-
PDF
- Split View
-
Views
-
Cite
Cite
Yadava Bapurao Jeve, Tarek Gelbaya, Muhammad Fatum, Time to consider ovarian tissue cryopreservation for girls with Turner’s syndrome: an opinion paper, Human Reproduction Open, Volume 2019, Issue 3, 2019, hoz016, https://doi.org/10.1093/hropen/hoz016
Close - Share Icon Share
Abstract
Turner’s syndrome (TS) is the most common sex chromosome abnormality in women. In addition to short stature and gonadal dysgenesis, it is associated with cardiac and renal anomalies. Due to rapid follicular atresia, the majority of women with TS suffer from primary ovarian insufficiency around puberty. Thus far, donor oocyte conception has been the key fertility option for these women. With advancing technology, ovarian tissue cryopreservation (OTCP) has emerged as a clinically justifiable option especially for pre-pubertal girls with cancer. Recently published results following the use of cryopreserved ovarian tissue are reassuring. It would be prudent to consider the extension of these technological and scientific advances to other conditions, such as TS, where accelerated follicular atresia is suspected. It is possible to obtain competent oocytes from cryopreserved ovaries of girls with TS provided the ovaries were preserved before ovarian failure. However, it is a complex decision whether and when to offer OTCP as a fertility preservation (FP) option for girls with TS. The rate of decline in fertility is variable in girls with TS and can be more complex in cases with mosaicism. On the other hand, OTCP has shown some promising results in patients with cancer, which can potentially be replicated in TS and other benign indications of patients at risk of premature ovarian failure. There are proven psychological and clinical benefits of FP. Thus, an argument could be made for offering OTCP to these patients to endow these girls with the option of having biological fertility using this innovative technology. Ethical, clinical and psychological dilemmas should be considered, discussed and addressed before considering such a novel approach. We believe that the time has come to start this discussion and open this avenue of FP for girls with TS.
Introduction
Turner’s syndrome (TS) is the most common sex chromosome abnormality of human females with an incidence of approximately 1 in 2000 female live births (Gravholt et al., 2017). Short stature and gonadal dysgenesis are two of the characteristic clinical features of the syndrome, together with a broad range of other phenotypic characteristics, which include an increased risk for heart and renal defects (Ford et al., 1959). The range of morbidities associated with the syndrome can have a profound effect on quality of life. Infertility and short stature are major concerns for women diagnosed with TS, influencing their psychosocial development (Sylven et al., 1991). Growth hormone treatment at a young age results in accelerated growth velocity and an increase in overall height, as compared to untreated girls (Baxter et al., 2007). Consequently, oestrogen replacement and fertility treatment are currently the mainstay of their endocrine management. A spontaneous puberty occurs in 5–10% of girls with TS, but only 2–5% reaches menarche with the possibility of achieving pregnancy (Hovatta, 1999). A recent study showed an overall 5.6% (27/480) prevalence of spontaneous pregnancies in women with TS. Most of these pregnancies occurred in women with mosaic TS and only 0.4% (2/480) spontaneous pregnancies were reported in women with a non-mosaic (X0) karyotype (Bernard et al., 2016). Due to the accelerated follicular atresia seen in TS patients, the majority of adolescent girls undergo ovarian failure prior to or around the time of puberty (Modi et al., 2003). Traditionally, donor oocyte conception has been the predominant fertility option for such patients. Donor oocyte conception has its own limitations including various pregnancy complications (Jeve et al., 2016), difficulty in obtaining a suitable donor and psychological issues associated with the process (Bracewell-Milnes et al., 2016). Although technologically acquired parenthood may add a highly-desired dimension to their social identities, a sense of loss could persist in most of the women’s personal identities (Hallebone, 1991). With recent technological advancements, fertility preservation (FP) may be successfully achieved using oocyte cryopreservation for pubertal girls and ovarian tissue cryopreservation (OTCP) for pre-pubertal girls (Grynberg et al., 2016). Although the exponential development of technology in the past few decades has led to promising results in the field of FP with OTCP, there are many unanswered questions about the clinical use of OTCP in TS patients. The purpose of this article is to examine the current and likely future status of OTCP for girls with TS.
Biological mechanism of follicular atresia
In TS ovaries, the premature ovarian failure is believed to be the result of follicular depletion. The mechanism and onset of follicular depletion has been widely studied in literature. Follicular atresia is a highly orchestrated and periodic process that results in destruction and elimination of follicles and oocytes from the ovary. Apoptosis is recognized as a key factor in atresia of antral follicles (Tilly et al., 1991) and is carried out by several molecular pathways, including mainly the B-cell lymphoma (Bcl-2) family, tumour necrosis factor (TNF), caspases and transforming growth factor beta-β proteins (Hussein, 2005). Apoptosis is regulated by genes such as Bcl-2 (pro-survival), Bax (pro-apoptotic) and cellular myelocytomatosis (c-Myc), which are expressed in granulosa cells of both foetal and adult ovaries, suggesting their possible role in atresia (Nandedkar and Dharma, 2001). The ovarian microenvironment and the interplay between pro-apoptotic and anti-apoptotic molecules play a significant role in folliculogenesis. Apoptosis was studied by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL) in human foetal ovaries (Modi et al., 2003). TUNEL analysis of the TS (45X) ovaries revealed massive apoptosis of the oocytes. It was concluded that chromosomal defects, by some means, accelerates apoptosis that probably leads to gonadal dysgenesis later in life.
The unique chromosomal mark-up in TS is the presence of only one copy of the X chromosome. However, in 46 XX females, one copy of the X chromosomes is inactivated to achieve a balanced gene expression dosage between males (XY) and females (XX) (Berletch et al., 2011). Therefore, logically, the absence of one sex chromosome would not be predicted to have any deleterious effect. Nevertheless, this X inactivation is incomplete in persons with a normal karyotype; 15% of the genes on the silenced X chromosome escape inactivation and are expressed from both chromosomes (Berletch et al., 2011). Abnormalities associated with TS are thought to be caused by haploinsufficiency of genes that are normally expressed by both X chromosomes. Haploinsufficiency of multiple genes on both arms of the X chromosome (both X chromosomes remain active in germ cells), in addition to pairing failure during meiosis, leads to gonadal dysgenesis in TS (Davenport, 2010). X-linked inhibitor of apoptosis protein is one of the key intracellular pro-survival proteins and an intracellular modulator of the TNF-alpha death signalling pathway in granulosa cells (Xiao et al., 2001). Key genes determining ovarian reserve include oocyte-derived bone morphogenetic protein 15 (BMP15) as a critical signal promoting the growth and differentiation of ovarian follicles, and its action is intrinsically linked to oocyte-derived growth and differentiation factor 9 (GDF9). Alternations in the BMP15 and GDF9 genes are associated with different ovarian phenotypic abnormalities (Rossetti et al., 2014). The process of accelerated apoptosis in the aneuploid gonad starts already in foetal life (Modi et al., 2003) and continues throughout childhood; therefore, most of the girls with TS fail to achieve the spontaneous puberty. The overall incidence of spontaneous puberty in TS is reported to be 5–10% (Pasquino et al., 1997). Therefore, it would be sensible to discuss options of FP before accelerated apoptosis leaves no follicles in the ovaries.
Current status of FP through OTCP
The first case of FP using OTCP, in a young woman with mosaic TS, was reported in 2008 (Huang et al., 2008). It was proposed more than 10 years ago that the combination of ovarian tissue cryobanking and immature oocyte collection from the tissue followed by IVM and vitrification of matured oocytes represents a promising approach of FP for young women with mosaic TS. OTCP protocols and the evidence supporting its use are derived mainly from patients with cancer. OTCP involves laparoscopic removal of ovarian tissue. To obtain the best results for cryopreservation, it is beneficial to remove the ovarian cortex from the medulla, which helps extreme penetration of cryoprotectants into the cortical tissue (Fathi et al., 2011). As ovarian reserve is already low in girls with TS, the recommendation is to remove as much tissue as possible, typically an entire ovary (Oktay et al., 2016). This is followed by making small strips of ovarian tissue to allow the cryoprotectants to penetrate the tissue. Slow freezing in liquid nitrogen has been the main procedure for preserving the ovarian tissue (Silber, 2012). The standard protocol for ovarian cryopreservation is slow programmed freezing, using human serum albumin-containing medium and propanediol, dimethylsulphoxide or ethylene glycol as a cryoprotectant, combined with sucrose (Hovatta, 2005). When the woman is ready to attempt pregnancy, autotransplantation of the thawed pieces of ovarian tissues is performed. Autotransplantation of the ovarian tissue can be performed in the ovarian fossae beneath the pelvic peritoneum (Oktay and Karlikaya, 2000; Pacheco and Oktay, 2017). The first live birth after orthotopic autotransplantation of cryopreserved ovarian tissue was reported in 2004 (Donnez et al., 2004). In a recent meta analysis, 85.2% of women had restored endocrine function, a 57.5% (69 of 120) clinical pregnancy rate and a 37.7% (65 of 172) live birth rate and ongoing pregnancy rate were reported (Pacheco and Oktay, 2017). This suggests approximately one in three women attempting the ovarian tissue transplant being able to have at least one child. This data is mainly derived from OTCP for cancer patients in different age groups. A recently published retrospective case-control study of 15 girls and young women aged 5–22 years with TS showed evidence of follicles in 60% of the biopsies; however, a high rate of abnormal follicle morphology was noted. The author suggested that the benefits of OTCP may be limited to a highly selected group of TS mosaic patients (Mamsen et al., 2019). Cryopreservation of oocytes or ovarian tissue has been performed experimentally in more than 150 girls and adolescents with TS over the past 16 years (Schleedoorn et al., 2019). The efficacy is still unknown for this subgroup of patients due to the lack of follow-up data.
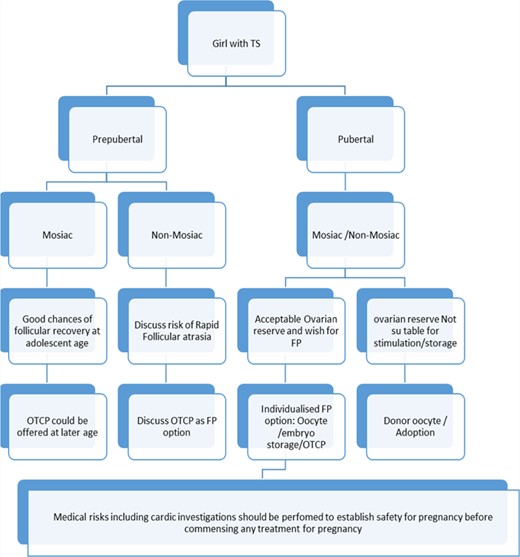
Fertility preservation (FP) options for girls with Turner's syndrome (TS). OTCP, ovarian tissue cryopreservation.
OTCP for girls with TS
Age to offer OTCP
The full biological development of ovaries is believed to be complete during the third trimester of pregnancy; therefore, it is biologically plausible that the ovarian tissue will be functional regardless of the age of the child that the tissue was cryopreserved. A confirmation of this notion was found by the report of the first live birth after an autograft of ovarian tissue that was cryopreserved before menarche, which was reported in 2015 (Demeestere et al., 2015). Recently, 84 children and eight ongoing pregnancies were reported following cryopreserved autologous ovarian tissue transplantation (Pacheco and Oktay, 2017), which includes adult and paediatric tissues. Chromosome abnormalities, such as TS, and trisomy 18 or 21, modify normal ovarian development by reducing the pool of available follicles and inhibiting follicular growth (Peters et al., 1978). Unless ovarian tissue is cryopreserved at a very early age, the success of this technology for girls with TS, especially 45X karyotype, may be thus predictably limited as a result of the accelerated follicular pool depletion. The chance of restoring fertility is related to the number and quality of follicles existing within the transplanted cortical tissue (Dolmans et al., 2009). It has been reported that primordial follicles can be found in ovarian tissue collected from girls (n = 57) with mosaic and non-mosaic TS up to the age of 17 years (Borgstrom et al., 2009). OTCP might be the only option for the paediatric and the pre-adolescent patients. Pre-pubertal girls with TS who are not timely assessed and considered for OTCP, could be missing the window of an opportunity for FP using OTCP, which could result in a TS generation without the real benefit of this technology. Figure 1 shows our suggested empirical approach for FP in girls with TS. Clinical decision making for FP is relatively clearer for pubertal girls with TS as it mainly comprises ovarian stimulation followed by oocyte or embryo vitrification. Patients with mosaic or non-mosaic TS with good ovarian reserve have the opportunity to undergo or not undergo FP depending on their ovarian reserve markers. There is a lack of conclusive data on ovarian reserve markers, such as antral follicle count or anti-Müullerian hormone (AMH), for young pubertal girls (Lie Fong et al., 2012). The study evaluating serum AMH as a marker for ovarian reserve in young girls and adolescents reported that, as a screening test of premature ovarian failure in TS, the sensitivity and specificity of AMH <8 pmol/l was 96 and 86%, respectively (Hagen et al., 2010). Fifty-seven girls with TS aged 8–19.8 years were studied (Borgstrom et al., 2009). The author concluded that spontaneous puberty, mosaicism and normal hormone concentrations were significant but not exclusive prognostic factors for finding follicles in TS ovaries (Borgstrom et al., 2009). Therefore, the decision should be based on considerations of the complete clinical picture, desire of the individual and opinion of the team involved. It is prudent to discuss FP as soon as possible in both mosaic and non-mosaic TS. The patients or their parents or guardians should be made aware of all the related risks, benefits and future chances of fertility before making any decision for FP, in the best interest of the child. A provision should be made for supportive counselling for both patients and parents, to aid in decision making, and multidisciplinary team agreement before a child is subjected to laparoscopy and oophorectomy for OTCP.
Amount of tissue for OTCP
The amount of tissue to be taken for OTCP is another debatable issue open for discussion. Currently, there is no consensus on a standard operative technique for surgical ovarian cortical tissue removal in adult females, and there are limited published reports of the surgical approach and outcomes in the paediatric population (Corkum et al., 2017). Ovaries are often small in girls with TS, and therefore unilateral oophorectomy would be advantageous to only having several ovarian strips sampled and would render more tissue for preservation. In addition, unilateral oophorectomy carries a relatively low risk of bleeding. Laparoscopic unilateral oophorectomy for OTCP can be performed safely and as a day case procedure in children (Rowell et al., 2019). On the other hand, partial ovarian biopsy, with some ovarian tissue left remaining, offers a potential site for future implantation of cryopreserved tissue. The evidence evaluated from various age groups showed that at least ½ to ⅔ of the ovary should be taken from the anti-mesenteric surface of the ovary (Beckmann et al., 2016). It was found that premenopausal unilateral oophorectomy significantly reduces the age of menopause by 1.8 years, and patients or their guardians should be counselled accordingly (Rosendahl et al., 2017). Partial ovarian cortical biopsy or unilateral oophorectomy could be planned after careful counselling and an agreed strategy for the OTCP programme.
Clinical dilemma
Three prominent clinical concerns are debated while offering OTCP for girls with TS: insufficient evidence for the efficacy of this technology for TS, safety of women with TS carrying a pregnancy and risk of chromosomal anomalies in children. The current literature has reported promising results following OTCP and subsequent transplantation of preserved tissue as a new option of FP. More than 130 births have been reported worldwide following orthotopic transplantation of cryopreserved ovarian tissue (Beckmann et al., 2019). These results are mainly derived from FP before cancer treatment and largely from adult patients who had their ovarian tissue cryopreserved during adulthood. The age of patients at the time of tissue retrieval varied. A cohort study of 545 patients who had OTCP (mean age ± SD of 22.3 ± 8.8 years) reported 33% (7 out of 21) live births (Jadoul et al., 2017). A single-centre study reported on OTCP for 418 girls and adolescents aged younger than 15 years. Three hundred and thirteen patients had malignant diseases, and 105 had benign conditions. Recently three patients had auto-transplantation but no pregnancy has been reported yet (Poirot et al., 2019). A systematic review evaluating FP options in women with TS included 67 studies. The efficacy of different FP options is still unknown due to the lack of follow-up data (Schleedoorn et al., 2019). Another review reported on 1019 patients undergoing OTCP with ages ranging from 0.4 to 20.4 years, with 298 aged younger than 13 years. Eighteen patients underwent auto-transplantation of thawed ovarian cortical tissue that was cryopreserved before the age of 21 years, resulting in 10 live births (Corkum et al., 2019). An update paper on worldwide OTCP activity and on the Danish cohort reported a total of 93 children born, and 51% pregnancies were achieved by natural conception. The age range of patients at the time of OTCP who succeeded in having a live birth or ongoing pregnancy was 9–38 years (Gellert et al., 2018). Fertility outcome including successful natural or assisted conception for girls with TS who were pre-pubertal at the time of FP remains limited. This could be due to the fact that OTCP is a relatively new technology, and the pre-pubertal girls who had cryopreserved ovarian tissue are not yet ready to start fertility treatment. Ovarian tissue of girls with TS could be biologically different from that of girls with cancer, and therefore the success with OTCP may be different. It may be possible that despite OTCP women with TS will still have accelerated atresia after implantation of ovarian tissue. This is especially important for women who are planning natural conception. There could be a better chance if assisted conception is planned shortly after autologous implantation, but there is lack of evidence to support this opinion.
Regardless of whether conception is natural or assisted, using their own eggs or donated eggs, pregnancies in women with TS carry higher risks than the general population, mainly due to coexistent medical morbidities. Potential pregnancy complications are related to cardiac, renal and other medical conditions. In women with TS, the risk for aortic dissection or rupture during pregnancy may be 2% or higher (Practice comittee 2005). Maternal mortality in women with TS has been reported to be as high as 1–2%, which is 100 times greater than general population (Grynberg et al., 2016). Therefore, appropriate pre-pregnancy counselling and screening is recommended before pregnancies are planned by women with TS. Such a pregnancy should be managed as a high-risk pregnancy by a multidisciplinary team (Grynberg et al., 2016). It would be important to put a comprehensive pregnancy plan in place before embarking on any fertility treatment for women with TS. These obstetric risks are present irrespective of the origin of the gametes. Therefore, similar concerns could be debated for cryopreservation of the patients’ oocytes or for donor eggs. If it is deemed relatively safe and clinically acceptable to use donor oocytes for conception for girls with TS, then the option of OTCP does not add any further risk for pregnancy. Additional increased obstetric risks are due to the donor origin of the oocytes. Donor oocyte pregnancy acts as an independent risk factor for pregnancy complications, including hypertensive disorders (odds ratio (OR), 3.92), small for gestational age (OR, 1.81) and preterm delivery (OR, 1.31), and these risks could possibly be eliminated by using autologous oocytes (Jeve et al., 2016). FP provides an opportunity for future fertility using a patient’s own gametes, should it be safe to carry a pregnancy in the future. For women who are at high risk of carrying a pregnancy because of cardiac or renal conditions, gestational surrogacy with preserved oocytes or ovarian tissue could be an option to have their own biological offspring. There is no conclusive data on the incidence of chromosomal abnormalities in biological children of women with TS. There is a possibility of meiotic non-disjunction in the oocytes of women with TS and chromosomal aberrations have been shown to be more common in children born after naturally conceived pregnancies (King et al., 1978; Swapp et al., 1989). It has been suggested that women with TS should be offered PGD, chorionic villous sampling or amniocentesis if fertilization of their own oocytes is successful (Karnis et al., 2003). The clinical dilemma becomes even more multifaceted if future research potential, such as oogonial stem cells (OSC), in-vitro activation (IVA) and oocyte–granulosa cell complexes are taken into consideration (Ghazal, 2013). However, these approaches are presently in their initial stages. OSC, identified in human ovaries (Kawashima and Kawamura, 2017), have been suggested to have the potential to develop into oocytes. The TS ovary contains a population of OSCs, but the ovarian stroma in TS does not support follicle formation. The IVA modality is based on fragmentation of ovarian tissue to disrupt Hippo signalling, with or without drug treatment to stimulate Akt signalling and resulting in IVA of folliculogenesis before autotransplantation (Kawashima and Kawamura, 2017).
Any clinical dilemma is often balanced on risk versus benefit ratio. OTCP has been demonstrated as a clinically viable and acceptable option of FP for children with cancer. We argue to extend its benefits to benign conditions, such as TS, as an option for future fertility.
Psychological dilemma
The psychological impact following the diagnosis of genetic conditions such as TS is under-reported. A significant psychological impact including social, behavioural and educational components is associated with TS (Saenger et al., 2001). Delayed initiation of sexual activities and an impaired sense of self-esteem in women with TS has been reported (McCauley et al., 1986a). Furthermore, it is thought that women with TS suffer from limited emotional arousal, high tolerance for adversity, unassertiveness and over compliance (McCauley et al., 1986b). This is compounded by the reports showing that premature menopause can impair sexual identity and sexual function along with a woman’s well-being and achievement of life goals (Graziottin, 2007). However, it is not clear whether these psychological issues are due to some underlying genetic or hormonal influence on behaviour or due to the issues of short stature, physical anomalies and infertility (Saenger et al., 2001). A study has shown that infertility is the most frequently cited concern followed closely by short stature, regardless of age (Sutton et al., 2005). Infertility alone is associated with significant psychological distress (Lukse and Vacc, 1999). Even for persons who may have not planned to have children, the threat of infertility can result in a deep sense of loss and anger. With wider acceptance of growth hormone treatment, short stature is being successfully treated. Psychological wellbeing of the girls with TS deserves further attention beyond hormone treatment. Given the complexity of a TS diagnosis and the psychosocial impact of the condition, counselling on psychosocial issues and addressing concerns about the girls’ daily life and future adult life needs to be integrated into standard paediatric endocrinologist care (Culen et al., 2017).
The inability to bear biological children was reported as the most prevalent and painful challenge endured by most of the adult women with TS (Sutton et al., 2005). Although the use of donor oocytes has been a widely used fertility option, evidence is limited on the psychological issues surrounding donor gamete conception (Bracewell-Milnes et al., 2016). Child-free living may be a reasonable choice for some women, but it would be helpful for girls and their parents to hear all life-plan and family-building options presented in a balanced manner. Girls in the peri-pubertal age group begin to realize the implication of TS, including reduced fertility potential. A clear guideline is required on how, when or what to discuss regarding fertility and potential FP options; and how to support them to accept their differences and empower them (Colindres et al., 2016).
The studies on the psychological and emotive effects of FP techniques are still scarce (Laganà et al., 2017). It can be argued that the primary benefit of OTCP is to promote autonomy by giving them the hope of having genetically concordant children in the future.
Ethical dilemma
Ethical dilemmas should be tested on the principle pillars of autonomy, beneficence, justice, non-maleficence and, additionally, confidentiality and honesty (Das and Sil, 2017). One of the top choices an individual makes in life is the opportunity to have one’s own children. An individual’s autonomy of choice, which is an essential foundation of a free society, should be respected (Carvalho et al., 2017). Unique ethical issues arise in the paediatric and adolescent population while applying these ethical principles. The moral and legal recognition of autonomy is achieved normally with informed consent. However, it would be difficult to establish autonomy in children in certain age groups especially when discussing future fertility and childbearing. It is commonly an easy decision when the child, parents and caring team agree on the best interest of the child but it would be difficult if there is a disagreement. The ESHRE Task Force on Ethics and Law recommended that the child’s decision should be respected if the child is mature and understands the issues at stake. When the child is immature, the decision to cryopreserve (or not) may be taken by the parents or the guardians, unless it poses grave prejudice to the well-being/welfare of the child. The child’s or adolescent’s opinion should be sought when they are able to understand the circumstances (de Carvalho et al., 2017). The importance of preserving the possibility of having genetically related offspring in the future is generally recognized, and the parents will have to decide whether this benefit outweighs the current risk of intervention for their child (ESHRE Task Force on Ethics and Law, 2004). The decision of FP is complex enough for an adult deciding for themselves, but parents face additional decisional conflict and regret when making the decision for their child (Li et al., 2017). On the principle of beneficence and non-maleficence, meaning risk and benefit analysis, it is recommended that discussion should include the potential risks emanating from application of the technique weighed against the proposed but as yet unproven benefits. It is important to note that the promising data published so far is derived from the cancer population and various age groups at the time of cryopreservation. FP could be justified on medical indications, social grounds and on the prevention of the biopsychosocial impact of a procreating disability (Carvalho et al., 2017). At the same time, the powerful desires for a biological child should be weighed against the risks of pregnancy in TS. Such desires may have biological roots, but it is reinforced by powerful social and cultural expectations about motherhood; these expectations should be satisfactorily questioned rather than uncritically accepted. The tissue storage period should be until the age at which it is considered acceptable for the tissue to be used for the achievement of a pregnancy, taking into account the welfare of the child and the risks to the pregnant mother (Carvalho et al., 2017). Further, consensus on posthumous reproduction is still required. A task force recommended that the parents do not have the right to decide about the (reproductive) use of the genetic material of their child after his/her death (ESHRE Task Force on Ethics and Law, 2004). All specialties present in the caring team (oncologists, paediatricians, reproductive specialists, psychologists/counsellors) should be heard during decision-making about the best procedure. Another important ethical aspect is the question of legal ownership and rights applying to the banked ovarian tissue. The legislation may vary in different countries or may be lacking in some countries. Parental consent should not be static and should be reviewed regularly with increasing involvement of the child where possible, especially as children become legally competent. These regulatory issues need to be addressed based on local and national guidance. Where appropriate, guidance could be requested from the local medical ethical committee. Although the ESHRE task force guidance provides a good ethical framework for cryopreserved ovarian tissue (ESHRE Task Force on Ethics and Law, 2004), there is a crucial role for a multidisciplinary Ethics Committee to guide clinical practice in individual circumstances (Voultsos et al., 2016).
Conclusions
Recent evidence has supported OTCP as one of the promising options for FP. Although there is no convincing evidence on fertility outcome for girls with TS, further studies are urged to evaluate the efficacy of OTCP in girls with TS. While waiting for the evidence to accumulate we feel that it is still important to discuss this option with children and their family. Current and future rapid development in the pertinent technologies would allow OTCP to be a successful and widely accepted method. However, young girls diagnosed with TS are faced with rapidly reducing fertility potential due to increased follicular depletion and atresia. Further delay in offering them OTCP may obviate their chances of FP. It could be argued that OTCP was offered to cancer patients a while ago despite lack of enough evidence at the time, which now is shown to be one of the successful methods. Therefore, the discussion on FP using OTCP for these girls should be started as soon as the diagnosis is established. When it comes to FP, all treatment options should be discussed along with their efficacy. Our duty is to fully inform patients about the various options, including OTCP, and enable them and their families to make an informed decision based on available evidence. At some point in their life, this may be their only option to preserve their fertility. In the present review, we have argued the case for offering OTCP to girls with TS after a well-informed discussion and counselling by the relevant multidisciplinary team. A multidisciplinary team should be actively involved in the decision-making, addressing all clinical, psychological, legal and ethical aspects while keeping the patient’s own interests at the centre of the discussion.
Authors’ roles
Y.J. contributed significantly in design, acquisition, analysis and interpretation of current evidence, along with drafting the article. T.G. contributed substantially by revising the manuscript critically and adding significant intellectual contents. M.F. contributed substantially in conception and design of the manuscript in addition to critically revising it with further addition of valuable contents.
Funding
None.
Conflict of interest
No conflict of interest.