-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey S. Borer, Angiotensin-converting enzyme inhibition: a landmark advance in treatment for cardiovascular diseases, European Heart Journal Supplements, Volume 9, Issue suppl_E, September 2007, Pages E2–E9, https://doi.org/10.1093/eurheartj/sum037
Close - Share Icon Share
Abstract
Angiotensin-converting enzyme-inhibitors (ACE inhibitors) were first introduced into clinical practice for the relief of hypertension (HT) more than a quarter of a century ago. Since that time, as the basic pathobiology of cardiovascular (CV) diseases has been increasingly understood, new applications for ACE inhibitors have been developed. Indeed, through their action on the renin–angiotensin system (RAS) and their local actions, ACE inhibitors have substantially improved the prognosis of patients with diseases over the entire continuum of CV disease, including HT, stable coronary artery disease, myocardial infarction (MI), and heart failure (HF), and have been applied specifically to prevent stroke, although also preventing diabetes and delaying or reducing renal dysfunction. The strength of available clinical data is such that guidelines developed for cardiologists by professional societies now recommend the use of ACE inhibitors for the management of HT, coronary and atherosclerotic vascular disease, MI, and HF. Newer ACE inhibitors with pharmacological profiles differing from those of older agents have added to the ease of application and relative safety of these drugs both for reduction of symptoms and for improvement of outcomes. Although other agents modulating RAS can provide some overlapping pharmacological effects and important clinical benefits, ACE inhibitors remain unique in the range of their proven benefits, justifying their central role in the armamentarium of the CV specialist.
Introduction
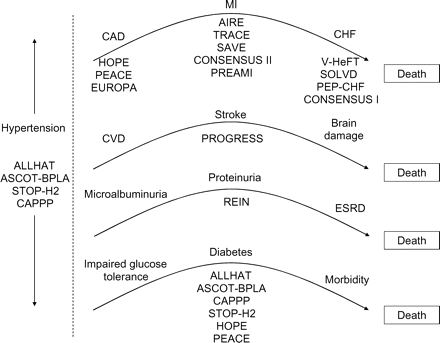
Angiotensin-converting enzyme-inhibitors (ACE inhibitors) were first introduced into clinical practice for hypertension (HT) relief more than a quarter of a century ago. Since that time, as the basic pathobiology of cardiovascular (CV) diseases has been increasingly better understood and the likely physiological and pathophysiological roles of ACE have been more fully elucidated, new applications regularly have been developed, including therapies for heart failure (HF)/left ventricular (LV) dysfunction, renal diseases of various aetiologies, myocardial infarction (MI), and stable coronary artery disease (CAD). As a result, during the ensuing decades, this group of drugs has been responsible for substantial improvement in outcome of patients with various CV and associated diseases. Indeed, ACE inhibitors have demonstrated efficacy throughout the continuum of CV disease from underlying risk factors such as HT prior to the development of overt CV disease, through end-stage CAD and HF (Figure 1), in addition to retarding renal dysfunction and the appearance of diabetes mellitus.
History
The first clinically available ACE inhibitor, captopril, was initially developed for the treatment of HT, focusing specifically on patients believed to have a predominantly renin-dependent pathophysiology.1 It may be of interest, given the widespread application of ACE inhibitors during the succeeding 25 years, that initial approval by the US Food and Drug Administration (FDA) was associated with intense debate and discussion. The drug's developer had performed initial approvability trials in a population including a relatively high proportion of patients with ‘collagen-vascular’ diseases; drug-associated renal dysfunction in this population was disturbingly frequent. Indeed, after prolonged discussion, the FDA's Cardio-Renal Advisory Committee (on which I was serving my first term at the time) finally voted for approval, but required the label to carry a warning and the admonition that the drug should only be used after two other drugs had failed to achieve acceptable blood pressure (BP) control! Four years later, the second ACE inhibitor to come to the FDA, enalapril, was introduced with a dossier including far fewer patients afflicted with collagen-vascular diseases and, perhaps as a result, with very little evidence of renal dysfunction. Enalapril was quickly recommended for approval by the Cardio-Renal panel—but its consideration sparked a debate about the importance of differences in the molecular structures of different ACE inhibitors to account for differences in adverse pharmacological effects. In this case, the sulfhydril groups of captopril (not present in enalapril) were putatively, although perhaps incorrectly, associated with the adverse renal experiences. Differences in the pharmacological effects of different ACE inhibitors remain an important theme today—but the overwhelming benefits associated with these drugs, as a group, have earned them an important place as first-line agents throughout the CV continuum.
Following captopril, several more ACE inhibitors were developed, with somewhat different pharmacological characteristics (longer duration of action, different adverse profiles, more or less efficacy vs. risk for various outcomes, etc.). During this interval, considerable evidence accumulated to support the therapeutic effects of ACE inhibitor beyond BP lowering (vascular, cardiac, and renal), even in the absence of systemic hyperactivity of the renin–angiotensin system (RAS). Indeed, as is the case with all drugs, ACE inhibitors have multiple pharmacological effects; both the benefits and the risks of ACE inhibitors cannot be attributable solely to systemic ACE inhibition.
Benefits at the early stage of the cardiovascular disease continuum—hypertension
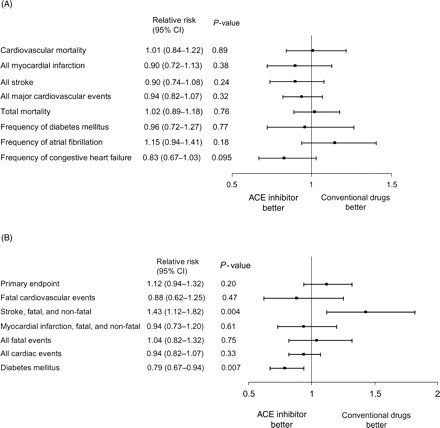
ACE inhibitors were first developed for the treatment of HT, for which they remain mainstays of therapy. Indeed, multiple studies have demonstrated the efficacy of ACE inhibitor for chronic BP reduction, particularly among patients with renin-dependent HT; systemic renin excess incontrovertibly heightens the adverse outcome risks of HT.2–6 However, BP reduction in patients with HT is therapeutic for stroke, MI, and CV death; BP reduction in such patients is a well-demonstrated surrogate for reduction in the risks of these outcome events. Consistent with this concept, morbidity–mortality trials in HT have demonstrated the efficacy of ACE inhibitor. The first such trials, Swedish Trial in Old Patients with Hypertension (STOP-H2) and Captopril Prevention Project (CAPPP) (Figure 2),3,4 demonstrated equivalent benefits of ACE inhibitor to those of earlier established antihypertensive agents, diuretics, and beta-blockers. The later Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) found a similar effect on major CV outcomes with the ACE inhibitor, lisinopril, in comparison with a thiazide diuretic, despite significantly greater BP reduction with the diuretic.5 Indeed, owing to the accumulated evidence of efficacy in minimizing specific sequelae of HT as well as of other CV diseases, ACE inhibitors have the broadest spectrum of indications for use, as endorsed by peer-selected expert guidelines committees, among all antihypertensive agents.7 Finally, in the recently published Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA) in which ACE inhibitor perindopril was coupled with calcium channel blockade by a dihydropyridine (amlodipine), the newer combination strategy was convincingly demonstrated to provide greater reduction in total mortality (11%), major CV events and procedures (16%), and new-onset diabetes (30%), than equivalent BP lowering with the combination of a beta-blocker and thiazide diuretic.2
Heart failure
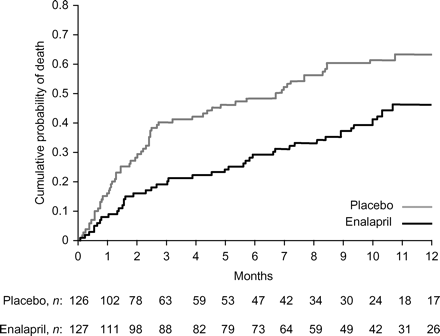
Since 1987, several large, prospective, randomized, placebo-controlled clinical trials have demonstrated that treatment with several different ACE inhibitors reduces mortality rates in patients who have HF associated with systolic dysfunction.8–11 In Cooperative North Scandinavian Enalapril Survival Study I (CONSENSUS I), substantial mortality benefit was conferred by administration of enalapril when compared with placebo, when administered to patients with severe HF (Figure 3).8 The Studies of Left Ventricular Dysfunction (SOLVD) extended these benefits to a broader range of patients, including not only those with symptomatic HF but also those with LV dysfunction who were asymptomatic (‘Functional Class 0’ HF).9,10 Vasodilator-Heart Failure Trial II (V-HeFT II) compared enalapril with hydralazine plus nitrates, a combination that improved outcome vs. placebo in the earlier Vasodilator-Heart Failure Trial I (V-HeFT I), and demonstrated significantly enhanced survival with the ACE inhibitor.11 As a result of these and other parallel studies, ACE inhibitors have been recommended for indefinite administration to patients with chronic HF and systolic dysfunction, both to minimize symptoms and to prolong survival.12,13
Although, as of yet, less compellingly demonstrated, HF associated primarily with diastolic dysfunction may respond to ACE inhibitor. This putative benefit may result from drug-mediated cardiac remodelling, with reduction of myocardial mass, fibrosis (possibly a direct effect of ACE inhibitors, as discussed subsequently), and stiffness. Consistent with this hypothesis, a meta-analysis of studies of anti-HT drugs has found ACE inhibitors to be the most effective of these agents in reversing LV hypertrophy.14 More importantly, in a study of the ACE inhibitors, perindopril [Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF)], among patients with HF and diastolic dysfunction, benefit was suggested, although not conclusively proven.15 Although the study lost its randomization power after 1 year, relative risk reduction was 31% for the primary outcome (all-cause mortality or unplanned HF hospitalization) when perindopril was administered rather than placebo (P = 0.055). Symptomatic improvement, including more normal functional class (P = 0.030) and enhanced 6-min walk distance (P = 0.011), was also noted.
Coronary artery disease: myocardial infarction and cardiac remodelling
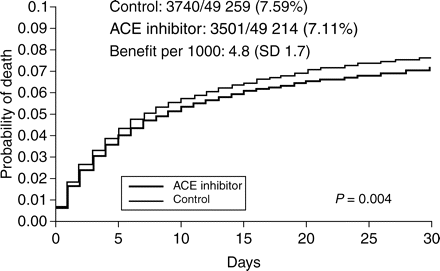
ACE inhibitors improve outcome in patients who have suffered MI. Although Cooperative North Scandinavian Enalapril Survival Study II (CONSENSUS II) failed to demonstrate a benefit,16 a meta-analysis including data from more than 98 000 patients with acute MI entered into four ACE inhibitor trials showed that early ACE inhibitor treatment (≤36 h post-MI) is associated with significantly lower mortality both in the first week and in the first month after MI (Figure 4).17 Long-term studies of ACE inhibitor in patients who have suffered MI [Acute Infarction Ramipril Efficacy Study (AIRE), Trandolapril Cardiac Evaluation Study (TRACE), Survival and Ventricular Enlargement study (SAVE)] have confirmed maintenance of these benefits over years.18–20 Consequently, ACE inhibitor have become an indispensable part of post-MI treatment in patients with subnormal LV function.
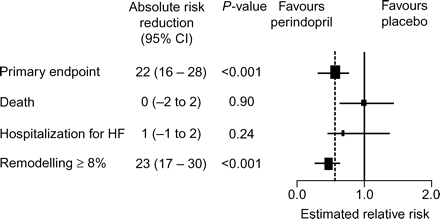
ACE inhibitor prevent or delay the detrimental cardiac remodelling after MI that appears to contribute to worsening prognosis after MI. For example, the recent Perindopril and Remodeling in Elderly with Acute Myocardial infarction (PREAMI) trial in 1252 elderly patients with preserved LV function, which studied post-MI, has shown that perindopril can prevent or delay cardiac remodelling (Figure 5).21 After 1 year, perindopril reduced the composite primary endpoint of death, HF hospitalization, and cardiac remodelling by 38% (P = 0.001) vs. placebo.
Coronary artery disease: outcome in stable coronary artery disease
Certain ACE inhibitors have been compellingly demonstrated to decrease the frequency of ischaemic events. These benefits presumably are based at least in part on drug-mediated effects that have been demonstrated experimentally, including retardation of atherosclerosis development, reversal of endothelial dysfunction, and favourable alteration of fibrinolytic balance; effects that are presumed to involve inhibiting both angiotensin II (Ang II) and bradykinin (BK) pathways.22–25
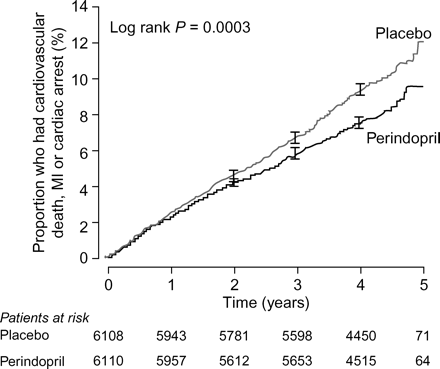
These pharmacological effects formed the basis for large trials of ACE inhibitor in atherosclerotic vascular disease. These include the HOPE trial, in patients at high risk of CV events, which showed that ramipril at a dose of 10 mg daily decreases the risk of stroke, MI, and death by 22% in comparison with placebo (P < 0.001).26 The European Trial on Reduction of Cardiac Events with Perindopril among Patients with Stable Coronary Artery Disease (EUROPA) trial,27 aimed at a population with stable CAD and somewhat less evidence of risk than the HOPE population, confirmed and extended the findings of HOPE (Figure 6). Treatment with perindopril 8 mg daily on a background of now-standard preventive therapy (antiplatelet drugs, lipid-lowering agents, and beta-blockers if MI had occurred) reduced risk of cardiac death, non-fatal MI, or resuscitated cardiac arrest by 20% in comparison with placebo (P = 0.0003).27
The Prevention of Events with ACE Inhibition (PEACE) trial failed to show the benefit of trandolapril in patients with stable CAD.28 This finding may relate to the dose selected for study (4 mg) or to intrinsic differences in the pharmacological profile of this ACE inhibitor vs. ramipril and perindopril. Nonetheless, the usefulness of ACE inhibitors, as a group, for secondary prevention in patients with established, stable CAD has been demonstrated by results of meta-analyses of all relevant trials.29,30 These results have been recognized by the European Society of Cardiology and the American College of Cardiology/American Heart Association.31,32
Beyond cardiac protection and treatment: kidneys, diabetes, and brain
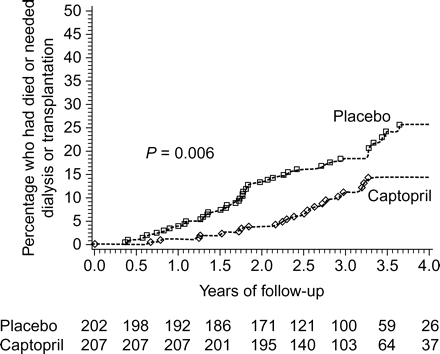
The CV disease continuum can be understood to extend to target organs beyond the heart, including the brain and kidneys.33,34 Indeed, CV dysfunction is an important cause of renal dysfunction; renal disease is also a risk factor for adverse outcomes in patients with established CAD.35 ACE inhibitors are plausible therapies for renal disease as RAS is implicated in the progression of renal dysfunction in patients with established renal disease. Perhaps, as a consequence, ACE inhibitors have unique nephroprotective properties in comparison with other anti-HT agents. Thus, in patients with type I diabetes, the ACE inhibitor captopril reduced the rate of the combined endpoint of dialysis, renal transplantation, and death (Figure 7).36 These benefits were confirmed in additional studies of diabetic patients37,38 and have been extended to non-diabetic renal disease.39–41 In patients with end-stage renal disease, treatment with the ACE inhibitor perindopril significantly reduced both CV and total mortality.40
A direct effect on diabetes may also exist. In a meta-analysis, regardless of indication for use (HT or CAD), reductions in new-onset type 2 diabetes were noted when ACE inhibitors were administered.42 In addition, certain ACE inhibitor may be particularly effective in preventing CV events in diabetics (Figure 2B).
In the Perindopril Protection against Recurrent Stroke Study (PROGRESS), the first study of ACE inhibitors in patients with cerebrovascular disease, 6105 patients were treated with a perindopril-based therapy (addition of indapamide was allowed) or placebo.43 After 3.9 years, perindopril-based treatment reduced the primary endpoint and recurrent fatal or non-fatal stroke by 28% (P vs. placebo < 0.0001). Active treatment also reduced the risk of major vascular events by 26%. Similar reductions in the risk of stroke in hypertensive and non-hypertensive subgroups were noted (P < 0.01).
Mode of action: differentiation with other renin–angiotensin system inhibitors
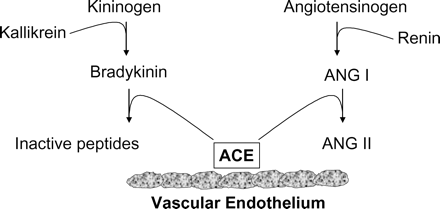
Like all drugs, ACE inhibitors have many pharmacological effects, some of which have not yet been clarified.44,45 However, most of these effects have been extensively studied and, plausibly, are at least in part responsible for the clinical benefits provided by these drugs. ACE inhibitors regulate the balance between RAS and the kallikrein–kinin system. RAS is centrally involved in BP regulation. ACE regulates the balance between the vasodilatory and natriuretic properties of BK and the vasoconstrictive and salt-retentive properties of Ang II. ACE inhibitors alter this balance by decreasing the formation of Ang II and the degradation of BK (Figure 8). Their effects on the BK pathway differentiate ACE inhibitors from other agents modulating RAS [angiotensin receptor blockers (ARBs) and renin inhibitors] and probably contribute to the clinical benefits of ACE inhibitor, particularly to the prevention of MI in patients with stable CAD.46 Evidence favouring such differentiation has been recently provided by a series of meta-regression analyses using data from 26 large trials (involving 146 838 patients and 22 666 events) comparing an ACE inhibitor or an ARB with placebo or active drug, prepared by the Blood Pressure Lowering Treatment Trialists Collaboration.47 This analysis revealed that both types of drugs produce a BP-dependent event reduction, but that ACE inhibitors, and not ARBs, provide an additional 9% risk reduction, which is independent of BP lowering effects. In addition, probably acting by impeding transforming growth actor (TGF)-β activity, which is modulated by local Ang II concentrations, ACE inhibitor blocks collagen synthesis,48 potentially beneficial in patients with HF because of cardiomyopathy or CAD (but possibly deleterious in regurgitant valvular diseases in which this effect may impair necessary LV structural support to resist dilatation).49
Although much of this review has discussed the effects of ACE inhibitors as a group, all ACE inhibitors are not equivalent.45 Pharmacologically, different types of ACE inhibitors exist: captopril-like compounds are administered in active form, but are further metabolized, with potential modulation of effects by metabolites; prodrugs such as enalapril, perindopril, ramipril, and trandolapril must be converted to their active form, leading to potential variability in activity based on individual metabolic characteristics; lisinopril-like compounds are active as administered and are not further metabolized.50 Of course, together with these structural differences, half-lives, plasma-levels, trough/peak relations, absorption characteristics, intermediary metabolism, and elimination vary among the compounds. Perhaps, as a result, differences in clinical actions have also been observed. For example, perindopril and ramipril reduced CV morbidity and mortality in patients with CAD in the EUROPA and HOPE trials,27,37 but in PEACE, trandolapril did not.28 Considerable work remains to elucidate the links between the pharmacokinetic and pharmacodynamic properties and clinical effects.
Conclusion
Almost three decades after their introduction into clinical use, ACE inhibitors have become recognized therapeutic choices throughout the entire spectrum of CV diseases. Newer ACE inhibitors with pharmacological profiles differing from those of older agents have added to the ease of application and relative safety of these drugs both for reduction of symptoms and for improvement of outcomes. Although other agents modulating RAS (ARBs and renin inhibitors) can provide some overlapping pharmacological effects and important clinical benefits, ACE inhibitors remain unique in the range of their proven benefits, justifying their central role in the armamentarium of the CV specialist.
Major studies of angiotensin-converting enzyme-inhibitors along the cardiovascular disease continuum. AIRE, Acute Infarction Ramipril Efficacy Study; ALLHAT, Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial; ASCOT-BPLA, Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm; CAD, coronary artery disease; CAPPP, Captopril Prevention Project; CHF, congestive heart failure; CONSENSUS, Cooperative North Scandinavian Enalapril Survival Study; CVD, cerebrovascular disease; EUROPA, European Trial on Reduction of Cardiac Events with Perindopril among Patients with Stable Coronary Artery Disease; ESRD, end-stage renal disease; HOPE, Heart Outcomes Prevention Evaluation; MI, myocardial infarction; PEACE, Prevention of Events with ACE Inhibition; PEP-CHF, Perindopril in Elderly People with Chronic Heart Failure; PREAMI, Perindopril and Remodeling in Elderly with Acute Myocardial infarction; PROGRESS, Perindopril Protection against Recurrent Stroke Study; REIN, Ramipril Efficacy in Nephropathy; SAVE, Survival and Ventricular Enlargement study; SOLVD, Studies of Left Ventricular Dysfunction; STOP-H2, Swedish Trial in Old Patients with Hypertension; TRACE, Trandolapril Cardiac Evaluation Study; V-HeFT, Vasodilator-Heart Failure Trial.
(A) Relative risk of cardiovascular mortality and morbidity for ACE inhibitor vs. conventional therapy in the STOP-H2 trial. Data are adjusted for age, sex, diabetes, diastolic blood pressure, and smoking. Adapted with permission from Figure 3.4 (B) Relative risk of cardiovascular mortality and morbidity for captopril vs. conventional therapy in the CAPPP trial. The primary endpoint was a composite of fatal and non-fatal myocardial infarction, stroke, and other cardiovascular deaths. ACE inhibitor, angiotensin-converting enzyme-inhibitors; CAPPP, Captopril Prevention Project; CI, confidence interval; STOP-H2, Swedish Trial in Old Patients with Hypertension. Adapted with permission from Table 4.3
Effect of ACE inhibitor therapy started in the acute phase (0–36 h) of myocardial infarction and continued for a short time (4–6 weeks) on cumulative mortality during days 0–30 in all trials combined: systematic overview of individual data from 100 000 patients in randomized trials. ACE inhibitor, angiotensin-converting enzyme-inhibitor. Reproduced with permission from Figure 1.17
Effect of perindopril treatment on left ventricular remodelling and clinical outcome: the Perindopril and Remodelling in Elderly with Acute Myocardial Infarction (PREAMI) study. The primary endpoint was combination of death, hospitalization for heart failure, or left ventricular remodelling. Size of squares is proportional to the number of patients in that group. Dashed line indicates overall relative risk. CI, confidence interval; HF, heart failure. Reproduced with permission from Figure 2.21
Time to the first occurrence of the primary endpoint after perindopril treatment of patients with stable coronary artery disease: the European Trial on Reduction of Cardiac Events with Perindopril among Patients with Stable Coronary Artery Disease (EUROPA). The primary endpoint was a composite of cardiovascular death, MI, or cardiac arrest. Error bars depict standard error. MI, myocardial infarction. Reproduced with permission from Figure 2.27
Cumulative percentage of patients with diabetic nephropathy in the captopril and placebo groups who died, or required dialysis or renal transplantation. The numbers at the bottom of the panel represent the numbers of patients in each group at risk for the event at baseline and after each 6-month period. Reproduced with permission from Figure 1.36
Acknowledgement
J.S.B. is a consultant to Servier Laboratoires, Neuilly sur Seine, France; the preparation of this manuscript was supported in part by Servier.
Conflict of interest: none declared.
References
ALLHAT officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS).
The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.
ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials.
The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure.
Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy.
Progress Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack.