-
PDF
- Split View
-
Views
-
Cite
Cite
Jiayin Wang, Noriyuki Ohara, Zhuo Wang, Wei Chen, Akira Morikawa, Hiroko Sasaki, Deborah A. DeManno, Kristof Chwalisz, Takeshi Maruo, A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFβ3 and their receptors in cultured uterine leiomyoma cells, Human Reproduction, Volume 21, Issue 7, 1 July 2006, Pages 1869–1877, https://doi.org/10.1093/humrep/del035
Close - Share Icon Share
Abstract
BACKGROUND: This study was conducted to evaluate the effects of a novel selective progesterone receptor modulator (SPRM) asoprisnil on the expression of growth factors and their receptors and on growth factor-induced proliferation of cultured uterine leiomyoma and matching myometrial cells. METHODS: The expression of epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I) and transforming growth factor (TGFβ3) was assessed by immunocytochemistry and semi-quantitative RT–PCR. The expression of phosphorylated EGF receptor (p-EGFR), IGF-I receptor α subunit (IGF-IRα) and phosphorylated TGFβ receptor type II (p-TGFβ RII) was assessed by Western blot analysis. Cell proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium assay. RESULTS: Treatment with 10−7 M asoprisnil decreased EGF, IGF-I and TGFβ3 mRNA and protein expression as well as p-EGFR, IGF-IRα and p-TGFβ RII protein expression in leiomyoma cells cultured for 72 h. EGF (100 ng/ml), IGF-I (100 ng/ml) and TGFβ3 (10 ng/ml) increased the number of viable leiomyoma cells cultured for 72 h, whereas the concomitant treatment with 10−7 M asoprisnil antagonized the growth factor-induced increase in leiomyoma cell proliferation. In cultured myometrial cells, however, asoprisnil affected neither the growth factor and their receptor expression nor the cell proliferation. CONCLUSION: Asoprisnil inhibits the expression of EGF, IGF-I, TGFβ3 and their receptors in cultured leiomyoma cells without affecting their expressions in myometrial cells.
Introduction
Uterine leiomyomata are benign sex-steroid-dependent neoplasms occurring in the majority of women by the time they reach menopause. Although historically estrogen has been attributed to the pathogenesis of leiomyomata, there is a growing body of evidence indicating that progesterone plays an important role in regulating the growth of uterine leiomyomata (Rein et al., 1995; Matsuo et al., 1997; Shimomura et al., 1998; Kurachi et al., 2001; Maruo et al., 2004). Several growth factors have been demonstrated to play an important role in leiomyoma growth, including epidermal growth factor (EGF) (Shimomura et al., 1998), insulin-like growth factor-I (IGF-I) (Gao et al., 2001) and transforming growth factor β3 (TGFβ3) (Arici and Sozen, 2003). These growth factors are known to modulate multiple cellular responses through interaction with their receptors.
Uterine leiomyomata are shown to have elevated levels of EGF mRNA (Harrison-Woolrych et al., 1994), IGF-I peptides (Van der Ven et al., 1997), IGF-I mRNA (Boehm et al., 1990; Giudice et al., 1993; Englund et al., 2000), IGF-I receptor (IGF-IR) mRNA and protein (Chandrasekhar et al., 1992; Van der Ven et al., 1997; Dixon et al., 2000), TGFβ3 mRNA (Dou et al., 1996; Lee and Nowak, 2001; Arici and Sozen, 2003) and TGFβ receptor type I (TGFβ RI) and type II (TGFβ RII) mRNA (Dou et al., 1996), compared with those in the adjacent normal myometrium. We have previously demonstrated that progesterone up-regulates the expression of proliferating cell nuclear antigen (PCNA) and EGF in cultured leiomyoma cells (Shimomura et al., 1998) but down-regulates IGF-I expression in those cells without affecting IGF-IR expression (Yamada et al., 2004), suggesting a progesterone-mediated role in the regulation of growth factor signalling in leiomyoma cell proliferation.
Asoprisnil, a member of the novel class of 11β-benzaldoxime-substituted selective progesterone receptor modulators (SPRMs), was developed for the treatment of symptomatic uterine leiomyomata and endometriosis (DeManno et al., 2003; Schubert et al., 2005). SPRMs represent a class of progesterone receptor (PR) ligands that exert clinically relevant tissue-selective progesterone agonist, antagonist, partial or mixed agonist/antagonist effects on various progesterone-target tissues in an in vivo situation depending on the biological action studied (Chwalisz et al., 2005). Early clinical studies with asoprisnil in patients with leiomyomata demonstrated a significant dose- and time-dependent reduction in leiomyoma volume as well as an improvement in leiomyoma symptoms such as menorrhagia and pressure-related symptoms (Chwalisz et al., 2003; Chwalisz et al., 2003, 2004).
However, little is known about the molecular mechanism by which asoprisnil causes the shrinkage of uterine leiomyomata. We have recently demonstrated that asoprisnil inhibits the proliferation of cultured human leiomyoma cells and induces apoptosis of those cells, without having an effect on the matched myometrial cells (Chen et al., 2006). In this context, it is possible that asoprisnil may inhibit leiomyoma growth by down-regulating the expression of growth factors and their receptors, which are known to exert stimulatory effects on leiomyoma cell proliferation. In the present study, we therefore investigated the effects of asoprisnil on the expression of EGF, IGF-I, TGFβ3, phosphorylated EGF receptor (p-EGFR), IGF-IR α subunit (IGF-IRα) and TGFβ RII in cultured human uterine leiomyoma cells and on the growth factor-induced proliferation of cultured leiomyoma cells in comparison with cultured human normal myometrial cells.
Materials and methods
Tissue collection
After informed consent and approval from Kobe University Hospital Institutional Review Board was obtained, 16 independent uterine surgical specimens were collected from hysterectomies performed for leiomyomata in Japanese women with regular menstrual cycles. Uterine leiomyoma and adjacent normal myometrium tissue samples were obtained from each hysterectomy specimen. The patients ranged in age from 29 to 46 years, with a mean age of 36.5 years, and had received no hormonal therapy for at least 6 months before surgery. The histological diagnosis of each uterine specimen was examined. Samples were excluded from the study if accurate menstrual cycle dates could not be assigned or if unexpected pathology was found (e.g. adenomyosis). Each uterine specimen was examined by a pathologist for histological evaluation. Endometrial tissues were obtained from the extirpated uterus, and the day of the menstrual cycle was determined by endometrial histological dating according to the method of Noyes et al. (1950). Eight samples were collected from the proliferative phase of the menstrual cycle, and eight samples were from the secretory phase of the menstrual cycle.
Cell culture
Uterine leiomyoma tissues and adjacent normal myometrium were obtained from the same individual uterus in the proliferative phase or secretory phase of the menstrual cycle. The central parts of leiomyoma tissues were collected with a careful removal of pseudo-capsules and fibrous septa materials. Tissues obtained were dissected from endometrial layers, cut into small pieces and digested in 0.2% collagenase (wt/vol) at 37°C for 3–5 h (Matsuo et al., 1997; Chen et al., 2005). The collagenase treatment was shown to provide a pure population with smooth muscle cell characteristics without stromal or glandular epithelial cell contamination (Matsuo et al., 1997). The leiomyoma cells and normal myometrial cells were collected by centrifugation at 460 g for 5 min and were washed three times with phosphate-buffered saline containing 1% antibiotic solution, respectively. Cell viability was determined by Trypan Blue exclusion test. The isolated leiomyoma cells and normal myometrial cells were plated at densities of approximately 1 × 106 cells/dish in 10-cm2 culture dishes, 4 × 104 cells/well in two-well chamber glass slides and 1 × 104/well in 96-well tissue culture plates. The isolated leiomyoma cells and normal myometrial cells in culture dishes and two-well chamber slides were subcultured at 37°C for 120 h in a humidified atmosphere of 5% CO2 and 95% air in Phenol Red-free Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal bovine serum (v/v; Invitrogen, Life Technologies, Grand Island, NY, USA). Because the PCNA-positive rate of leiomyoma cells was shown to be higher in the secretory phase than in the proliferative phase of the menstrual cycle (Shimomura et al., 1998), we subcultured isolated cells in Phenol Red-free DMEM supplemented with 10% fetal bovine serum for 120 h to abrogate the menstrual cycle-dependent influence on the biological characteristics of cells, confirming that the 120 h subculture produced no differences in the PCNA-positive rate of cultured leiomyoma cells obtained from the different phases (Shimomura et al., 1998). The monolayer cultures reaching approximately 70% confluence were stepped down to serum-free conditions for an additional 72 h in the absence or presence of 10−7 M asoprisnil (TAP Pharmaceutical Products, Lake Forest, IL, USA). We used 10−7 M asoprisnil as a treatment dose in the present study, because we have demonstrated that this concentration is efficacious both in inhibiting proliferation and in inducing apoptosis in cultured leiomyoma cells (Chen et al., in press). Asoprisnil was dissolved in absolute ethanol. Final concentration of ethanol in culture media was <0.01%, and the same concentration of ethanol was used as a vehicle in control cultures.
Cell proliferation assay
The cell viability of cultured cells was colorimetrically determined using a cell proliferation assay kit (CellTiter 96R AQueous One Solution Cell Proliferation assay, Promega, Madison, WI, USA). After cultured leiomyoma and normal myometrial cells were treated with recombinant 100 ng/ml EGF (236-EG, R&D Systems, Minneapolis, MN, USA), 100 ng/ml IGF-I (AF-291-NA, R&D Systems) or 10 ng/ml TGF3 (243-B3, R&D Systems) in the absence or presence of 10−7 M asoprisnil in serum-free, Phenol Red-free DMEM media in a 96-well tissue culture plate for 72 h, 20 µl of CellTiter 96R AQueous One Solution Reagent containing 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and phenazine ethosulfate was added to each well, and cultured cells were incubated at 37°C in a humidified, 5% CO2 atmosphere for 4 h. The absorbance of soluble formazan produced by cellular reduction of the MTS was measured at 490 nm using an MTP-120 ELISA plate reader (Corona Electric, Osaka, Japan). The quantity of formazan product as measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture. The experiments were repeated with at least six different cultured specimens in triplicate.
Immunocytochemical staining for EGF, IGF-I and TGFβ3
Immunocytochemical staining for EGF, IGF-I and TGFβ3 was performed using a polyvalent immunoperoxidase kit (Omnitags, Lipshow, MI, USA), as described previously (Chen et al., 2005). A mouse monoclonal antibody to human EGF (MAB236, R&D Systems), a goat polyclonal antibody to human IGF-I (AF-291-NA, R&D Systems) and a mouse monoclonal antibody to TGFβ3 (MAB643, R&D Systems) were used as the primary antibodies at a dilution of 1:20, 1:50 and 1:50, respectively. Cultured cells were subjected to the same immunoperoxidase method, except that the primary antibody was replaced by non-immune murine immunoglobulin G (IgG) or goat IgG (Miles, Erkhardt, IN, USA) at the same dilution as the primary antibody, as a negative control, resulting in a lack of positive immunostaining for EGF, IGF-I and TGFβ3.
Semi-quantitative RT–PCR
Total RNA was obtained from cultured leiomyoma cells and normal myometrial cells using RNeasy Mini Kit (Qiagen, Chatsworth, CA, USA). First strand cDNA for EGF, IGF-I and TGFβ3 was synthesized from 2 µg total RNA using an Omniscript RT Kit (Qiagen). PCR was performed using 6.25 pmol/l of each primer as described previously (Chen et al., 2005). The amplification conditions consisted of initial denaturation at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s and extension at 72°C for 30 s. The reactions were subjected to 35 cycles using specific PCR primers for EGF (sense primer: 5′-ACATCAAATATCCTCAATGG-3′, antisense primer: 5′-GTGGCATCAAGACCGGGCTGC-3′), IGF-I (sense primer: 5′-AAAATCAGCAGTCTTCCAAC-3′, antisense primer: 5′-AGATCACAGCTCCGGAAGCA-3′) and TGF3 (sense primer: 5′-CCTAAAGGAATTACCTCCAA-3′, antisense primer: 5′-TTCCTCTGACCCCCCTGGCC-3′). Tubes containing all PCR components except the RT reaction mixture were amplified, which served as a negative control to check for the presence of DNA that may have been carried over from a previous reaction. PCR for β-actin (sense primer: 5′-CTTCTACAATGAGCTGCGTG-3′, antisense primer: 5′-TCATGAGGTAGTCAGTCA-3′) was performed as a positive control to exclude potential RNA degradation or RNA transcription default. The PCR-amplified products were visualized on 3% agarose gel and stained with ethidium bromide. The bands were scanned with GT-9700F (Epson, Tokyo, Japan) and qualified with NIH Image version 1.60 (National Institutes of Health, Bethesda, MD, USA). The PCR products were cloned, and sequence analysis revealed their specificity. The intensities of the bands representing EGF, IGF-I and TGFβ3 mRNA were expressed as the ratio to the intensities of the bands representing β-actin mRNA performed in the same experiment. The experiments were repeated with at least three different cultured specimens in triplicate with similar results, and the reported results are representative.
Western blot analysis for p-EGFR, IGF-I Rα and p-TGFβ RII
Western blot analysis was performed as described previously (Chen et al., 2005). At the termination of the cultures, cultured leiomyoma and myometrial cells were incubated at 4°C for 20 min in the presence of lysis buffer consisting of 150 mM/l NaCl, 2 mM phenylmethylsulfonyl fluoride, 1% Nonidet P-40, 0.5% deoxycholate, 1 mg/l aprotinin, 0.1% sodium dodecyl sulphate (SDS) and 50 mM Tris–HCL (pH 7.5). Cells were subsequently scraped off the plates. The lysates were centrifuged at 13 000 g for 30 min, and the supernatants were collected. Protein contents in the supernatants were determined by the Bradford (1976) assay. Each 60 µg aliquot of the proteins extracted from cultured leiomyoma and normal myometrial cells was electrophoresed on a 10% SDS–polyacrylamide gel electrophoresis under reducing conditions. The proteins were electrotransferred onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA, USA). Blots were incubated with a goat polyclonal antibody against p-EGFR (Tyr1173, sc-12351, Santa Cruz Biotechnology, Santa Cruz, CA, USA), a mouse monoclonal antibody against IGF-IRα (1H7, sc-461, Santa Cruz Biotechnology) and a goat polyclonal antibody against p-TGFβ RII (Tyr259, sc-17005, Santa Cruz Biotechnology) at a dilution of 1:400, 1:200 and 1:400, respectively. Antibody binding was detected with horse-radish peroxidase-conjugated anti-mouse or anti-goat secondary antibody (Amersham Biosciences, Arlington Heights, IL, USA) diluted at 1:1000. Antigen–antibody complexes were detected with the enhanced chemiluminescence detection system (Amersham Biosciences). Membranes were visualized by exposure to X-OMAT film (Eastman Kodak, Rochester, NY, USA). The radioautograms were scanned and quantified with ChemiImager 4400 (Astec, Osaka, Japan). The experiments were repeated with at least six different cultured specimens with the similar results, and a representative result was reported.
Statistical analysis
The data were expressed as the mean + SD from at least three independent experiments. Statistical significance was determined using one-way analysis of variance. A difference with a P < 0.05 was considered statistically significant.
Results
Effects of 10−7 M asoprisnil on the expression of immunoreactive EGF, IGF-I and TGFβ3 protein in cultured leiomyoma and normal myometrial cells, as assessed by immunocytochemistry
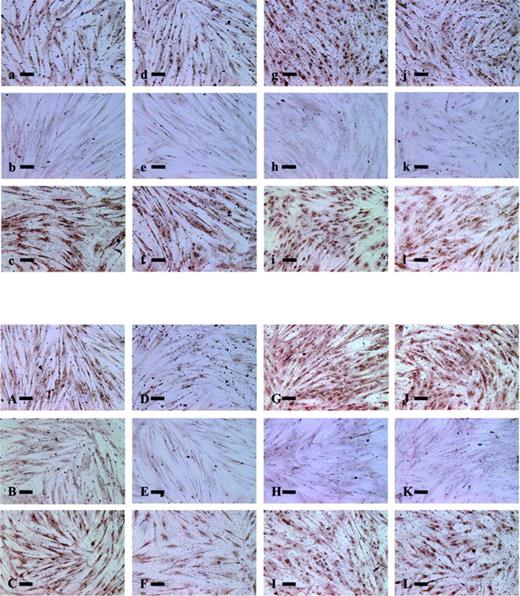
The expression of immunoreactive EGF, IGF-I and TGFβ3 protein in leiomyoma and normal myometrial cells cultured for 48 and 72 h in the absence or presence of 10−7 M asoprisnil was assessed by immunocytochemistry (Figure 1). In leiomyoma cells cultured for 48 h, 10−7 M asoprisnil treatment did not affect immunostaining for EGF (d), IGF-I (e) and TGFβ3 (f) compared with untreated control cultures (EGF, a; IGF-I, b; TGFβ3, c, upper panel). Similarly, in normal myometrial cells cultured for 48 h, 10−7 M asoprisnil treatment had no apparent effects on the immunostaining for EGF (j), IGF-I (k) and TGFβ3 (l) compared with untreated control cultures (EGF, g; IGF-I, h; TGFβ3, i, upper panel).
Effects of asoprisnil on the expression of immunoreactive epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I) and transforming growth factor β (TGFβ3) protein in leiomyoma and normal myometrial cells cultured for 48 or 72 h, as assessed by immunocytochemistry. The expression of immunoreactive EGF, IGF-I and TGFβ3 protein in leiomyoma and normal myometrial cells cultured for 48 h (upper panel) and for 72 h (lower panel). EGF in leiomyoma cells cultured without any treatment (a, A), EGF in leiomyoma cells cultured in the presence of 10−7 M asoprisnil (d, D), IGF-I in leiomyoma cells cultured without any treatment (b, B), IGF-I in leiomyoma cells cultured in the presence of 10−7 M asoprisnil (e, E), TGFβ3 in leiomyoma cells cultured without any treatment (c, C) and TGFβ3 in leiomyoma cells cultured in the presence of 10−7 M asoprisnil (f, F). EGF in normal myometrial cells cultured without any treatment (g, G), EGF in normal myometrial cells cultured in the presence of 10−7 M asoprisnil (j, J), IGF-I in normal myometrial cells cultured without any treatment (h, H), IGF-I in normal myometrial cells cultured in the presence of 10−7 M asoprisnil (k, K), TGFβ3 in normal myometrial cells cultured without any treatment (i, I) and TGFβ3 in normal myometrial cells cultured in the presence of 10−7 M asoprisnil (l, L). Bars represent 50 µm. Original magnification 40.
By contrast, in leiomyoma cells cultured for 72 h, 10−7 M asoprisnil treatment attenuated the expression of immunoreactive EGF (D), IGF-I (E) and TGFβ3 (F) compared with untreated control cultures (EGF, A; IGF-I, B; TGFβ3, C, lower panel). In normal myometrial cells cultured for 72 h, however, asoprisnil treatment had no apparent effects on the immunostaining expression of EGF (J), IGF-I (K) and TGFβ3 (L) compared with untreated control cultures (EGF, G; IGF-I, H; TGFβ3, I, lower panel). Non-immune murine IgG or goat IgG, used as a negative control, showed a lack of positive immunostaining in leiomyoma and normal myometrial cells (data not shown).
Effects of 10−7 M asoprisnil on the expression of mRNAs encoding for EGF, IGF-I and TGFβ3 in cultured leiomyoma cells, as assessed by semi-quantitative RT–PCR
On the basis of the results of immunocytochemical analysis (Figure 1), we further examined the expression of mRNAs encoding for EGF, IGF-I and TGFβ3 in leiomyoma and normal myometrial cells cultured for 72 h in the absence or presence of 10−7 M asoprisnil.
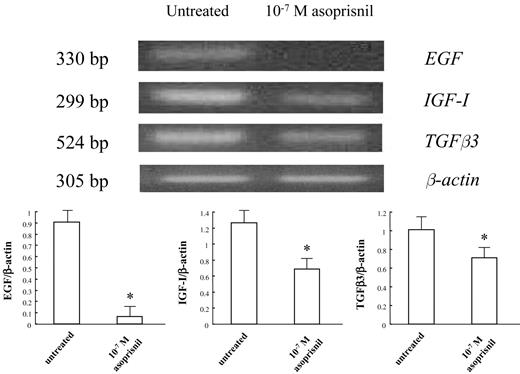
Semi-quantitative RT–PCR analysis demonstrated the presence of a 330 bp fragment of EGF mRNA, 299 bp fragment of IGF-I mRNA and 524 bp fragment of TGFβ3 in untreated leiomyoma cells cultured for 72 h (Figure 2, upper panel). Treatment with 10−7 M asoprisnil resulted in a significant decrease (P < 0.01) in the expression of EGF, IGF-I and TGFβ3 mRNA in leiomyoma cells cultured for 72 h compared with untreated control cultures (Figure 2, lower panel). However, asoprisnil treatment did not affect the expression of EGF, IGF-I and TGFβ3 mRNA in normal myometrial cells cultured for 72 h (data not shown).
Effects of asoprisnil on the expression of mRNAs encoding for epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I) and transforming growth factor β (TGFβ3) in leiomyoma cells cultured for 72 h, as assessed by semi-quantitative RT–PCR. Semi-quantitative RT–PCR analysis demonstrated the presence of a 330 bp fragment of EGF mRNA, 299 bp fragment of IGF-I mRNA and 524 bp fragment of TGFβ3 mRNA in untreated cultured leiomyoma cells (upper panel). The lower panel shows the fold increase of EGF, IGF-I and TGFβ3 mRNA normalized to the respective β-actin in leiomyoma cells cultured for 72 h in the presence of 10−7 M asoprisnil. Treatment with 10−7 M asoprisnil resulted in a significant decrease in EGF, IGF-I and TGFβ3 mRNA expression in leiomyoma cells cultured for 72 h compared with untreated control cultures (lower panel). Densitometric analysis of EGF, IGF-I and TGFβ3 mRNA was performed as described in Materials and methods. β-Actin mRNA was used to ensure the even loading of each specimen. Results represent the mean + SD of the fold increase over the control value of at least three independent experiments. *P < 0.01 versus untreated control cultures.
Effects of 10−7 M asoprisnil on the expression of p-EGFR, IGF-IRα and p-TGFβ RII in cultured leiomyoma and normal myometrial cells, as assessed by Western blot analysis
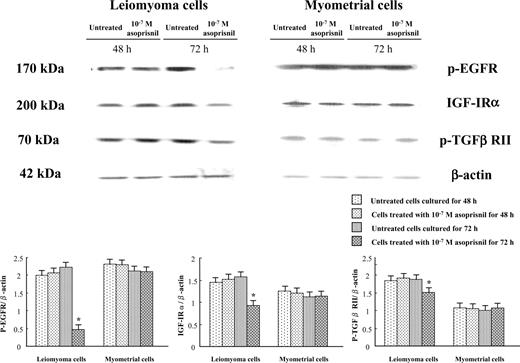
The expression of p-EGFR, IGF-IRα and p-TGFβ RII protein in leiomyoma and normal myometrial cells cultured for 48 and 72 h in the absence or presence of 10−7 M asoprisnil was assessed by Western blot analysis (Figure 3). Treatment with 10−7 M asoprisnil did not affect the expression of p-EGFR, IGF-IRα and p-TGFβ RII in leiomyoma and normal myometrial cells cultured for 48 h but resulted in a significant (P < 0.01) decrease in the expression of p-EGFR, IGF-IRα and p-TGFβ RII in leiomyoma cells cultured for 72 h. In normal myometrial cells cultured for 72 h, however, treatment with 10−7 M asoprisnil did not affect the expression of p-EGFR, IGF-IRα and p-TGFβ RII.
Effects of asoprisnil on the expression of phosphorylated epidermal growth factor receptor (p-EGFR), insulin-like growth factor-I receptor α subunit (IGF-IRα) and phosphorylated transforming growth factor β receptor type II (p-TGFβ RII) in leiomyoma and normal myometrial cells cultured for 48 and 72 h, as assessed by Western blot analysis. Treatment with 10−7 M asoprisnil resulted in a significant decrease in the expression of p-EGFR, IGF-IRα and p-TGFβ RII in leiomyoma cells cultured for 72 h. In cultured normal myometrial cells, however, treatment with 10−7 M asoprisnil did not affect the expression of p-EGFR, IGF-IRα and p-TGFβ RII during the treatment periods of 72 h. Densitometric analysis of p-EGFR, IGF-IRα and p-TGFβ RII was performed as described in Materials and methods. β-Actin was used to ensure the even loading of each specimen. Results represent the mean + SD of the fold increase over the control value of at least six independent experiments performed in triplicate. *P < 0.01 versus untreated control cultures for 72 h.
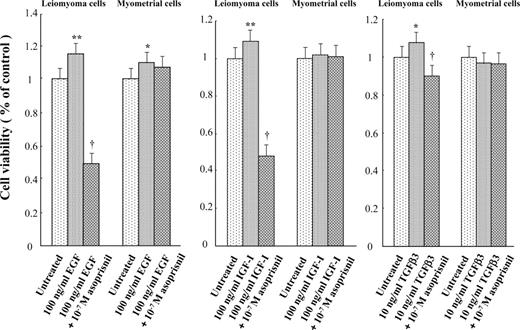
Effects of asoprisnil on the number of viable leiomyoma and normal myometrial cell cultured for 72 h in the presence of either recombinant EGF, IGF-I or TGFβ3, as assessed by MTS assay
Treatment with 100 ng/ml recombinant EGF significantly (P < 0.01) increased the number of viable leiomyoma cells cultured for 72 h compared with untreated control cultures, whereas the concomitant treatment with 10−7 M asoprisnil significantly (P < 0.01) reduced the EGF-induced increase in the viable cultured cells (Figure 4, left panel). In cultured normal myometrial cells, treatment with 100 ng/ml recombinant EGF significantly (P < 0.05) increased the number of viable cells compared with untreated control cultures, whereas the concomitant treatment with 10−7 M asoprisnil did not affect the EGF-induced increase in the viable cultured cells (Figure 4, left panel).
Effects of asoprisnil on the number of viable leiomyoma and normal myometrial cells cultured for 72 h in the presence of either recombinant epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I) or transforming growth factor β (TGFβ3), as assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Treatment with 100 ng/ml recombinant EGF significantly increased the number of viable cultured leiomyoma cells compared with untreated control cultures, and the concomitant treatment with 10−7 M asoprisnil significantly reduced the EGF-induced increase in the viable cultured cells (left panel). In cultured normal myometrial cells, treatment with 100 ng/ml recombinant EGF significantly increased the number of viable cultured cells compared with untreated control cultures, but the concomitant treatment with 10−7 M asoprisnil did not affect the EGF-induced increase in the viable cultured cells (left panel). Treatment with either 100 ng/ml recombinant IGF-I or 10 ng/ml recombinant TGFβ3 significantly increased the number of viable cultured leiomyoma cells compared with untreated control cultures, and the concomitant treatment with 10−7 M asoprisnil significantly decreased either the IGF-I- or TGFβ3-induced increase in the viable cultured cells (middle panel and right panel). In cultured normal myometrial cells, however, treatment with 100 ng/ml recombinant IGF-I or 10 ng/ml TGFβ3 did not affect the cell viability (middle panel and right panel). Results represent the mean + SD of at least six independent experiments performed in triplicate. *P < 0.05, **P < 0.01 versus untreated control cultures, †P < 0.01 versus cultured leiomyoma cells treated with recombinant growth factor alone.
Treatment with 100 ng/ml recombinant IGF-I significantly (P < 0.01) increased the number of viable leiomyoma cells cultured for 72 h compared with untreated control cultures, whereas the concomitant treatment with 10−7 M asoprisnil significantly (P < 0.01) decreased the IGF-I-induced increase in the viable cultured cells (Figure 4, middle panel). In cultured normal myometrial cells, however, treatment with 100 ng/ml recombinant IGF-I did not affect the cell viability either in the absence or in the presence of 10−7 M asoprisnil (Figure 4, middle panel).
Treatment with 10 ng/ml recombinant TGFβ3 significantly (P < 0.05) increased the number of viable leiomyoma cells cultured for 72 h compared with untreated control cultures, whereas the concomitant treatment with 10−7 M asoprisnil significantly (P < 0.01) decreased the TGFβ3-induced increase in the viable cultured cells (Figure 4, right panel). In cultured normal myometrial cells, however, treatment with 10 ng/ml recombinant TGFβ3 did not affect the cell viability either in the absence or in the presence of 10−7 M asoprisnil (Figure 4, right panel).
Discussion
In the present study, we demonstrated that EGF, p-EGFR, IGF-I, IGF-IRα, TGFβ3 and p-TGFβ RII are expressed in both cultured human leiomyoma and normal myometrial cells. This suggests that EGF, IGF-I and TGFβ3 may be involved in the regulation of various cell responses in the two types of cells in an autocrine/paracrine manner. We showed for the first time that treatment with a novel SPRM asoprisnil decreases immunoreactive EGF, IGF-I and TGFβ3 protein expression, EGF, IGF-I and TGFβ3 mRNA expression as well as p-EGFR, IGF-IRα and p-TGFβ3 RII protein expression in leiomyoma cells cultured for 72 h but that asoprisnil treatment did not affect those expressions in cultured normal myometrial cells.
It has been suggested that EGF, IGF-I and TGFβ3 may stimulate leiomyoma cell growth by augmenting the proliferative potential (Maruo et al., 1996; Gao et al., 2001; Lee and Nowak, 2001) and that IGF-I may also act to inhibit apoptosis in cultured leiomyoma cells (Gao et al., 2001). Furthermore, IGF-IR mRNA (Chandrasekhar et al., 1992; Van der Ven et al., 1997; Dixon et al., 2000) and TGF receptor mRNA (Dou et al., 1996) are reported to be overexpressed in uterine leiomyomas compared with adjacent normal myometrium. These data suggest that the growth potential in leiomyoma cells may be augmented compared with normal myometrial cells. However, little is known about the effects of progesterone antagonists or SPRMs on the expression of growth factors and their receptors in cultured leiomyoma and normal myometrial cells.
EGFR and IGF-IR are transmembrane tyrosine kinases (Singh and Harris, 2005; Yakar et al., 2005). Binding of EGF to EGFR causes autophosphorylation of the carboxy-terminal tyrosine residues on EGFR. Activation of EGFR-signalling pathways results in cell-cycle progression, survival, proliferation and cell migration (Singh and Harris, 2005). IGF-IR undergoes the cleavages of α and β subunits. The extracellular domain consists of an α subunit which binds to IGF-I ligand, and the intracellular domain consists of a β subunit which possesses the kinase activity (Yakar et al., 2005). Activation of the IGF-IR-signalling pathways leads to cell survival, proliferation and cell-cycle progression (Yakar et al., 2005). In addition, the TGFβ superfamily of receptors comprises two groups of serine–threonine kinase receptors, TGFβ RI and TGFβ RII (Caestecker, 2004). TGFβ binds to the active TGFβ RII, and then TGFβ RII recruits TGFβ RI (Lutz and Knaus, 2002). The phosphorylation of TGFβ RII plays an autoregulatory role for the kinase activity of this receptor (Wrana et al., 1994; Lawler et al., 1997). Activation of the TGFβ-signalling pathways leads to cellular processes such as proliferation, differentiation and apoptosis (Lutz and Knaus, 2002).
The down-regulation by asoprisnil of the expression of EGF, IGF-I, TGFβ3 and their receptors may disrupt the signalling of the EGF/EGFR system, IGF-I/IGF-IR system and TGFβ3/TGFβ RII system in cultured leiomyoma cells. Because EGF is shown to increase not only the uptake of [3H]thymidine by cultured leiomyoma cells but also PCNA-positive nuclei of those cells compared with untreated control cultures (Maruo et al., 1996), the disruption of the EGFR-mediated signalling may result in the inhibition of cell proliferation in cultured leiomyoma cells. In particular, the decrease in p-EGFR, a bioactive form, may attenuate the delivery of mitogenic signals. Moreover, we have previously demonstrated that IGF-I significantly increases cell proliferation and inhibits apoptosis in cultured leiomyoma cells compared with untreated control cultures (Gao et al., 2001). On the basis of these findings, the down-regulation of the IGF-I/IGF-IR system may attenuate the cell-proliferative and anti-apoptotic activities of IGF-I in cultured leiomyoma cells. On the other hand, TGFβ3 is shown to inhibit the DNA synthesis in myometrial cells but increases the DNA synthesis in leiomyoma cells (Lee and Nowak, 2001). The disruption of the TGFβ3/TGFβ RII system may cause the inhibition of downstream signalling such as cell proliferation in cultured leiomyoma cells. Collectively, the EGF/EGFR system, IGF-I/IGF-IR system and TGFβ3/TGFβ RII system may be the targets of asoprisnil in cultured leiomyoma cells, leading to the inhibition of leiomyoma cell proliferation.
Actually, we found that treatment with either recombinant EGF, IGF or TGFβ3 alone significantly increased the cell number of viable cultured leiomyoma cells, and that the concomitant treatment with asoprisnil antagonized the EGF-, IGF-I- and TGFβ3-induced increases in the cell number of viable cultured leiomyoma cells. By contrast, in cultured normal myometrial cells, treatment with recombinant EGF alone only increased the viability of cultured cells without apparent effects of treatment with either recombinant IGF-I or TGFβ3 alone on the cell viability. Furthermore, in cultured normal myometrial cells, the concomitant treatment with asoprisnil did not affect the cell number of cultured cells. This suggests that asoprisnil may exert tissue-selective, antiproliferative effects in cultured leiomyoma cells by down-regulating the signalling of the EGF/EGFR system, IGF-I/IGF-IR system and TGFβ3/TGFβ RII system. Because IGF-I inhibits apoptosis of cultured leiomyoma cells, it is conceivable that asoprisnil-induced suppression of IGF-I/IGF-IR system may make cultured leiomyoma cells susceptible to apoptosis.
The diverse effects of progesterone on target tissues are mediated by PR, a nuclear receptor family of ligand-activated transcription factor (Leonhardt and Edwards, 2002). In addition to the regulation of target gene transcription by nuclear PR, several protein kinases are activated in response to progesterone binding to cognate cytoplasmic or membrane-associated receptors (Lange, 2004). Moreover, a cross talk between PR- and growth factor-initiated intracellular-signalling pathways is suggested to play a role in coordinately regulating gene subsets in hormonally responsive tissues (Lange, 2004; Faivre et al., 2005). Several investigators proposed an integrated PR activation of cellular-signalling pathways in breast cancer model (Lange, 2004; Faivre et al., 2005). PR and growth factors activate mitogen-activated protein kinases (MAPKs), resulting in positive regulation of PR action via direct PR phosphorylation. PR phosphorylation in turn acts on progesterone response element-containing gene promoters or other PR-regulated genes. Activation of MAPKs by PR provides for regulation of gene promoters independently of the transcriptional PR functions. Cell-cycle proteins such as cyclin D1, cyclin E and p21WAF1 are synergistically up-regulated by EGF in the presence of progestin in a MAPK-dependent manner in T47D human breast cancer cells (Lange et al., 1998), whereas antiprogestin RU486 was shown to prevent progestin activation of Src/p21/MAPK pathways (Migliaccio et al., 1998). It remains unknown whether asoprisnil affects the cross talk between progesterone and growth factors in leiomyoma cells. On the basis of our results presented in this study, however, it seems reasonable to speculate that asoprisnil may inhibit not only progesterone-regulated gene transcription at nuclear PR levels but also MAPK activation caused by cytoplasmic PR and growth factors in cultured leiomyoma cells, thereby down-regulating leiomyoma cell proliferation.
The reasons responsible for the differential effects of asoprisnil between cultured leiomyoma and normal myometrial cells remain unknown. Nevertheless, the cell-type selective activities of asoprisnil in cultured leiomyoma cells might be due at least in part to the difference in the cell-reactivity to progesterone, the difference in the mechanism by which asoprisnil modulates the gene transcription of EGF, IGF-I, TGFβ3 and their receptor protein expressions and the difference in the cell sensitivity to the signalling of the EGF/EGFR system, IGF-I/IGF-IR system and TGFβ3/TGFβ RII system. In addition, the milieu of transcriptional cofactors in leiomyoma cells compared with myometrial cells, although undefined at this time, is another possible factor in the differential response of these cells to asoprisnil. It has been hypothesized that the complex of liganded receptor and the balance of coactivators and corepressors imparts cell-specific effects of SPRMs (Smith and O’Malley, 2004). Taken together, asoprisnil may act to inhibit leiomyoma cell proliferation by targeting the growth factor/receptor systems in those cells without affecting the cell proliferation of cultured normal myometrial cells.
In conclusion, we have demonstrated that asoprisnil down-regulates the expression of EGF, IGF-I, TGFβ3, p-EGFR, IGF-IRα and p-TGFβ RII in cultured leiomyoma cells and inhibits the growth factor-induced increase in cell proliferation of those cells without affecting those in cultured normal myometrial cells. These data suggest that the cell-type-specific inhibition of growth factor expression and action in leiomyoma cells may play a role in suppression of leiomyoma volume observed in clinical studies. Asoprisnil may open a new avenue for a novel tissue-selective approach to the treatment of uterine leiomyomas. Further studies will be necessary to elucidate the molecular mechanism by which asoprisnil exerts the cell-type-specific effects on uterine leiomyoma cells but not on normal myometrial cells.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research 1437053 from the Japanese Ministry of Education, Science and Culture, the Ogyaa-Donation Foundation of the Japan Association of Obstetricians and Gynecologists and TAP Pharmaceutical Products Inc.