-
PDF
- Split View
-
Views
-
Cite
Cite
A.K. Ludwig, J.M. Weiss, S. Tauchert, T. Dietze, S. Rudolf, K. Diedrich, A. Peters, K.M. Oltmanns, Influence of hypo- and hyperglycaemia on plasma leptin concentrations in healthy women and in women with polycystic ovary syndrome, Human Reproduction, Volume 22, Issue 6, June 2007, Pages 1555–1561, https://doi.org/10.1093/humrep/dem041
Close - Share Icon Share
Abstract
Insulin resistance and obesity play an important role in the pathogenesis of polycystic ovary syndrome (PCOS). It is known that experimentally induced insulin resistance diminishes the stimulatory effect of insulin on leptin secretion. It is not yet known whether the long-term insulin resistance as found in PCOS patients alters the leptin response to hypo- and hyperglycaemia.
We induced hyper- and hypoglycaemia by glucose clamp technique in 7 patients with PCOS and 20 healthy controls. After a plasma glucose level of 8.8 mmol/l was reached, the plasma glucose level was reduced stepwise to 6.8, 4.8 and 2.8 mmol/l.
The PCOS patients required lower glucose infusion rates to reach the glycaemic targets (P < 0.05). Serum insulin and C-peptide concentrations increased significantly during the clamp compared with the baseline in both groups (P < 0.001 for insulin, and P < 0.001, P < 0.005 for C-peptide control and PCOS, respectively) and increased significantly more in PCOS patients compared with the control group (both P < 0.05). Basal leptin levels were significantly higher in the PCOS group than in the control group (P = 0.005). In the controls, the leptin concentration increased significantly during the clamp (P < 0.001 for each glycaemic target), whereas in the PCOS group, leptin secretion increased only during hypoglycaemia (P = 0.04).
Compared with the healthy controls, the response of leptin secretion to hyper- and hypoglycaemia was diminished in PCOS patients. Changes in leptin secretion seem not to be caused by hyper- and hypoglycaemia, but rather by hyperinsulinaemia. Reduced insulin sensitivity seems to be responsible for the diminished leptin response, which might contribute to the obesity found in PCOS patients.
Introduction
Polycystic ovary syndrome (PCOS) is a common endocrine disorder characterized by menstrual dysfunction and hyperandrogenism. An association of PCOS with insulin resistance, hyperinsulinaemia and alterations in β- cell function has been established and predisposes PCOS patients to develop a metabolic syndrome (Schröder et al., 2004). Although also lean patients suffer from PCOS, the majority of PCOS patients are overweight. Adipose tissue has been established as a major endocrine organ. Leptin is a product of the ob gene and provides the central nervous system with feed-back information about fat storage of the body. Thus, leptin is thought to be a part of the regulation of appetite, food intake and the metabolic rate (Janeckova, 2001). Recent research has demonstrated that leptin plays an integral role in the normal physiology of the reproductive system. Observational studies have demonstrated that states of leptin excess, deficiency or resistance can be associated with abnormal reproductive function (Moschos et al., 2002).
Although insulin resistance and obesity play an important role in the pathophysiology of PCOS, a definitive role for has not been established, yet. Most studies did not find a PCOS-specific influence on leptin, as no significant differences were found between body mass index (BMI)-matched PCOS patients and healthy controls (Laughlin et al., 1997; Mantzoros et al., 1997; Rouru et al., 1997). The main determinants of leptin concentrations in these studies were fat mass and the degree of obesity and of insulin resistance. However, Brzecheffa et al., (1996) found significantly higher leptin levels in lean PCOS patients compared to lean controls (P < 0.005) and comparable leptin levels compared to obese controls. Twenty-nine percentage of PCOS patients had leptin levels above the 99% prediction interval for their BMI (Brzechffa et al., 1996).
Insulin is thought to be an important regulator of leptin secretion. However, increasing evidence suggests that insulin-mediated glucose uptake, rather than insulin per se, regulates circulating leptin concentration (Frühwald-Schultes et al., 2002). It has been shown that experimentally induced insulin resistance diminishes the stimulatory effect of insulin on the leptin secretion. So far it has not been studied, whether the long-term insulin resistance found in PCOS patients alters the leptin response to hypo- and hyperglycaemia. An alteration in leptin response to hyper- and hypoglycaemia might contribute to the pathogenesis of obesity in PCOS patients.
Therefore, we performed hyperinsulinaemic clamp experiments in healthy women and in PCOS patients in order to study the influence of hyperglycaemia and hypoglycaemia on leptin secretion and to determine possible differences between healthy women and women with PCOS.
Material and Methods
Study population
Twenty healthy women and seven patients with PCOS participated in the study. The diagnosis of PCOS was based on the criteria established by the National Institutes of Health (NIH) in 1990 [oligomenorrhoea with a menstrual cycle length >35 days or amenorrhoea and either clinical signs of hyperandrogenism (hirsutism, obvious acne, alopecia) and/or an elevated total testosterone]. Non-classical congenital adrenal hyperplasia, androgen-secreting neoplasms and hyperprolactinaemia were excluded previously. All patients underwent a transvaginal ultrasonography. The free androgen index (FAI) was estimated prior to the clamp. The FAI was estimated as follows: testosterone (ng/ml) × 347/sex hormone-binding globulin (nmol/l).
Inclusion criteria for the control group were:
regular menstrual cycle (cycle length 21–35 days)
BMI: 18–24 kg/m2.
Exclusion criteria for the control and the PCOS group were:
chronic or acute illnesses
current medication of any kind
contraception or hormonal therapy
smoking, alcohol or drug abuse
diabetes or hypertension in first degree relatives.
Experimental design
The clamps were performed on days 3–7 of the menstrual cycle or on days 3–7 after induction of a withdrawal bleeding in amenorrhoeic PCOS patients. All subjects were requested to abstain from alcohol, not to perform any kind of exhausting physical activity and to go to bed no later than 22:00 h on the day preceding the study. On the day of the study the participants came to the medical research unit at 08:00 h after an overnight fast of at least 10 h. A cannula was inserted into a vein on the back of the hand, which was placed in a heated box (50–60°C) to obtain arterialized blood. A second cannula was inserted in an antecubital vein of the contralateral arm. Both cannulas were connected to long thin tubes that enabled on the one side blood sampling and on the other side an adjustment of the rate of glucose infusion to reach and maintain a specific blood glucose level. These tubes went to an adjacent room so that the participating women did not notice when taking blood and changing the glucose infusion rate.
After a 30 min baseline period, insulin was infused at a continuous rate of 2.5 mU/min−1 × kg−1. A 20% glucose solution was simultaneously infused at a variable rate to control the plasma glucose concentration. During the clamp, blood glucose levels were repeatedly determined every 5 min by a glucose analyzer (Hemocue®). During the hyperglycaemic clamps, plasma glucose of 8.8 mmol/l was reached during a period of 30 min and it was held for 30 min. The plasma glucose was reduced in a stepwise manner to achieve three more plateaus: 6.8, 4.8 and 2.8 mmol/l. Each plateau was maintained for a 30 min period, and the next lower plateau was induced gradually within the next 30 min.
During the baseline and the four plateaus blood samples were drawn every 15 min for the determination of serum insulin, C-peptide and leptin concentrations.
Assays
All samples were immediately centrifuged and the supernatants were stored at −24°C until assay. Serum insulin was measured by immunoassay using the LKIN1 kit [Immulite, DPC Biermann, Los Angeles, USA; inter‐assay coefficient of variation (CV): <6.1%; intra assay CV: <5.2%]. C-peptide was measured by immunoassay using the LKPE1 kit (Immulite, DPC Biermann, Los Angeles, USA; inter‐assay CV: <6.0%; intra‐assay CV: <5.1). Plasma leptin was measured by enzyme-linked immunosorbent assay (Active®, Webster, TX, USA; inter‐assay CV: <4.9%, intra‐assay CV: <4.4%).
Ethical approval
The study protocol was approved by the Ethics Committee of the University of Schleswig-Holstein, Campus Lübeck, Germany. All participants gave written informed consent before entering the study.
Statistical analysis
Values are presented as mean ± SE. The repeated measurements for insulin, C-peptide and leptin were averaged for the baseline period and each glycaemic target. Statistical analysis was based on analysis of variance for repeated measurements. Concentrations of insulin, C-peptide and leptin were compared by paired Student's t-test, unpaired Student's t-test and Wilcoxon test, as appropriate. P < 0.05 was considered to be statistically significant.
Results
Patients' characteristics
Twenty healthy controls and seven women with PCOS could be included in the study. There were no differences in age and BMI between the control group and the PCOS group. The FAI was significantly higher in the PCOS group than in the controls (P = 0.002). The PCOS patients showed a significantly higher waist-to-hip-ratio (P < 0.05) and a bigger neck circumference (P = 0.002) compared to control patients. All PCOS patients showed polycystic ovaries in the transvaginal ultrasound while none of the controls had polycystic ovaries. There were no significant differences in total serum cholesterol, high- or low-density lipoprotein and triglyceride concentrations (Table I).
Characteristics of patients with PCOS
| . | Control group . | PCOS group . | P-value . |
|---|---|---|---|
| n | 20 | 7 | |
| Age (years) | 25.6 ± 2.2 | 26.8 ± 3.8 | 0.51 |
| BMI (kg/m2) | 21.6 ± 2.1 | 28.0 ± 5.3 | 0.08 |
| Waist-to-hip-ratio | 0.74 ± 0.5 | 0.79 ± 0.9 | 0.03 |
| Neck circumference (cm) | 33.7 ± 1.2 | 36.8 ± 2.8 | 0.002 |
| Fasting glucose level (nmol/l) | 4.33 ± 0.31 | 4.17 ± 0.53 | 0.73 |
| Total testosterone (ng/ml) | 0.38 ± 0.17 | 0.92 ± 0.18 | <0.001 |
| Free androgen index | 3.49 ± 2.25 | 9.68 ± 6.31 | 0.002 |
| Total cholesterol | 4.21 ± 0.4 | 5.04 ± 1.1 | 0.23 |
| LDL | 2.31 ± 0.5 | 3.26 ± 0.9 | 0.11 |
| HDL | 1.58 ± 0.2 | 1.36 ± 0.2 | 0.15 |
| Triglycerides | 0.78 ± 0.3 | 1.49 ± 0.8 | 0.18 |
| . | Control group . | PCOS group . | P-value . |
|---|---|---|---|
| n | 20 | 7 | |
| Age (years) | 25.6 ± 2.2 | 26.8 ± 3.8 | 0.51 |
| BMI (kg/m2) | 21.6 ± 2.1 | 28.0 ± 5.3 | 0.08 |
| Waist-to-hip-ratio | 0.74 ± 0.5 | 0.79 ± 0.9 | 0.03 |
| Neck circumference (cm) | 33.7 ± 1.2 | 36.8 ± 2.8 | 0.002 |
| Fasting glucose level (nmol/l) | 4.33 ± 0.31 | 4.17 ± 0.53 | 0.73 |
| Total testosterone (ng/ml) | 0.38 ± 0.17 | 0.92 ± 0.18 | <0.001 |
| Free androgen index | 3.49 ± 2.25 | 9.68 ± 6.31 | 0.002 |
| Total cholesterol | 4.21 ± 0.4 | 5.04 ± 1.1 | 0.23 |
| LDL | 2.31 ± 0.5 | 3.26 ± 0.9 | 0.11 |
| HDL | 1.58 ± 0.2 | 1.36 ± 0.2 | 0.15 |
| Triglycerides | 0.78 ± 0.3 | 1.49 ± 0.8 | 0.18 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein, Data are mean ± SE.
Characteristics of patients with PCOS
| . | Control group . | PCOS group . | P-value . |
|---|---|---|---|
| n | 20 | 7 | |
| Age (years) | 25.6 ± 2.2 | 26.8 ± 3.8 | 0.51 |
| BMI (kg/m2) | 21.6 ± 2.1 | 28.0 ± 5.3 | 0.08 |
| Waist-to-hip-ratio | 0.74 ± 0.5 | 0.79 ± 0.9 | 0.03 |
| Neck circumference (cm) | 33.7 ± 1.2 | 36.8 ± 2.8 | 0.002 |
| Fasting glucose level (nmol/l) | 4.33 ± 0.31 | 4.17 ± 0.53 | 0.73 |
| Total testosterone (ng/ml) | 0.38 ± 0.17 | 0.92 ± 0.18 | <0.001 |
| Free androgen index | 3.49 ± 2.25 | 9.68 ± 6.31 | 0.002 |
| Total cholesterol | 4.21 ± 0.4 | 5.04 ± 1.1 | 0.23 |
| LDL | 2.31 ± 0.5 | 3.26 ± 0.9 | 0.11 |
| HDL | 1.58 ± 0.2 | 1.36 ± 0.2 | 0.15 |
| Triglycerides | 0.78 ± 0.3 | 1.49 ± 0.8 | 0.18 |
| . | Control group . | PCOS group . | P-value . |
|---|---|---|---|
| n | 20 | 7 | |
| Age (years) | 25.6 ± 2.2 | 26.8 ± 3.8 | 0.51 |
| BMI (kg/m2) | 21.6 ± 2.1 | 28.0 ± 5.3 | 0.08 |
| Waist-to-hip-ratio | 0.74 ± 0.5 | 0.79 ± 0.9 | 0.03 |
| Neck circumference (cm) | 33.7 ± 1.2 | 36.8 ± 2.8 | 0.002 |
| Fasting glucose level (nmol/l) | 4.33 ± 0.31 | 4.17 ± 0.53 | 0.73 |
| Total testosterone (ng/ml) | 0.38 ± 0.17 | 0.92 ± 0.18 | <0.001 |
| Free androgen index | 3.49 ± 2.25 | 9.68 ± 6.31 | 0.002 |
| Total cholesterol | 4.21 ± 0.4 | 5.04 ± 1.1 | 0.23 |
| LDL | 2.31 ± 0.5 | 3.26 ± 0.9 | 0.11 |
| HDL | 1.58 ± 0.2 | 1.36 ± 0.2 | 0.15 |
| Triglycerides | 0.78 ± 0.3 | 1.49 ± 0.8 | 0.18 |
LDL, low-density lipoprotein; HDL, high-density lipoprotein, Data are mean ± SE.
Glucose, insulin and C-peptide
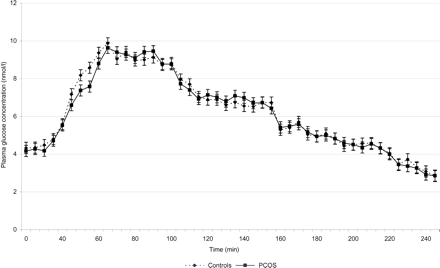
The control group and the PCOS group showed no differences in the fasting plasma glucose concentration at baseline (4.33 ± 0.31 versus 4.17 ± 0.53; P = 0.48). The glucose concentrations during the baseline period of 30 min and during the clamp are shown in Fig. 1.
Glucose concentration (nmol/l) in the control group and the PCOS group (mean ± SE).
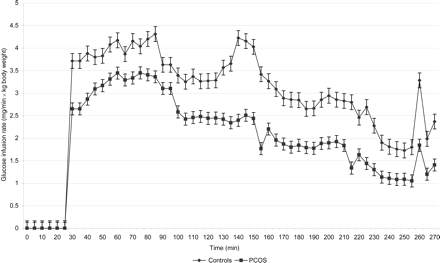
The PCOS patients required less glucose per kg body weight and minute to reach the glycaemic targets than the controls. At 30–40 min (adjusting period for the glycaemic target of 8.8 mmol/l) and from 135 min (during the plateau of the glycaemic target of 6.8 mmol/l) to the end of the clamp, the differences in glucose infusion rates were significant (P < 0.05) (Fig. 2).
The infusion rate of glucose in the control group and the PCOS group per kg body weight (mean ± SE). At 30–40 min and from 135 min to the end of the clamp, the difference in glucose infusion rate was significant (P < 0.05).
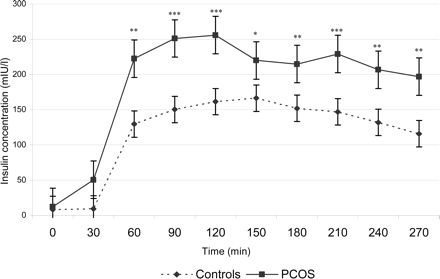
Serum insulin concentrations increased during the clamps significantly compared to the baseline period in both groups (P < 0.001 in both groups). Neither in the control group nor in the PCOS group, was there a significant difference in insulin concentration between the hyperglycaemic state (glycaemic target: 8.8 nmol/l) and the hypoglycaemic state (glycaemic target: 2.8 nmol/l). The control group and the PCOS group showed no significant difference in the insulin concentration at baseline (P = 0.18). However, during the whole clamp period, insulin concentrations remained significantly higher in PCOS patients compared to the control group (P < 0.05) (Fig. 3).
Serum insulin concentration in the PCOS group and the control group (mean ± SE). Significant differences between PCOS and control group are indicated (*P < 0.05; **P < 0.005; ***P < 0.001).
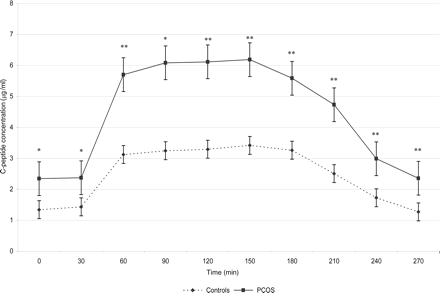
The C-peptide concentrations increased significantly during the hyperglycaemic state (8.8 nmol/l) compared to the baseline period in both groups (P < 0.001 for the control group and P < 0.005 for the PCOS group). During the hypoglycaemic state (2.8 nmol/l), C-peptide concentrations remained above baseline levels in both groups (P = 0.022 for the control group and P = 0.025 for the PCOS group). But the C-peptide levels during the hyperglycaemic state were significantly higher than during the hypoglycaemic state in both groups (P < 0.005 in both groups). During the baseline period and throughout the clamp, the C-peptide concentration of the PCOS patients was significantly higher than of the controls (P < 0.05) (Fig. 4).
C-peptide concentration in the PCOS group and the control group (mean ± SE). Significant differences between PCOS and control group are indicated (*P < 0.05; **P < 0.005).
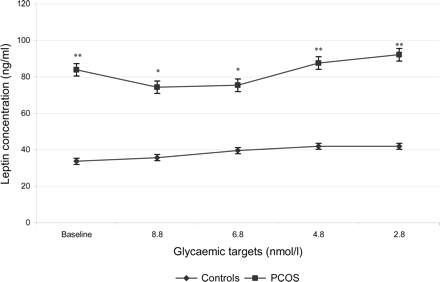
Leptin
At baseline the leptin concentration was significantly higher in the PCOS patients compared to the healthy controls (83.9 ± 68.9 ng/ml versus 33.7 ± 15.3 ng/ml; P = 0.005).
During the clamp the leptin concentration steadily increased above the baseline level in the control group (P < 0.001 for each glycaemic target). In the control group the leptin concentration was significantly higher during hypoglycaemia (2.8 nmol/l) than during hyperglycaemia (8.8 nmol/l) (P < 0.05).
In the PCOS patients the leptin concentration did not change significantly during hyperglycaemia and euglycaemia. During hyperglycaemia, there was a trend towards a decrease in leptin secretion compared with baseline levels in the PCOS group (P = 0.09). The leptin concentration increased significantly above baseline levels during hypoglycaemia in the PCOS group (P = 0.04).
During hyperglycaemia (8.8 nmol/l), mild hyperglycaemia (6.8 nmol/l), euglycaemia (4.8 nmol/) and during hypoglycaemia (2.8 nmol/l), the leptin concentrations remained significantly higher in the PCOS patients compared to the controls (P < 0.05) (Fig. 5).
Leptin concentration in the PCOS group and the control group (mean ± SE). Significant differences between PCOS and control group are indicated (*P < 0.05, **P < 0.005).
Due to baseline differences, the response of leptin to the clamp are more pronounced in the percent changes of leptin concentration than in the absolute levels. In the control group the leptin concentration increased by 6.8% during hyperglycaemia (8.8 mmol/l), 23.1% during mild hyperglycaemia (6.8 mmol/l), 24.1% during euglycaemia (4.8 mmol/l) and 32.5% during hypoglycaemia (2.8 mmol/l) in relation to baseline levels. During hypoglycaemia the increase of leptin concentration was significantly higher than during hyperglycaemia (P < 0.01).
In the PCOS group the leptin concentration decreased initially by 15.8% during hyperglycaemia (8.8 mmol/l) and by 13.6% during mild hyperglycaemia (6.8 mmol/l). Then leptin levels rose by 8.4% during euglycaemia (4.8 mmol/l) and by 15.3% during hypoglycaemia (2.8 mmol/l) in relation to baseline levels. The response of the leptin concentration to hypoglycaemia was significantly different to the response to hyperglycaemia in the PCOS group (P < 0.05).
Discussion
Our results show that the response of leptin secretion to hyper- and hypoglycaemia is diminished in PCOS patients. The long-term insulin resistance might be responsible for the diminished response. The decreased leptin response might contribute to the obesity found in PCOS patients.
Compared to the control group, the PCOS group showed higher levels of insulin and C-peptide during the clamp. The increase of the C-peptide concentration during the clamp is reflecting the endogenous insulin production in spite of the exogenous insulin infusion during the clamp in contrast to the control group that showed only a slight increase in the C-peptide concentration. Moreover, to achieve comparable plasma glucose levels during the clamp, the glucose demand was significantly lower in the PCOS group than in the control group. Together, these results confirm previous findings (Schröder et al., 2004) of a lower insulin sensitivity in the PCOS group. The lower insulin sensitivity might be caused by the PCOS itself. However, it is likely that the higher BMI in the PCOS group contributes to the reduced insulin sensitivity. Campbell and Gerich (1990) could show that insulin sensitivity decreases significantly over a threshold BMI of 26.8 kg/m2.
The increased leptin concentration in PCOS patients compared to healthy controls can be explained by the reduced insulin sensitivity in PCOS patients. Although PCOS and control group showed no significant differences in BMI, the leptin concentration might also be influenced by the BMI. In the PCOS group the leptin concentration correlated to the BMI, while there was no correlation in the control group. However, as an aim of the study was to study a cohort of healthy, non-obese women, the maximum BMI in the control group was 24 kg/m2.
In the healthy controls, the leptin concentration increased during hyperglycaemia and increased further during euglycaemia and hypoglycaemia, while in the PCOS group there was only a significant increase of leptin secretion during hypoglycaemia.
In the literature there are very few data on leptin levels in hyperglycaemic clamp studies. In a 72-h euglycaemic and hyperglycaemic clamp study with five to seven non-obese males per group Boden et al., (1997) found that 72 h of hyperinsulinaemia increased serum leptin levels dose-dependently, but hyperglycaemia or high plasma free fatty acid levels did not affect leptin release (Boden et al., 1997). Ryan and Elahi (1996) examined the acute effect of hyperinsulinaemia (2 h) and hyperglycaemia (1 h) on leptin levels during glucose clamps in 42 athletes and 14 control women. Neither short-term hyperglycaemia nor hyperinsulinaemia affected plasma leptin levels (Ryan and Elahi, 1996). As the leptin level increased further during euglycaemia and hypoglycaemia in our clamp, it is most likely that the hyperinsulinaemia is responsible for the increase in leptin levels. This is in accordance with the study by Boden et al., (1997). In the study by Ryan and Elahi (1996) the duration of the hyperinsulinaemia might have been too short to observe effects on leptin levels.
Although leptin is known to decrease during hypoglycaemia (Boden et al., 1996, Sandoval et al., 2003), we observed an increase in leptin concentration in the control group. The shorter duration of our hypoglycaemic plateau might be responsible for the missing decline of leptin secretion in response to hypoglycaemia in our study. Sandoval et al., (2003) analysed leptin levels during a hyperinsulinaemic hypoglycaemic (2.8 nmol/l) clamp of 120 min. Leptin levels declined significantly in 24 health men and 23 healthy women (BMI: 23 ± 0.5 kg/m2) during the last 30 min of the clamp (5.4 versus 4.0 ng/dl; P < 0.05) (Sandoval et al., 2003).
Therefore, we postulate that the changes of plasma glucose concentration seem not to be responsible for the increase in leptin secretion during our clamp. The changes in leptin secretion seem rather to be caused by the hyperinsulinaemia during the clamp. An increase in leptin concentration during the hyperinsulinaemic clamp is in accordance with pervious data (Schmitz et al., 1997; Wellhoerner et al., 2000; Frühwald-Schultes et al., 2002).
The response of leptin secretion during the clamp was diminished in the PCOS group in which insulin sensitivity and glucose demand were reduced compared to the control group. This is in accordance with observations of Frühwald-Schultes et al., (2002). They performed a 6 h hypoglycaemic clamp in 15 healthy men and in 15 healthy men, in whom an insulin resistance was induced experimentally by a 2.5 h hypoglycaemia on the day before the experiment. During the hypoglycaemic clamp leptin levels increased by 25.4% in healthy men, but not in the experimentally-induced insulin resistant men. The authors suggested that insulin resistance diminishes the stimulatory effect of insulin on leptin secretion (Frühwald-Schultes et al. 2002).
In the PCOS group the leptin concentration showed a trend towards an initial decrease during hyperglycaemia. Interestingly, a transitory decline in leptin levels was also observed by Frühwald-Schultes et al., (2002) in healthy men with experimentally induced insulin resistance. The transitory decrease in leptin levels can most likely be explained by diurnal changes in leptin levels in combination with a strongly reduced stimulatory effect of insulin due to insulin resistance.
Although PCOS and control group show no significant differences in BMI, a weakness of this study lies in the wide range of the BMI. However, our intention was to study a healthy non-obese cohort of women in order to obtain an optimal control group. Because of the nature of PCOS, most PCOS patients presenting to the department are obese. Therefore, only two PCOS patients had a BMI under 24 kg/m2.
Another weakness of the study is the small sample size of PCOS patients. This study was designed as a pilot study focusing on healthy women, but including also PCOS patients. In further studies lean and obese PCOS patients as well as lean and obese weight-matched controls should be studied. The duration of the hyperglycaemic and the hypoglycaemic plateaus should be longer in further studies.
In conclusion, short-term variations of the plasma glucose concentration seem neither to influence leptin secretion in healthy women nor in PCOS patients. The increase of the leptin secretion seems not to be caused by hyper- and hypoglycaemia, but rather by the hyperinsulinaemia.
The response of leptin secretion is diminished in PCOS patients. The long-term insulin resistance might be responsible for the diminished response which might contribute to the obesity found in PCOS patients.