follicle is normal (Hughesdon, 1982; Webber et al., 2003). With regard to the important intra-ovarian effects of androgens on the small follicle growth (Vendola et al., 1998; Weil et al., 1998), the hyperandrogenism (HA) of PCOS is designated as the main culprit for this follicle excess (Jonard and Dewailly, 2004). However, evidence for this is mainly experimental, based on animal studies (Vendola et al., 1998; Weil et al., 1998). Little clinical data documenting such a phenomenon is available in women with PCOS. Nevertheless, in a previous study using a selective follicular counting by ultrasound (i.e. counting separately the 2–5 and 6–9 mm follicles), we reported that the 2–5 mm follicle number (FN) was positively correlated to the serum testosterone and androstenedione levels in patients with PCOS, all three parameters being in excess in comparison to controls (Jonard et al., 2003a).
In contrast, we also showed in this previous study that the proportion of larger (6–9 mm) antral follicles, relative to the 2–5 mm FN, was much less than in control women. Further, in a subsequent preliminary study (Jonard et al., 2003b), we observed a negative and significant correlation between the two follicle ranges. This may reflect an impaired transition from the 2–5 to 6–9 mm follicle stage and suggests that the excess of 2–5 mm follicles in PCOS could exert an inhibitory effect on further follicular growth. This study was designed to revisit this preliminary data and to analyse whether the contribution of the excess of 2–5 mm follicles to the FA of PCOS was direct, or indirect through other known contributors of follicular derangement in PCOS, such as HA and/or HI (Franks et al., 1998).
Patients and Methods
This study was approved by the Institutional Review Board of the University Hospital of Lille. All patients and controls gave an informed consent before their inclusion in this study. Data were obtained from a database including clinical, hormonal and ultrasound features that were consecutively recorded between 2000 and 2005 in patients referred to our department for HA and/or OA, as previously reported (Dewailly et al., 2006).
Patient population
Controls
The control population consisted of 188 women who were referred to our department for IVF because of tubal and/or male infertility. Exclusion criteria were a history of menstrual disturbances (i.e. cycle length either <25 days or >35 days), hirsutism, abnormal serum level of prolactin or androgens (i.e. serum testosterone above 0.7 ng/ml), PCO at ultrasonography and hormonal treatment during the 3 months before the study.
Patients with PCOS
Four hundred and fifty-seven women were selected for this study. The diagnosis of PCOS was based on the association of at least two of the three following criteria according to the Rotterdam classification (The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group, 2004): (i) ovulatory disturbances, mainly oligomenorrhoea or amenorrhoea; (ii) HA as defined either clinically by hirsutism (modified Ferriman and Gallwey score >8) or severe acne/seborrhoea, and biologically by a testosterone serum level >0.7 ng/ml and (iii) more than 12 follicles in the 2–9 mm range in each ovary at ultrasonography and/or an ovarian volume higher than 10 ml.
Amenorrhoea, oligomenorrhoea or regular cycles were defined as no menses during >3 months, <8 menses in the preceding year or cycle length between 25 and 35 days, respectively.
Investigation procedures
Blood sampling was performed in the early follicular phase, between 8.00 and 9.00 AM, between day 2 and day 7 after the last menstrual period both in PCOS patients and in controls. In PCOS patients, the last menstrual period was either spontaneous or induced by the administration of didrogesterone (10 mg/day for 7 days).
Ultrasound examination was performed with a 7-MHz transvaginal transducer (Logic 400 General Electric, Milwaukee, WI, USA), the same day as blood sampling. Ultrasound measurements were taken in real time, according to a standardized protocol allowing counting separately the 2–5 and 6–9 mm follicles, as previously described (Jonard et al., 2003a). The ovarian area was measured by outlining the ovarian picture on a longitudinal plane, as previously described (Jonard et al., 2003a).
Any patient with at least one follicle with a diameter greater than 9 mm or a serum estradiol level above 80 pg/ml was excluded from the study.
Hormone immunoassays
LH and FSH were measured using chemiluminescent two sites immunoassays on a multi-parameter system (Axsym, Abbott Laboratories, Rungis, France), while testosterone was measured in duplicate using a radioimmunoassay (Coat-a-count, total testosterone kit) provided by Diagnostic Products Corporation (La Garenne Colombes, France). Fasting serum insulin levels were measured in duplicate by an immunoradiometric assay (Bi-Insulin IRMA Pasteur, Bio-Rad, Marnes la Coquette, France) that uses two monoclonal anti-insulin antibodies. Intra- and inter-assay coefficients of variation for the insulin assay were <3.8 and 7.5%, respectively as previously described (Pigny et al., 2003). Results are expressed as mIU/l in terms of the World Health Organization 66/304 reference insulin preparation.
Statistical methods
Comparisons between two independent groups were performed using the non-parametric Mann–Whitney test. One-factor analysis of variance (ANOVA) was used for comparisons between PCOS subgroups with post hoc Bonferroni's correction for 2 × 2 comparisons between each group. A stepwise discriminant analysis was applied to the set of variables that differed significantly by ANOVA between PCOS subgroups. Significant and independent relationships between variables were evaluated by multiple linear regression with partial correlations allowing to control for confounding effect from any variable on the others. All statistical analyses were performed using the Statistical Package for the Social Sciences 11.5 (SPSS Inc., Chicago, IL, USA). A P-value of <0.05 was considered significant.
Results
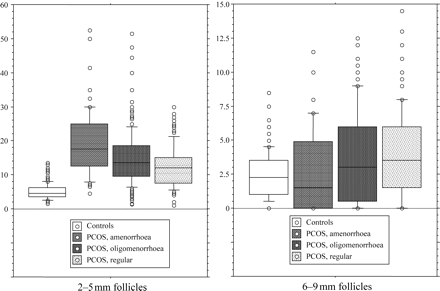
The menstrual status was used as an index of the severity of the FA. Therefore, the whole PCOS group was split into three subgroups according to the menstrual status, i.e. amenorrhoea, oligomenorrhoea or regular cycles, each including 63 (13.8%), 284 (62.1%) and 110 (24%) patients, respectively.
Table 1 shows the median values with 5th–95th percentiles of the main clinical, hormonal and ultrasound parameters in controls and each PCOS subgroup. By the Mann–Whitney test, the rank of values from every PCOS subgroup differed significantly from controls for all parameters, except for age in the regular cycle subgroup (P = 0.054) and for the 6–9 mm FN in the amenorrhoeic subgroup (P = 0.063).
Median values with 5th–95th percentiles (in parenthesis) of the tested variables in PCOS and control groups
| . | PCOS (n = 457) . | Controls (n = 188) . | ||
|---|---|---|---|---|
| . | Amenorrhea (n = 63) . | Oligomenorrhea (n = 284) . | Regular cycles (n = 110) . | |
| Age (years) | 26.0 (17.2–36.2)a,b | 28.0 (20.0–35.5) | 28.0 (21.4–35.2) | 29.0 (22.4–36.0) |
| BMI (kg/m2) | 28.2 (19.4–42.5) | 25.2 (18.8–41.9) | 24.6 (18.4–39.9) | 22.9 (18.1–35.0) |
| WC (cm) | 90.0 (64.0–123.0)b | 82.0 (64.3–117.7) | 80.0 (62.5–113.5) | 73.0 (60.0–102.2) |
| Testosterone (ng/ml) | 0.58 (0.28–1.16)a,b | 0.46 (0.13–.92)c | 0.38 (0.16–.75) | 0.25 (0.08–.54) |
| LH (IU/l) | 6.8 (2.2–17.5) | 6.2 (2.1–14.8) | 4.9 (1.8–13.8) | 3.9 (2.0–7.3) |
| FSH (IU/l) | 4.8 (2.8–7.1) | 5.2 (3.3–7.7) | 5.3 (3.9–7.9) | 5.9 (4.3–8.5) |
| Insulin (mIU/l) | 7.8 (1.2–34.4)a,b | 5.2 (1.0–20.3) | 4.4 (0.9–15.3) | 3.4 (0.9–10.1) |
| 2–5 mm FN | 17.5 (6.6–40.2)a,b | 13.5 (4.0–28.4)c | 11.5 (3.7–24.6) | 4.5 (2.2–9.5) |
| 6–9 mm FN | 1.5 (0–10.0)a,b | 3.5 (0–12.0) | 4.0 (0–12.2) | 2.3 (0–5.5) |
| Ovarian area (cm2) | 6.68 (3.9–9.3)a,b | 5.9 (3.8–8.6)c | 5.47 (3.7–7.4) | 3.9 (2.8–5.1) |
| . | PCOS (n = 457) . | Controls (n = 188) . | ||
|---|---|---|---|---|
| . | Amenorrhea (n = 63) . | Oligomenorrhea (n = 284) . | Regular cycles (n = 110) . | |
| Age (years) | 26.0 (17.2–36.2)a,b | 28.0 (20.0–35.5) | 28.0 (21.4–35.2) | 29.0 (22.4–36.0) |
| BMI (kg/m2) | 28.2 (19.4–42.5) | 25.2 (18.8–41.9) | 24.6 (18.4–39.9) | 22.9 (18.1–35.0) |
| WC (cm) | 90.0 (64.0–123.0)b | 82.0 (64.3–117.7) | 80.0 (62.5–113.5) | 73.0 (60.0–102.2) |
| Testosterone (ng/ml) | 0.58 (0.28–1.16)a,b | 0.46 (0.13–.92)c | 0.38 (0.16–.75) | 0.25 (0.08–.54) |
| LH (IU/l) | 6.8 (2.2–17.5) | 6.2 (2.1–14.8) | 4.9 (1.8–13.8) | 3.9 (2.0–7.3) |
| FSH (IU/l) | 4.8 (2.8–7.1) | 5.2 (3.3–7.7) | 5.3 (3.9–7.9) | 5.9 (4.3–8.5) |
| Insulin (mIU/l) | 7.8 (1.2–34.4)a,b | 5.2 (1.0–20.3) | 4.4 (0.9–15.3) | 3.4 (0.9–10.1) |
| 2–5 mm FN | 17.5 (6.6–40.2)a,b | 13.5 (4.0–28.4)c | 11.5 (3.7–24.6) | 4.5 (2.2–9.5) |
| 6–9 mm FN | 1.5 (0–10.0)a,b | 3.5 (0–12.0) | 4.0 (0–12.2) | 2.3 (0–5.5) |
| Ovarian area (cm2) | 6.68 (3.9–9.3)a,b | 5.9 (3.8–8.6)c | 5.47 (3.7–7.4) | 3.9 (2.8–5.1) |
aAmenorrhoea subgroup significantly different from oligomenorrhoea subgroup (ANOVA with post hoc analysis, P < 0.05).bAmenorrhoea subgroup significantly different from regular cycle subgroup (ANOVA with post hoc analysis, P < 0.05).cOligomenorrhoea subgroup significantly different from regular cycle subgroup (ANOVA with post hoc analysis, P < 0.05); See Results for the comparisons between controls and each PCOS subgroup.
Median values with 5th–95th percentiles (in parenthesis) of the tested variables in PCOS and control groups
| . | PCOS (n = 457) . | Controls (n = 188) . | ||
|---|---|---|---|---|
| . | Amenorrhea (n = 63) . | Oligomenorrhea (n = 284) . | Regular cycles (n = 110) . | |
| Age (years) | 26.0 (17.2–36.2)a,b | 28.0 (20.0–35.5) | 28.0 (21.4–35.2) | 29.0 (22.4–36.0) |
| BMI (kg/m2) | 28.2 (19.4–42.5) | 25.2 (18.8–41.9) | 24.6 (18.4–39.9) | 22.9 (18.1–35.0) |
| WC (cm) | 90.0 (64.0–123.0)b | 82.0 (64.3–117.7) | 80.0 (62.5–113.5) | 73.0 (60.0–102.2) |
| Testosterone (ng/ml) | 0.58 (0.28–1.16)a,b | 0.46 (0.13–.92)c | 0.38 (0.16–.75) | 0.25 (0.08–.54) |
| LH (IU/l) | 6.8 (2.2–17.5) | 6.2 (2.1–14.8) | 4.9 (1.8–13.8) | 3.9 (2.0–7.3) |
| FSH (IU/l) | 4.8 (2.8–7.1) | 5.2 (3.3–7.7) | 5.3 (3.9–7.9) | 5.9 (4.3–8.5) |
| Insulin (mIU/l) | 7.8 (1.2–34.4)a,b | 5.2 (1.0–20.3) | 4.4 (0.9–15.3) | 3.4 (0.9–10.1) |
| 2–5 mm FN | 17.5 (6.6–40.2)a,b | 13.5 (4.0–28.4)c | 11.5 (3.7–24.6) | 4.5 (2.2–9.5) |
| 6–9 mm FN | 1.5 (0–10.0)a,b | 3.5 (0–12.0) | 4.0 (0–12.2) | 2.3 (0–5.5) |
| Ovarian area (cm2) | 6.68 (3.9–9.3)a,b | 5.9 (3.8–8.6)c | 5.47 (3.7–7.4) | 3.9 (2.8–5.1) |
| . | PCOS (n = 457) . | Controls (n = 188) . | ||
|---|---|---|---|---|
| . | Amenorrhea (n = 63) . | Oligomenorrhea (n = 284) . | Regular cycles (n = 110) . | |
| Age (years) | 26.0 (17.2–36.2)a,b | 28.0 (20.0–35.5) | 28.0 (21.4–35.2) | 29.0 (22.4–36.0) |
| BMI (kg/m2) | 28.2 (19.4–42.5) | 25.2 (18.8–41.9) | 24.6 (18.4–39.9) | 22.9 (18.1–35.0) |
| WC (cm) | 90.0 (64.0–123.0)b | 82.0 (64.3–117.7) | 80.0 (62.5–113.5) | 73.0 (60.0–102.2) |
| Testosterone (ng/ml) | 0.58 (0.28–1.16)a,b | 0.46 (0.13–.92)c | 0.38 (0.16–.75) | 0.25 (0.08–.54) |
| LH (IU/l) | 6.8 (2.2–17.5) | 6.2 (2.1–14.8) | 4.9 (1.8–13.8) | 3.9 (2.0–7.3) |
| FSH (IU/l) | 4.8 (2.8–7.1) | 5.2 (3.3–7.7) | 5.3 (3.9–7.9) | 5.9 (4.3–8.5) |
| Insulin (mIU/l) | 7.8 (1.2–34.4)a,b | 5.2 (1.0–20.3) | 4.4 (0.9–15.3) | 3.4 (0.9–10.1) |
| 2–5 mm FN | 17.5 (6.6–40.2)a,b | 13.5 (4.0–28.4)c | 11.5 (3.7–24.6) | 4.5 (2.2–9.5) |
| 6–9 mm FN | 1.5 (0–10.0)a,b | 3.5 (0–12.0) | 4.0 (0–12.2) | 2.3 (0–5.5) |
| Ovarian area (cm2) | 6.68 (3.9–9.3)a,b | 5.9 (3.8–8.6)c | 5.47 (3.7–7.4) | 3.9 (2.8–5.1) |
aAmenorrhoea subgroup significantly different from oligomenorrhoea subgroup (ANOVA with post hoc analysis, P < 0.05).bAmenorrhoea subgroup significantly different from regular cycle subgroup (ANOVA with post hoc analysis, P < 0.05).cOligomenorrhoea subgroup significantly different from regular cycle subgroup (ANOVA with post hoc analysis, P < 0.05); See Results for the comparisons between controls and each PCOS subgroup.
One-factor ANOVA displayed significant variations between PCOS subgroups for all variables except body mass index (P = 0.063). The results of the 2 × 2 comparisons of PCOS subgroups after post hoc analysis with Bonferroni's correction are indicated in Table 1. Interestingly, the 2–5 and 6–9 mm FNs varied inversely according to the menstrual status (Fig. 1).
Box-and-whisker plots showing the distribution of individual values for 2–5 (left) and 6–9 mm (right) FN in patients with PCOS, according to their menstrual status. Horizontal small bars represent the 5th–95th percentile range, and the boxes indicate the 25th–75th percentile range. The horizontal line in each box corresponds to the median. Open circles represents values beyond the 95th percentile. See Table 1 for comparisons between groups.
After stepwise discriminant analysis, the 2–5 mm FN displayed the strongest association to the menstrual status (step 1: F to eliminate = 12.63), followed by age (step 2: F to eliminate = 6.31) and then by insulin (step 3: F to eliminate = 5.33; F minimum to include = 3.84). The other variables [waist circumference (WC), testosterone, FSH, LH and ovarian area] were rejected by the analysis. When the data were reanalysed by selecting the PCOS patients with a testosterone level > 0.5 ng/ml (n = 139), the discriminant analysis selected only the 2–5 mm FN and rejected all the other variables.
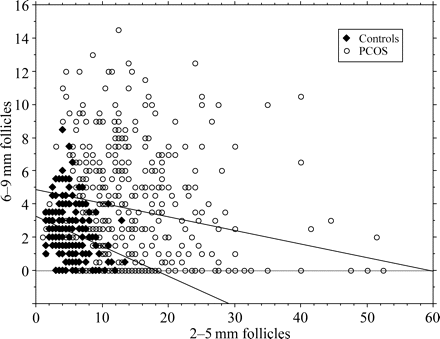
Multiple linear regression was performed in an attempt to explain the relationship between FA and the 2–5 mm FN. A significant and independent negative relationship was found between the 2–5 mm and 6–9 mm FN in the whole PCOS group (r = − 0.186, P < 0.01) (Fig. 2). The 2–5 mm FN was also negatively related to age and positively related to testosterone, independently from the other variables (r = − 0.104, P < 0.05 and r = 0.154, P < 0.01, respectively). The 6–9 mm FN was negatively related to WC, independently from the 2–5 mm FN (r = −0.108, P < 0.05).
Negative relationship between the 2–5 and 6–9 mm FN in controls (n = 188) and in patients with PCOS (n = 457). The upper and lower regression lines apply to the PCOS and control group, respectively. See the text for the values of the correlation coefficients.
A similar significant and independent negative relationship between the 2–5 and 6–9 mm FN was found in controls (r = −0.281, P < 0.01) (Fig. 2). In this group, the 2–5 mm FN was also significantly and negatively related to age independently from the other variables (r = −0.238, P < 0.01).
Discussion
Multivariate analysis in our patients with PCOS showed that the 2–5 mm FN was associated very strongly with the severity of the menstrual disorder that we used as a marker of the FA. In our stepwise discriminant analysis, this relationship was the strongest (step 1) and it was independent from two other major determinants of the FA, namely age and fasting insulin level, that were also kept in the model (steps 2 and 3, respectively). Therefore, our data confirm the well-known improving effect of age (Elting et al., 2003) as well as the worsening effect of HI (Franks et al., 1998) on the FA of PCOS. In addition, it shows that the excess in 2–5 mm follicles is another independent and important contributor to the FA of PCOS.
Putatively explaining such an effect, we observed an independent and strong negative relationship between the 2–5 and 6–9 mm FN, confirming our preliminary report (Jonard et al., 2003b). Interestingly, this relationship was also found in controls, as shown in Fig. 2, thus suggesting the presence of a physiological negative influence from the 2–5 mm follicle pool on the terminal follicle growth at the time of selection, independently from the intra- or extra-ovarian factors known to intervene in this process. Putatively, the larger the number of 2–5 mm follicles, the lower the proportion of them growing to 6–9 mm and further follicles. In the normal situation, such a regulation at the time of follicle selection for dominance would serve to prevent multiple ovulation. In PCOS, this natural phenomenon would be overdriven and would thus impair any follicle to become dominant. Among the factors that may drive this regulation, the anti-Müllerian hormone (AMH) presents itself as a good candidate since its expression is maximal in granulosa cells of this follicle class (Weenen et al., 2004), where it inhibits FSH-induced effects such as aromatase expression (Durlinger et al., 2001). In patients with PCOS, there is a marked increase in the serum AMH level (Pigny et al., 2003; Laven et al., 2004) and we have shown previously (Pigny et al., 2003) that it is tightly related to the 2–5 mm FN but not to the 6–9 mm FN. In addition, we recently reported in a smaller series of patients that serum AMH level was closely related to the severity of the menstrual disorder, along with the 2–5 mm FN (Pigny et al., 2006). We hypothesize, therefore, that in PCO the excess of small antral follicles would create an exaggerated AMH tone within the microenvironment of the selectable follicles. This would impair the action of FSH and would thus contribute to the aromatase inhibition that characterizes the FA of PCOS (Jakimiuk et al., 1997). However, we recognize that this assumption is based on correlation data that do not imply causality.
Surprisingly, serum testosterone was not retained by the stepwise discriminant analysis as a variable contributing to the FA. This disagrees at first sight with many previous reports establishing a link between HA and OA in patients with PCOS (reviewed in Diamanti-Kandarakis et al., 2006). However, the explanation may reside in the fact that HA would contribute to FA via the 2–5 mm follicle excess that it favours (Vendola et al., 1998; Weil et al., 1998). Therefore, this variable did not contribute enough by itself in our stepwise discriminant analysis when challenged to stronger variables such as the 2–5 mm FN. In addition, we recently reported that this link is not constant since some patients with PCO are oligomenorrhoeic despite the absence of overt HA (Dewailly et al., 2006). Conversely, other patients with HA and PCO are eumenorrhoeic (Carmina and Lobo 2001). Altogether, these observations may indicate that in many situations the peripheral androgenic status poorly reflects the intra-ovarian androgen effects. However, the fact that only the 2–5 mm FN was selected after including only those PCOS patients with a testosterone level > 0.5 ng/ml strengthens our conclusion that HA influences the menstrual cycle abnormalities through its effect on the follicle excess in PCOS. In the more frankly hyperandrogenic patients, this relationship overcomes the ones involving age or HI. Lastly, that serum LH and FSH were not retained by the analysis fits with the current opinion that the FA of PCOS is mainly driven by intra-ovarian abnormalities and that the gonadotropin derangement is secondary (Franks et al., 1998).
In this study, the independent relationship between FA and insulin confirms the worsening effect of HI and/or IR on the FA of PCOS (Franks et al., 1998). This effect does not seem to act through amplification of the negative effect exerted by the excess of 2–5 mm follicles. Rather, it would be additive through different mechanisms, as suggested in this study by the inverse relationship between the 6–9 mm FN and the WC, independently from the 2–5 mm FN. Since WC is strongly associated to HI and/or IR, this finding is also in line with numerous reports about the negative effect of the metabolic component of PCOS on the ovulatory status (reviewed in Pasquali et al., 2006). Further to a quantitative effect (i.e. reduced proportion of 6–9 mm follicles), this metabolic influence may also exert a qualitative derangement of granulosa cells, as suggested by an insufficient production of inhibins at that follicle stage in PCO (Welt et al., 2005). This could explain why in our study patients were amenorrhoeic or oligomenorrhoeic despite the presence of an absolute number of 6–9 mm follicles that was normal or greater than controls, respectively.
To conclude, this study shows that the size of the 2–5 mm follicle pool has a very strong and specific impact on the FA of PCOS. We propose the hypothesis of an autoinhibiting effect within the selectable follicle pool that exists in normal women but is exaggerated in PCOS. However, further research is warranted to support this hypothesis and clearly, more experimental data are needed in humans to elucidate which factor(s) is involved in this inhibition.
Acknowledgements
We thank Mrs Francine Becquin for her excellent technical help, Ms Sophie Delva, Céline Vandaele and Claire Vandamme for collecting the clinical data and Mrs Lydie Lombardo and Sylvie Vanoverschelde for collecting the blood samples. We thank Dr Patrick Devos, Centre d'études et de Recherches en Informatique Médicale, University of Lille 2, for his help in the Statistics.