-
PDF
- Split View
-
Views
-
Cite
Cite
Nil Ozyuncu, Menekse Sahin, Timucin Altin, Remzi Karaoguz, Muharrem Guldal, Omer Akyurek, Cardiac metastasis of malignant melanoma: a rare cause of complete atrioventricular block, EP Europace, Volume 8, Issue 7, July 2006, Pages 545–548, https://doi.org/10.1093/europace/eul058
Close - Share Icon Share
Abstract
Malignant melanoma has an aggressive biological behaviour and a high rate of cardiac involvement. As shown from post-mortem studies, metastases of melanoma can involve any organ and cardiac metastases are frequent. This report describes a case of widespread malignant melanoma in a patient with clinical presentation of complete atrioventricular (AV) block. Thorax CT and transthoracic echocardiography revealed a mass involving the conduction system. By VDD permanent pacing (atrial synchronous ventricular pacing), haemodynamic stability was maintained and the patient remains under follow-up receiving chemo-immunotherapy. In the retrospective analysis of the patient's records, we realized that the AV conduction delay had been progressing for at least 7 months. Cardiac metastasis of malignant melanoma is a common finding and can proceed in the absence of overt clinical manifestations. Therefore, the clinician should be alert to the development of cardiac signs and symptoms in a metastatic melanoma patient and should perform a detailed cardiac examination to exclude cardiac metastasis of the tumour.
Introduction
Cardiac metastases occasionally complicate the course of neoplastic diseases. Malignant melanoma is the neoplasm which most frequently involves the heart.1 It metastasizes to the heart in nearly 64% of cases.2 A diagnosis of cardiac involvement before death is infrequent at less than 16% of patients with melanoma present with cardiac symptoms.2 Non-specific clinical signs which are usually thought to be the result of the associated neoplasm and symptoms frequently being masked by other visceral metastases lead to the rarity of antemortem diagnosis.2
Metastatic melanoma, typically refractory to chemotherapy, carries a poor prognosis, especially with cardiac involvement. Here, we report a case with cardiac metastasis of melanoma, leading to complete atrioventricular (AV) block requiring permanent pacing.
Case report
A 56-year-old male was first seen at the general surgery clinic of Ankara University Hospital in October 2002. On his right proximal thigh there was a lobular, ulcerated, tan-brown coloured lesion with irregular borders. On physical examination, he had marked lymphadenopathy in the right inguinal region. The primary lesion, a 4.5×4×2 cm tumour infiltrating the adipose tissue, was removed with wide surgical excision. The pathological examination of the tumour revealed a malignant melanoma. No distant metastasis was found on detailed clinical and laboratory examination and therapeutic right inguinal lymph node dissection was performed. Eight lymph nodes were excised, five of them were shown to have metastases of melanoma. The primary tumour's Clarke's level was V and the Breslow thickness was ≥4 mm. There was regional lymph node involvement with no distant metastasis. The tumour was defined as stage III according to American Joint Committee on Cancer staging method. One month later, he started to receive adjuvant interferon alpha-2b therapy, but it was stopped 3 months later because of intolerance. In April 2003, he underwent surgery for right rectus muscle metastasis and 4 months later new local excisions of cutaneous metastases were performed. They all proved histopathologically to be metastases of melanoma. He also received intralesional interferon therapy. Since December 2003, he has been on a temozolamide treatment protocol. In June 2004, local excisions were performed for multiple recurrences on the right thigh, following which the treatment protocol was changed to thalidomide. On October 2004, he also received radiotherapy on the right inguinal region for 10 days.
On 29 December 2004, he was admitted to the emergency service because of nausea and vomiting. It was learned that he had had nausea for 2 months and had lost 8 kg during that period. His cranial CT, performed on 7 December 2004 because of his symptoms, was normal and showed no signs of metastasis. He presented pre-syncope which was aggravated by standing upright. He was unable to maintain the upright position because of both light-headedness and nausea. Symptoms were partially relieved by lying down.
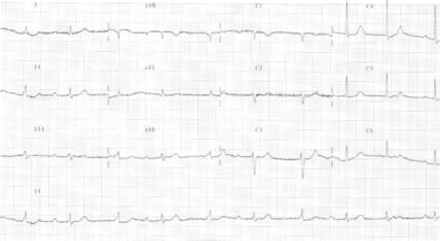
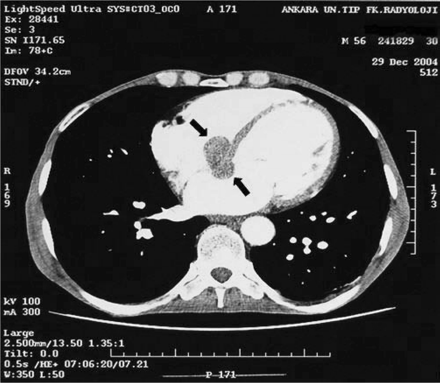
On clinical examination, he was conscious and cooperative, had multiple cutaneous metastases in the axillary and inguinal regions; he also had widespread lymphadenopathy. He had bradycardia with heart rate 45/min, and the blood pressure was 90/60 mmHg. On cardiovascular examination, there was a grade 1/6 pansystolic apical murmur. His neurological examination was normal. In the laboratory examinations; haemoglobin 11.2 g/dL, white cell count 7800/mm3, platelets 250 000/mm3, blood urea nitrogen 35 mg/dL, creatinine 1.3 mg/dL, Na 146 mmol/L, K 3.8 mmol/L, glucose 81 mg/dL. Electrocardiography showed complete AV block (Figure 1) and intermittent 2:1 AV block with a ventricular rate 45–50/min. Because of the increased D-dimer level (D-dimer=7119 ng/mL) detected and mild dyspnoea at presentation, pulmonary artery CT angiography and thorax CT imaging of the patient was arranged to examine the lung fields, but these showed no signs of pulmonary thromboembolism or pulmonary metastasis. However, in the CT images, a 3.5×2 cm low-density soft-tissue lesion in interventricular and interatrial septum was detected (Figure 2).
3.5×2 cm low-density soft-tissue mass (shown with arrows) in the interventricular and interatrial septum of the heart detected on thorax CT imaging.
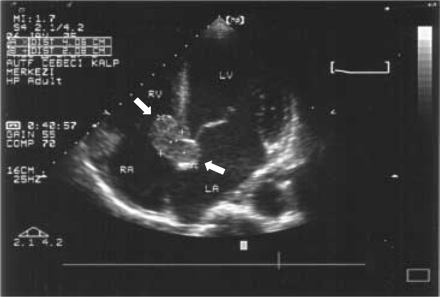
A temporary pacing system was inserted in the emergency room and the patient was admitted to the cardiology service. On echocardiography, a 4×2 cm non-pedunculated and non-mobile, homogeneous, smooth-surfaced echogenic mass on the membranous interventricular septum, protruding into the right ventricle and also involving the distal one-third of interatrial septum was revealed (Figure 3). Apical and anterior wall motion was hypokinetic, the remaining wall motion was normal. There was grade 1 mitral regurgitation and a minimal pericardial effusion.
Transthoracic echocardiogram showing 4×2 cm smooth surfaced, homogeneous echogenic mass (shown with arrows) involving the lower one-third of the interatrial septum, membranous interventricular septum, and protruding into the right ventricle.
On 6 January 2005, VDD permanent pacemaker was implanted without any complication. After the implant, the patient showed amelioration of light-headedness in the upright position. His oral intake improved and there was no problem in wound healing. He was discharged 12 days after the implantation of the pacemaker.
On retrospective analysis of the patient's data, we noticed that his ECG taken in January 2004 had a normal PR interval of 0.18 s; however, on the ECG taken in May 2004, he had first degree atrioventricular (AV) block with a PR interval of 0.26 s ultimately presenting with complete AV block in December 2004. Thus, progression of AV block was occurring for at least 7 months.
Discussion
Malignant melanoma is the tumour with highest rate of cardiac metastasis. Most frequently, the right side of the heart is affected. Metastatic involvement occurs in the myocardium most commonly with a rate of 98%, also in epicardium and endocardium with frequency rates of 78 and 73%, respectively.2,3 The clinical signs and symptoms are unclear and non-specific, leading to rare antemortem diagnosis. Asymptomatic massive involvement of the myocardium has been termed as ‘charcoal heart’.4 When present, the clinical signs and symptoms include fatigue, weakness, pericardial effusion, congestive heart failure, cardiac arrhythmia, superior vena cava syndrome, right ventricular outflow and inflow obstruction, and transient ischaemic attack.2,5,6 Clinical features such as tachycardia, dyspnoea, and oedema are usually attributed to non-cardiac involvement.
We report a case of malignant melanoma, presenting with complete AV block due to cardiac metastasis. In the literature, cardiac metastasis of malignant melanoma leading to arrhythmia is very rare.6,7 Matturi et al.6 performed post-mortem examinations of the conduction systems in the arrhythmic hearts of two cases of melanoma. One of the cases showed 1° AV block and atrial fibrillation, metastasis involved the myocardium at the AV node, left and right bundle branches.6 Our case had a similar mass involving the conduction system leading to complete AV block. The patient had widespread metastatic disease and there was no intracavitary lesion in the heart, so surgical intervention was not considered. Malouf et al.5 described a successful transoesophageal echocardiographically guided transvenous biopsy for histological diagnosis in a case with right atrial metastatic melanoma. Gibbs et al.8 described cardiac tamponade during transvenous biopsy in a malignant melanoma patient with a mass occupying more than 90% of the right ventricular cavity. The mass was confirmed to be malignant melanoma histopathologically in the post-mortem study.8 We do not have a histopathological diagnosis, but we accept the mass as a malignant melanoma metastasis because of the widespread metastatic primary tumour and the cardiac involvement similar to the melanoma metastases described in the literature.3,5,6,8,9 After the pacemaker implantation, the patient has been stable during 2 months of follow-up under chemo-immunotherapy.
In conclusion, clinicians should be alert to the possibility of cardiac metastasis in a patient with malignant melanoma even without clinical symptoms. Small prolongations in PR interval on routine ECGs during follow-up visits may be due to involvement of the conduction system, which may later lead to complete AV block, as in our case. Echocardiography, which is an easy and sensitive technique for detection of cardiac metastasis, should be performed when any suspicion exists in order to exclude cardiac involvement during melanoma patient follow-up.
References
- complete atrioventricular block
- hemodynamics
- atrium
- chemotherapy regimen
- follow-up
- immunotherapy
- melanoma
- neoplasm metastasis
- signs and symptoms
- heart
- neoplasms
- chest
- metastatic melanoma
- echocardiography, transthoracic
- ventricular pacing
- cardiovascular findings
- cisplatin/dacarbazine/vinblastine protocol
- examination of heart