-
PDF
- Split View
-
Views
-
Cite
Cite
Christoph Kaiser, Jens Bremerich, Sabine Haller, Hans Peter Brunner-La Rocca, Georg Bongartz, Matthias Pfisterer, Peter Buser, Limited diagnostic yield of non-invasive coronary angiography by 16-slice multi-detector spiral computed tomography in routine patients referred for evaluation of coronary artery disease, European Heart Journal, Volume 26, Issue 19, October 2005, Pages 1987–1992, https://doi.org/10.1093/eurheartj/ehi384
Close - Share Icon Share
Abstract
Aims Multislice spiral computed tomography (MSCT) is a promising non-invasive method to diagnose coronary artery disease (CAD). As no detailed comparative evaluation in consecutive patients referred for evaluation of CAD has been reported, this prospective study evaluating 2384 coronary segments in 149 consecutive patients was performed.
Methods and results The coronary artery tree was analysed in 16 segments both for coronary angiography (CA) and MSCT; a luminal narrowing ≥50% based on visual assessment was considered significant. By MSCT, 77% of 2110 angiographically assessable segments could be evaluated, 94% per patient in proximal and 70% in distal segments (P<0.001). Sensitivity of MSCT to detect significant stenoses was 30% in all, but only 10% in peripheral segments. The main limitations were calcifications in 34% of segments and motion artefacts in 24% of patients. Overall diagnostic sensitivity for the presence of significant CAD was 86% but specificity was only 49%.
Conclusion When compared with invasive CA, 16-slice MSCT is of limited diagnostic value for the diagnosis of CAD in consecutive patients. Despite a clinically useful sensitivity for the overall diagnosis of significant CAD, specificity is low. Thus, relevant decisions regarding the need of and suitability for possible revascularization procedures cannot be based on MSCT findings alone.
See page 1942 for the editorial comment on this article (doi:10.1093/eurheartj/ehi463)
Introduction
Invasive coronary angiography (CA) is the current gold-standard for the assessment of coronary anatomy and diagnosis of coronary artery disease (CAD) with a low but definite complication rate.1 Multislice spiral computed tomography (MSCT) CA is a promising non-invasive technique for the detection of CAD. To make MSCT a clinically useful tool for the evaluation of patients with suspected or known CAD, complete visualization of all clinically relevant segments of coronary arteries and a reliable quantification of coronary artery stenoses within these segments are mandatory. This is particularly true if revascularization procedures such as coronary angioplasty or bypass surgery are also to be planned on the basis of MSCT findings. Previous studies with relatively small numbers of selected patients reported a high sensitivity and specificity for the detection of significant obstructive coronary lesions.2–6 In a recently published study, 16-slice MSCT was shown to reliably detect significant obstructive CAD in patients with stable angina in sinus rhythm, with a high negative predictive value (NPV) and a lower sensitivity for the detection of non-calcified lesions.7 However, no prospective series of unselected patients referred for the evaluation of suspected or known CAD in view of further therapeutic interventions have been studied or reported. Therefore, we set out to test the diagnostic yield of 16-slice MSCT in the routine work-up of consecutive patients referred for conventional X-ray CA for the evaluation of known or suspected CAD in view of possible therapeutic interventions.
Methods
Patients
In this prospective study, 149 consecutive patients referred for elective invasive evaluation of known or suspected CAD were included between November 2002 and September 2003. Exclusion criteria were women in childbearing age, known hypersensitivity against iodine-based contrast agents, serum creatinine >130 µmol/L, fasting serum glucose >13 mmol/L, and no consent. All patients were studied on their prescribed medication and, in fact, 69% were taking beta-blocking drugs. The study protocol was approved by the Ethics Committee of the states of Basel according to the Helsinki Declaration (1975/1983), and all patients gave written informed consent.
Procedures
Diagnostic invasive CA was performed according to standard Judkins procedure using 6F catheters the day following MSCT. MSCT data were acquired using a 16-slice scanner (Sensation 16, Siemens Medical Systems, Forchheim, Germany). To calculate the bolus arrival time for the contrast-enhanced scan, 20 mL of contrast media were injected at a rate of 4 mL/s with a power injector (Ulrich, Ulm, Germany), 300 mg iodine/mL (Ultravist 300, Schering, Germany), followed by a chaser bolus of 20 mL saline in the antecubital vein. CT attenuation values were measured at the level of the ascending aorta to identify the first slice with an opacification of ≥100 HE and to calculate the scan delay time. Subsequently, the contrast media bolus was injected (80 mL at the rate of 4 mL/s followed by a saline chaser of 20 mL at the rate of 4 mL/s). A contrast-enhanced retrospectively ECG-gated scan (16×0.75 mm collimation, table feed 2.8 mm/rotation, effective tube current 400 mA at 120 kV) was acquired. A typical scan length was 25 s. No additional beta-blocker was administered to modulate heart rate. Images were reconstructed with the multisegment algorithm with retrospective ECG gating. Reconstruction started at −600, −500, −400, and −300 ms. Images were reconstructed with 1 mm slice thickness and 0.5 mm increment to obtain isotropic voxels of 1×1×1 mm3. For analysis, the reviewers were allowed to choose the data set with the fewest artefacts.
Analysis of data
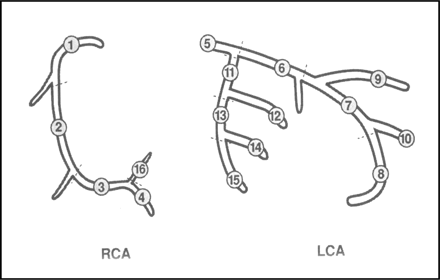
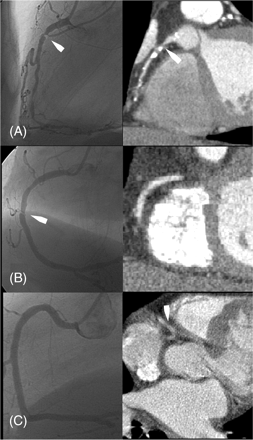
All angiograms were analysed post hoc by an experienced interventional cardiologist. MSCT images were analysed by an experienced radiologist and an experienced cardiologist in consensus, the latter being experienced in interventional cardiology. Data sets were reviewed offline at a workstation (Leonardo, Siemens, Germany). Reading physicians were blinded to invasive CA results and were allowed to use axial source images at all reconstructed phases, as well as to reconstruct these 3D data sets in multiplanar mode. For the analysis of MSCT and CA, the coronary artery tree was broken down in 16 segments after a modified American Heart Association (AHA) classification (Figure 1)8 and all segments were analysed individually. A luminal narrowing of >50% based on visual estimation was considered significant in both modalities. Three typical examples of corresponding views by CA and MSCT are shown in Figure 2.
Statistical analysis
Data are expressed as frequencies, mean±standard deviation, or median (interquartile range) as appropriate. Kolmogorov–Smirnov test was used to test categorical and continuous variables for normal distribution. If not normally distributed, non-parametric tests were used and data are expressed as median (adjusted for group mid-points) and interquartile range. Categorical variables were compared using χ2 and Fisher's exact test or Yates' corrected χ2 as appropriate. Receiver–operator characteristics curve was used to test sensitivity and specificity to diagnose significant CAD by number of positive segments in MSCT. For the analysis of the diagnostic accuracy of MSCT per coronary artery segments, only segments which could be adequately assessed by CA were considered. Only descriptive data are provided for segments because of potential within patient correlations. All calculations were done with the use of a commercially available statistical program (SPSS v 12 for Windows). Two-sided tests were used. A P-value of <0.05 was considered statistically significant. Bonferroni adjustment was made for comparison of per cent of assessable segments in the three main vessels of each patient.
Showing a significant relationship between stenoses seen by MSCT and CA would have needed a substantially smaller number of patients. However, we aimed to compare the two methods in a clinically meaningful number of patients. Hence, we included approximately 150 patients, which is comparable with previous studies addressing this and other comparable issues.
Results
Patients' characteristics are depicted in Table 1. The CA revealed CAD in 113 (76%) patients (one-vessel disease n=25; two-vessel disease n=38; three-vessel disease n=50), whereas 36 patients had no significant coronary obstructions (severe mitral or aortic valve disease n=9; cardiomyopathy n=8; hypertensive heart disease n=2; no discernible heart disease n=17).
Visibility and evaluability of coronary artery segments by MSCT vs. CA
Of a total of 2384 coronary artery segments, 2110 (89%) were visible by CA. Of these, 1854 (88%) were visible and 1619 (77%) could be assessed by MSCT [median per patient 80% (interquartile range 28%)]. Factors independently influencing the per cent of assessable segment per patient were motion artefacts (P≤0.0001) and per cent of calcified segments (P≤0.05). Motion artefacts were noted in 35 patients (24%). In these patients, heart rate was significantly higher (72±13 b.p.m.): in 66% (23 of 35), heart rate was ≥65 b.p.m. when compared with a heart rate of 61±10 and in only 30% (33 of 109), heart rate was ≥65 b.p.m. without motion artefacts (both P≤0.001).
If the heart rate was ≥65 b.p.m., 248 of 797 (31%) segments were not assessable compared with 243 of 1313 (19%) segments with lower heart rate. Thus, in patients with heart rate ≥65 b.p.m., a median of 71% of the segments (interquartile range 28%) were assessable vs. 86% (interquartile range 25%) in patients with lower heart rate (P≤0.001).
Most segments were assessable in the LAD [median per patient 88% (interquartile range 29%)], followed by the right coronary artery (RCA) [84 (41%), P≤0.01 vs. LAD], and least in the LCX [71 (44%), P≤0.01 vs. LAD and RCA]. As expected, the number of assessable segments was larger for proximal (1,2,5,6,7,11, and 13) than for distal segments [median of assessable segments per patient (interquartile range) 94 (15%) vs. 70 (46%), P≤0.001].
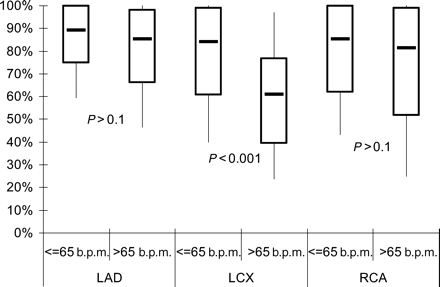
Heart rate significantly influenced assessable segments per patient in LCX, but not in LAD and RCA (Figure 3). Also, proximal segments were influenced less than distal segments by heart rate (median of assessable segments per patient with heart rate ≤65 vs. >65 b.p.m.; proximal 95 vs. 91%, P=0.08; distal 77 vs. 56%, P<0.001).
Detection of significant stenoses by MSCT vs. CA
Binary stenoses were found by CA in 432 (20%) and by MSCT in 274 (13%) segments. Detailed results of analyses of all segments and subdivided for the three main coronary vessels and proximal vs. distal segments are given in Table 2. The sensitivity of MSCT to detect significant lesions was highest in proximal LAD and RCA and lowest in distal segments of three coronary arteries. In contrast, specificity was higher in all vessels. The sensitivity of MSCT to detect proximal lesions was markedly better than to detect distal lesions (47 vs. 10%), but because of the lower frequency of stenoses in the distal segments, the specificity was somewhat lower.
False-positive findings were seen more often in the presence of calcifications noted by MSCT (present in 34% of all segments). Thus, NPV was 77% in the presence of calcification but 86% in the absence (specificity 75 and 98%, respectively). In contrast, the positive predictive value (PPV) was substantially lower if calcifications were not seen (28 vs. 50%) and sensitivity was minimal (5 vs. 52%). Calcification per se in MSCT had a sensitivity of 52% and a specificity of 71% (PPV: 32%, NPV: 85%) in the presence of a significant stenosis by CA.
Sensitivity was identical in patients with heart rate ≤65 when compared with ≥65 b.p.m. (29 vs. 30%). Specificity (93 vs. 89%) as well as PPV (53 vs. 37%) was slightly higher, but NPV was lower (82 vs. 86%). Diagnostic yield per segment including only patients without motion artefacts (n=1535) was not substantially higher (sensitivity 31%, specificity 91%, PPV 49%, and NPV 83%).
A total of 45 segments (2.1%) were previously stented; diagnostic accuracy was lower in these segments (58%). However, excluding these segments from analysis did not influence the overall results at all (diagnostic accuracy 79% irrespective of inclusion or exclusion of these segments).
Diagnosing CAD
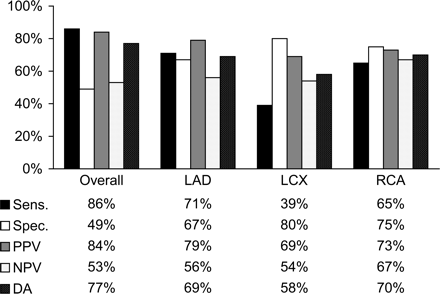
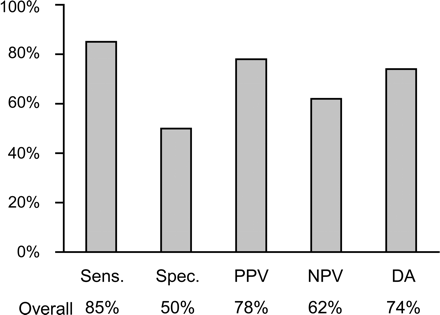
CAD with at least one coronary stenosis >50% was diagnosed in 113 (76%) patients by CA and in 115 (77%) by MSCT. Overall, the identification of patients with CAD by MSCT was reasonably good; the results of exclusion of relevant disease were however poor (Figure 3). Considering all the three individual vessels, the diagnosis of significant disease by MSCT was most difficult in the LCX (Figure 4). When considering only proximal stenoses [present in 101 patients (68%)], diagnostic yield was basically identical (Figure 5). In particular, the specificity remained low. Both the number of segments with stenosis and the calcification in MSCT were significantly related to the diagnosis of CAD by CA (P≤0.001), but area under curve in ROC analysis was relatively low (both 0.78±0.04).
In patients with heart rate ≤65 b.p.m., sensitivity was identical (86 vs. 86%), but specificity was slightly higher, though still insufficient (59 vs. 40%; PPV 90 vs. 72%, NPV 48 vs. 62%, diagnostic accuracy 81 vs. 70%). The influence of heart rate on diagnostic yield was not statistically significant, neither overall nor in one of the three main coronary arteries. The absence of motion artefacts tended to improve the diagnostic yield. Again, it remained clinically insufficient (sensitivity 87 vs. 82%, specificity 50 vs. 46%, PPV 86 vs. 72%, NPV 52 vs. 60%, diagnostic accuracy 79 vs. 69%).
In various subgroups of patients with lower probability of CAD, diagnostic yield was not substantially different in the whole group (Table 3).
Discussion
The most important finding of the study of MSCT in consecutive patients referred for the evaluation of CAD by CA is that MSCT yields reasonable sensitivity but low specificity for the diagnosis/exclusion of significant CAD. The main reasons for these limitations are the high rate of calcifications in such patients, which may mask or exist without relevant coronary lesions, and the high susceptibility of the method to motion artefacts. In addition, MSCT is limited in defining involved coronary segments. Particularly, distal segments and those of the LCX are not sufficiently well detected. Therefore, present-day 16-slice MSCT is of limited value for detailed analysis of the extent of CAD in view of need of and suitability for possible revascularization procedures in patients referred for the evaluation of CAD. The emerging 64-slice technology will provide better spatial and temporal resolution combined with faster acquisition times and therefore potentially improve diagnostic accuracy in calcified and peripheral segments.
Diagnosis of CAD
For the individual patient, a correct diagnosis or exclusion of significant CAD is most important. Kuettner et al.9 reported a correct diagnosis by MSCT in only 35% of all their patients. In the present study of unselected patients with a relatively high pre-test probability of CAD, an overall accuracy of 77% was found in the correct diagnosis of relevant CAD by MSCT. Considering the high rate of coronary risk factors and high prevalence of CAD in referral patients, a high sensitivity as reported here may be expected. The low specificity with many false-positive results was most likely due to the more frequent occurrence of calcifications in elderly and high-risk patients, which may lead to misinterpretations.10 Again, sensitivity remained particularly low in the LCX. Taking into account only proximal stenoses, diagnostic yield did not improve substantially, indicating that the rate of false-positive results remains unchanged compared with smaller peripheral segments. Comparing the results of the present study with other non-invasive tests for the detection of CAD as recently reported in a large pooled analysis,11 the sensitivity of MSCT may be even somewhat better than that of myocardial perfusion scintigraphy (79%) and stress echocardiography (76%), with, however, a markedly inferior specificity of 49% compared with 73% for scintigraphy and 88% for stress echocardiography. The diagnostic yield of MSCT improved to a certain extent in patients with low heart rates ≤65 b.p.m. and in patients with absence of motion artefacts. However, the overall NPV for MSCT also in these subgroups of patients limits this method as a screening tool for the exclusion of significant CAD.
Visibility and evaluability of coronary artery segments
Early studies in selected patients reported up to 94% of coronary segments evaluable by MSCT,5 whereas in the present study, only 77% of all angiographically visible segments were evaluable by MSCT. Evaluability was particularly impaired in peripheral segments. Similar findings have been reported by Gerber, Nieman, Achenbach, and others,2,3,9,12–15 who also included peripheral segments and side branches in their analyses as done in the present study. The most important reasons for impaired visibility and evaluability of coronary artery segments by MSCT may be motion artefacts, calcifications, and the presence of coronary stents.4,9,13 In the present study of unselected patients, these factors only partly explained the relatively low diagnostic yield of MSCT.
Furthermore, the influence of heart rate on the image quality of MSCT has been well described.2,9,14,16 Schroeder et al.14 suggested to lower the heart rate ≤65 b.p.m. to achieve best image quality. As half of our patients had known or suspected LAD, 69% were already treated with oral beta-blockers, resulting in a low mean heart rate of 63 b.p.m. during MSCT without additional i.v. beta-blockade. According to the findings by Gerber et al.12 patients with heart rates >65 b.p.m. showed a higher rate of motion artefacts, resulting in a lower rate of visible and evaluable coronary artery segments by MSCT, which is confirmed in this study. In most of the published studies,2,4,5 evaluability was particularly poor in coronary segments of the lateral wall because of the fact that the LCX easily blends with adjacent contrast-filled structures such as the great cardiac vein and the left atrium. This is also reflected in the present study by significantly impaired visibility and evaluability of the LCX when compared with the other two main coronary artery vessels, especially in patients with heart rates >65 b.p.m.
Detection of stenoses
The overall diagnostic yield of MSCT in each angiographically assessable segment in the present report is similar to that reported by Kuettner et al.9 and Nieman et al.17 but inferior to that published by other groups.2,3,5,7,13,15 The main reasons for these discrepancies are that most of those studies included highly selected patients and that for their analyses, peripheral segments were excluded or combined. In contrast to proximal vessels, MSCT yields a very poor sensitivity in the periphery of the coronary arteries. We could also confirm the limitations of MSCT in the lateral wall with a low sensitivity in the LCX. It is known that coronary calcifications are associated with the presence of significant coronary stenoses.10 This was true in the present report in 32% of calcifications. However, calcifications were also noted in 68% of segments in MSCT without significant stenoses. In non-calcified segments, however, the sensitivity of MSCT even dropped to an overall of 5% because of the low tissue contrast, particularly in small non-calcified segments.
Limitations
There are some limitations to the present study. Hence, we included only patients with relatively high probability of CAD in our study. MSCT might be contemplated in the presence of an intermediate likelihood of significant CAD and ambiguous prior test results. This, however, has not yet been investigated. We did not find any subgroup where MSCT yielded sufficient diagnostic accuracy. This also applies to subgroups with lower probability of CAD, such as patients with non-typical symptoms or limited number of cardiovascular risk factors, tempering the enthusiasm of using MSCT as a broad screening tool for CAD.
In some of our patients, heart rate was above the suggested limit of 65 b.p.m., resulting in a higher rate of motion artefacts and a somewhat lower diagnostic yield of MSCT. However, MSCT did not provide sufficiently high diagnostic accuracy, even after exclusion of patients with heart rate >65 b.p.m. from analysis. In particular, NPV was relatively low, thereby limiting the value of MSCT as a tool to exclude CAD. Routine administration of additional i.v. beta-blockers to every patient may have improved the image quality in some of the patients.
Moreover, the time points for image reconstruction in MSCT with 100 ms steps was rather crude; steps of 50 ms may be favourable to optimize the analysis.
Conclusions
When compared with gold-standard CA, 16-slice MSCT is of limited diagnostic value for the detailed diagnosis of CAD in non-selected patients with moderate-to-high probability of CAD. Despite a reasonable sensitivity for the overall diagnosis of ‘significant CAD’, specificity is low and lesions in distal segments and those of the LCX coronary artery are not sufficiently well detected. Factors limiting MSCT are poor visibility and evaluability of peripheral segments, misinterpretation of calcified segments, and a very low diagnostic accuracy in non-calcified vessels.
The first two authors contributed equally to this paper.
Figure 1 Segmental anatomy of the coronary arteries after a modified AHA classification. 1, RCA proximal; 2, RCA mid; 3, RCA distal; 4, right posterior descendens; 5, main stem; 6, LAD proximal; 7, LAD mid; 8, LAD distal; 9, first diagonal; 10, second diagonal; 11, LXC proximal; 12, obtuse marginal; 13, LCX distal; 14, LCX posterolateral branch; 15, LCX posterodescendens branch; 16, RCA posterolateral branch. RCA, right coronary artery; LCX, left circumflex artery; LAD, left anterior descending coronary artery.
Figure 2 Typical examples of corresponding views by CA (left) and MSCT (right). (A) True-positive MSCT with high-grade stenosis (arrows) of segment 1 visible by both CA and MSCT. (B) False-negative MSCT with high-grade eccentric web-shaped stenosis of segment 2 (arrow), clearly visible by CA but not visible by MSCT. (C) False-positive MSCT with normal CA of RCA. MSCT shows a lesion in segment 2 (arrow), which was interpreted as significant stenosis.
Figure 3 Per cent of assessable segments per patient in the three main coronary arteries subdivided in patients with heart rate above and below 65 b.p.m.
Figure 4 Diagnostic accuracy of MSCT for the detection of CAD in each patient compared with CA. Sens., sensitivity; Spec., specificity; PPV, positive predictive value; NPV, negative predictive value; DA, diagnostic accuracy.
Figure 5 Diagnostic accuracy of MSCT for the detection of CAD in proximal coronary arteries only compared with CA (abbreviations see Figure 4).
Baseline characteristics
| Total number of patients (n) | 149 |
| Male (%) | 110 (74) |
| Age (years; x̄±SD) | 63.9±9.0 |
| Current smoker (%) | 62 (42) |
| Arterial hypertension (%) | 94 (63) |
| Positive family history (%) | 57 (38) |
| Hypercholesterolaemia (%) | 96 (64) |
| Diabetes mellitus (%) | 26 (17) |
| Known CAD (%) | 62 (42) |
| Prior MI (%) | 43 (29) |
| Prior CABG/PCI (%) | 51 (34) |
| Typical angina ≥CCS II | 78 (52) |
| Atypical chest pain | 31 (21) |
| Dyspnoea | 28 (19) |
| Silent ischaemia | 12 (8) |
| Total number of patients (n) | 149 |
| Male (%) | 110 (74) |
| Age (years; x̄±SD) | 63.9±9.0 |
| Current smoker (%) | 62 (42) |
| Arterial hypertension (%) | 94 (63) |
| Positive family history (%) | 57 (38) |
| Hypercholesterolaemia (%) | 96 (64) |
| Diabetes mellitus (%) | 26 (17) |
| Known CAD (%) | 62 (42) |
| Prior MI (%) | 43 (29) |
| Prior CABG/PCI (%) | 51 (34) |
| Typical angina ≥CCS II | 78 (52) |
| Atypical chest pain | 31 (21) |
| Dyspnoea | 28 (19) |
| Silent ischaemia | 12 (8) |
CABG, coronary artery bypass graft; CCS, Canadian Cardiac Society; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Baseline characteristics
| Total number of patients (n) | 149 |
| Male (%) | 110 (74) |
| Age (years; x̄±SD) | 63.9±9.0 |
| Current smoker (%) | 62 (42) |
| Arterial hypertension (%) | 94 (63) |
| Positive family history (%) | 57 (38) |
| Hypercholesterolaemia (%) | 96 (64) |
| Diabetes mellitus (%) | 26 (17) |
| Known CAD (%) | 62 (42) |
| Prior MI (%) | 43 (29) |
| Prior CABG/PCI (%) | 51 (34) |
| Typical angina ≥CCS II | 78 (52) |
| Atypical chest pain | 31 (21) |
| Dyspnoea | 28 (19) |
| Silent ischaemia | 12 (8) |
| Total number of patients (n) | 149 |
| Male (%) | 110 (74) |
| Age (years; x̄±SD) | 63.9±9.0 |
| Current smoker (%) | 62 (42) |
| Arterial hypertension (%) | 94 (63) |
| Positive family history (%) | 57 (38) |
| Hypercholesterolaemia (%) | 96 (64) |
| Diabetes mellitus (%) | 26 (17) |
| Known CAD (%) | 62 (42) |
| Prior MI (%) | 43 (29) |
| Prior CABG/PCI (%) | 51 (34) |
| Typical angina ≥CCS II | 78 (52) |
| Atypical chest pain | 31 (21) |
| Dyspnoea | 28 (19) |
| Silent ischaemia | 12 (8) |
CABG, coronary artery bypass graft; CCS, Canadian Cardiac Society; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Diagnosis of significant stenosis by MSCT in all segments and subdivided in the three main vessels and in proximal and distal segment
| . | n . | TP . | TN . | FP . | FN . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . |
|---|---|---|---|---|---|---|---|---|---|
| All segments | 2110 | 128 | 1532 | 146 | 304 | 30 | 91 | 47 | 83 |
| LM | 145 | 4 | 122 | 8 | 11 | 27 | 94 | 33 | 92 |
| LAD | 684 | 58 | 439 | 70 | 117 | 33 | 86 | 45 | 79 |
| Proximal | 147 | 23 | 76 | 33 | 15 | 61 | 70 | 41 | 84 |
| Middle | 142 | 24 | 68 | 19 | 31 | 44 | 78 | 56 | 69 |
| Distal | 141 | 7 | 108 | 9 | 17 | 29 | 92 | 44 | 86 |
| Diagonal 1 | 138 | 4 | 88 | 9 | 37 | 10 | 91 | 31 | 70 |
| Diagonal 2 | 116 | 0 | 99 | 0 | 17 | 0 | 100 | 0 | 85 |
| LCX | 630 | 19 | 483 | 27 | 101 | 16 | 95 | 41 | 83 |
| Proximal | 145 | 12 | 104 | 13 | 16 | 43 | 89 | 48 | 87 |
| Middle | 104 | 5 | 80 | 7 | 12 | 29 | 92 | 42 | 87 |
| Distal | 110 | 0 | 91 | 0 | 19 | 0 | 100 | 0 | 83 |
| Marginal 1 | 142 | 1 | 95 | 7 | 39 | 3 | 93 | 13 | 71 |
| Marginal 2 | 129 | 1 | 113 | 0 | 15 | 6 | 100 | 100 | 88 |
| RCA | 651 | 47 | 488 | 41 | 75 | 39 | 92 | 53 | 87 |
| Proximal | 148 | 21 | 100 | 13 | 14 | 60 | 89 | 62 | 88 |
| Middle | 134 | 19 | 73 | 20 | 22 | 46 | 79 | 49 | 77 |
| Distal | 123 | 5 | 97 | 4 | 17 | 23 | 96 | 56 | 85 |
| RPD | 124 | 0 | 111 | 1 | 12 | 0 | 99 | 0 | 90 |
| RPL | 122 | 2 | 107 | 3 | 10 | 17 | 97 | 40 | 92 |
| Proximala | 965 | 108 | 623 | 113 | 121 | 47 | 85 | 49 | 84 |
| Distal | 1145 | 20 | 909 | 33 | 183 | 10 | 97 | 38 | 83 |
| . | n . | TP . | TN . | FP . | FN . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . |
|---|---|---|---|---|---|---|---|---|---|
| All segments | 2110 | 128 | 1532 | 146 | 304 | 30 | 91 | 47 | 83 |
| LM | 145 | 4 | 122 | 8 | 11 | 27 | 94 | 33 | 92 |
| LAD | 684 | 58 | 439 | 70 | 117 | 33 | 86 | 45 | 79 |
| Proximal | 147 | 23 | 76 | 33 | 15 | 61 | 70 | 41 | 84 |
| Middle | 142 | 24 | 68 | 19 | 31 | 44 | 78 | 56 | 69 |
| Distal | 141 | 7 | 108 | 9 | 17 | 29 | 92 | 44 | 86 |
| Diagonal 1 | 138 | 4 | 88 | 9 | 37 | 10 | 91 | 31 | 70 |
| Diagonal 2 | 116 | 0 | 99 | 0 | 17 | 0 | 100 | 0 | 85 |
| LCX | 630 | 19 | 483 | 27 | 101 | 16 | 95 | 41 | 83 |
| Proximal | 145 | 12 | 104 | 13 | 16 | 43 | 89 | 48 | 87 |
| Middle | 104 | 5 | 80 | 7 | 12 | 29 | 92 | 42 | 87 |
| Distal | 110 | 0 | 91 | 0 | 19 | 0 | 100 | 0 | 83 |
| Marginal 1 | 142 | 1 | 95 | 7 | 39 | 3 | 93 | 13 | 71 |
| Marginal 2 | 129 | 1 | 113 | 0 | 15 | 6 | 100 | 100 | 88 |
| RCA | 651 | 47 | 488 | 41 | 75 | 39 | 92 | 53 | 87 |
| Proximal | 148 | 21 | 100 | 13 | 14 | 60 | 89 | 62 | 88 |
| Middle | 134 | 19 | 73 | 20 | 22 | 46 | 79 | 49 | 77 |
| Distal | 123 | 5 | 97 | 4 | 17 | 23 | 96 | 56 | 85 |
| RPD | 124 | 0 | 111 | 1 | 12 | 0 | 99 | 0 | 90 |
| RPL | 122 | 2 | 107 | 3 | 10 | 17 | 97 | 40 | 92 |
| Proximala | 965 | 108 | 623 | 113 | 121 | 47 | 85 | 49 | 84 |
| Distal | 1145 | 20 | 909 | 33 | 183 | 10 | 97 | 38 | 83 |
aproximal segments: 1,2,5,6,7,11, and 13.
LM, left main coronary artery, RPD, right posterior descending; PRL, right posterolateral branch; TP, true positive; TN, true negative; FP, false positive; FN, false negative.
Diagnosis of significant stenosis by MSCT in all segments and subdivided in the three main vessels and in proximal and distal segment
| . | n . | TP . | TN . | FP . | FN . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . |
|---|---|---|---|---|---|---|---|---|---|
| All segments | 2110 | 128 | 1532 | 146 | 304 | 30 | 91 | 47 | 83 |
| LM | 145 | 4 | 122 | 8 | 11 | 27 | 94 | 33 | 92 |
| LAD | 684 | 58 | 439 | 70 | 117 | 33 | 86 | 45 | 79 |
| Proximal | 147 | 23 | 76 | 33 | 15 | 61 | 70 | 41 | 84 |
| Middle | 142 | 24 | 68 | 19 | 31 | 44 | 78 | 56 | 69 |
| Distal | 141 | 7 | 108 | 9 | 17 | 29 | 92 | 44 | 86 |
| Diagonal 1 | 138 | 4 | 88 | 9 | 37 | 10 | 91 | 31 | 70 |
| Diagonal 2 | 116 | 0 | 99 | 0 | 17 | 0 | 100 | 0 | 85 |
| LCX | 630 | 19 | 483 | 27 | 101 | 16 | 95 | 41 | 83 |
| Proximal | 145 | 12 | 104 | 13 | 16 | 43 | 89 | 48 | 87 |
| Middle | 104 | 5 | 80 | 7 | 12 | 29 | 92 | 42 | 87 |
| Distal | 110 | 0 | 91 | 0 | 19 | 0 | 100 | 0 | 83 |
| Marginal 1 | 142 | 1 | 95 | 7 | 39 | 3 | 93 | 13 | 71 |
| Marginal 2 | 129 | 1 | 113 | 0 | 15 | 6 | 100 | 100 | 88 |
| RCA | 651 | 47 | 488 | 41 | 75 | 39 | 92 | 53 | 87 |
| Proximal | 148 | 21 | 100 | 13 | 14 | 60 | 89 | 62 | 88 |
| Middle | 134 | 19 | 73 | 20 | 22 | 46 | 79 | 49 | 77 |
| Distal | 123 | 5 | 97 | 4 | 17 | 23 | 96 | 56 | 85 |
| RPD | 124 | 0 | 111 | 1 | 12 | 0 | 99 | 0 | 90 |
| RPL | 122 | 2 | 107 | 3 | 10 | 17 | 97 | 40 | 92 |
| Proximala | 965 | 108 | 623 | 113 | 121 | 47 | 85 | 49 | 84 |
| Distal | 1145 | 20 | 909 | 33 | 183 | 10 | 97 | 38 | 83 |
| . | n . | TP . | TN . | FP . | FN . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . |
|---|---|---|---|---|---|---|---|---|---|
| All segments | 2110 | 128 | 1532 | 146 | 304 | 30 | 91 | 47 | 83 |
| LM | 145 | 4 | 122 | 8 | 11 | 27 | 94 | 33 | 92 |
| LAD | 684 | 58 | 439 | 70 | 117 | 33 | 86 | 45 | 79 |
| Proximal | 147 | 23 | 76 | 33 | 15 | 61 | 70 | 41 | 84 |
| Middle | 142 | 24 | 68 | 19 | 31 | 44 | 78 | 56 | 69 |
| Distal | 141 | 7 | 108 | 9 | 17 | 29 | 92 | 44 | 86 |
| Diagonal 1 | 138 | 4 | 88 | 9 | 37 | 10 | 91 | 31 | 70 |
| Diagonal 2 | 116 | 0 | 99 | 0 | 17 | 0 | 100 | 0 | 85 |
| LCX | 630 | 19 | 483 | 27 | 101 | 16 | 95 | 41 | 83 |
| Proximal | 145 | 12 | 104 | 13 | 16 | 43 | 89 | 48 | 87 |
| Middle | 104 | 5 | 80 | 7 | 12 | 29 | 92 | 42 | 87 |
| Distal | 110 | 0 | 91 | 0 | 19 | 0 | 100 | 0 | 83 |
| Marginal 1 | 142 | 1 | 95 | 7 | 39 | 3 | 93 | 13 | 71 |
| Marginal 2 | 129 | 1 | 113 | 0 | 15 | 6 | 100 | 100 | 88 |
| RCA | 651 | 47 | 488 | 41 | 75 | 39 | 92 | 53 | 87 |
| Proximal | 148 | 21 | 100 | 13 | 14 | 60 | 89 | 62 | 88 |
| Middle | 134 | 19 | 73 | 20 | 22 | 46 | 79 | 49 | 77 |
| Distal | 123 | 5 | 97 | 4 | 17 | 23 | 96 | 56 | 85 |
| RPD | 124 | 0 | 111 | 1 | 12 | 0 | 99 | 0 | 90 |
| RPL | 122 | 2 | 107 | 3 | 10 | 17 | 97 | 40 | 92 |
| Proximala | 965 | 108 | 623 | 113 | 121 | 47 | 85 | 49 | 84 |
| Distal | 1145 | 20 | 909 | 33 | 183 | 10 | 97 | 38 | 83 |
aproximal segments: 1,2,5,6,7,11, and 13.
LM, left main coronary artery, RPD, right posterior descending; PRL, right posterolateral branch; TP, true positive; TN, true negative; FP, false positive; FN, false negative.
Diagnostic accuracy of diagnosing CAD in different subgroups of patients
| . | Overall . | No typical angina . | Risk factors 0–2a . | Age <65 years . |
|---|---|---|---|---|
| Number of patients (% of all patients) | 149 (100) | 72 (48) | 62 (42) | 78 (52) |
| Patients with stenosis by CA (%) | 113 (76) | 45 (63) | 39 (63) | 56 (72) |
| Sensitivity (%) | 86 | 89 | 79 | 79 |
| Specificity (%) | 49 | 52 | 57 | 50 |
| Positive predictive value (%) | 84 | 76 | 76 | 80 |
| Negative predictive value (%) | 53 | 74 | 62 | 48 |
| Diagnostic accuracy (%) | 77 | 75 | 71 | 71 |
| . | Overall . | No typical angina . | Risk factors 0–2a . | Age <65 years . |
|---|---|---|---|---|
| Number of patients (% of all patients) | 149 (100) | 72 (48) | 62 (42) | 78 (52) |
| Patients with stenosis by CA (%) | 113 (76) | 45 (63) | 39 (63) | 56 (72) |
| Sensitivity (%) | 86 | 89 | 79 | 79 |
| Specificity (%) | 49 | 52 | 57 | 50 |
| Positive predictive value (%) | 84 | 76 | 76 | 80 |
| Negative predictive value (%) | 53 | 74 | 62 | 48 |
| Diagnostic accuracy (%) | 77 | 75 | 71 | 71 |
CA, coronary angiography.
aPatients with a maximum of two cardiovascular risk factors (hypercholesterolaemia, arterial hypertension, current smoking, diabetes mellitus, and family history).
Diagnostic accuracy of diagnosing CAD in different subgroups of patients
| . | Overall . | No typical angina . | Risk factors 0–2a . | Age <65 years . |
|---|---|---|---|---|
| Number of patients (% of all patients) | 149 (100) | 72 (48) | 62 (42) | 78 (52) |
| Patients with stenosis by CA (%) | 113 (76) | 45 (63) | 39 (63) | 56 (72) |
| Sensitivity (%) | 86 | 89 | 79 | 79 |
| Specificity (%) | 49 | 52 | 57 | 50 |
| Positive predictive value (%) | 84 | 76 | 76 | 80 |
| Negative predictive value (%) | 53 | 74 | 62 | 48 |
| Diagnostic accuracy (%) | 77 | 75 | 71 | 71 |
| . | Overall . | No typical angina . | Risk factors 0–2a . | Age <65 years . |
|---|---|---|---|---|
| Number of patients (% of all patients) | 149 (100) | 72 (48) | 62 (42) | 78 (52) |
| Patients with stenosis by CA (%) | 113 (76) | 45 (63) | 39 (63) | 56 (72) |
| Sensitivity (%) | 86 | 89 | 79 | 79 |
| Specificity (%) | 49 | 52 | 57 | 50 |
| Positive predictive value (%) | 84 | 76 | 76 | 80 |
| Negative predictive value (%) | 53 | 74 | 62 | 48 |
| Diagnostic accuracy (%) | 77 | 75 | 71 | 71 |
CA, coronary angiography.
aPatients with a maximum of two cardiovascular risk factors (hypercholesterolaemia, arterial hypertension, current smoking, diabetes mellitus, and family history).
References
Noto TJ, Johnson LW, Krone R, Weaver WF, Clark DA, Kramer JR, Vetrovec GW. Cardiac catheterization 1990: a report of the registry of the society for cardiac angiography and interventions (SCA&I).
Nieman K, Cademartiri F, Lemos PA, Raaijmakers R, Pattynama PM, de Feyter PJ. Reliable noninvasive coronary angiography with fast submillimeter multislice spiral computed tomography.
Achenbach S, Giesler T, Ropers D, Ulzheimer S, Derlien H, Schulte C, Wenkel E, Moshage W, Bautz W, Daniel WG, Kalender WA, Baum U. Detection of coronary artery stenoses by contrast-enhanced, retrospectively electrocardiographically-gated, multislice spiral computed tomography.
Nieman K, Oudkerk M, Rensing BJ, van Ooijen P, Munne A, van Geuns RJ, de Feyter PJ. Coronary angiography with multi-slice computed tomography.
Knez A, Becker CR, Leber A, Ohnesorge B, Becker A, White C, Haberl R, Reiser MF, Steinbeck G. Usefulness of multislice spiral computed tomography angiography for determination of coronary artery stenoses.
Ropers D, Baum U, Pohle K, Anders K, Ulzheimer S, Ohnesorge B, Schlundt C, Bautz W, Daniel WG, Achenbach S. Detection of coronary artery stenoses with thin-slice multidetector row spiral computed tomography and multiplanar reconstruction.
Mollet NR, Cademartiri F, Nieman K, Saia F, Lemos PA, McFadden EP, Pattynama PM, Serruys PW, Krestin GP, de Feyter PJ. Multislice spiral computed tomography coronary angiography in patients with stable coronary artery disease.
Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association.
Kuettner A, Kopp AF, Schroeder S, Rieger T, Brunn J, Meisner C, Heuschmid M, Trabold T, Burgstahler C, Martensen J, Schoebel W, Selbmann HK, Claussen CD. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with angiographically proven coronary artery disease.
Kajinami K, Seki H, Takekoshi N, Mabuchi H. Coronary calcification and coronary atheroscleriosis: site by site comparative morphologic study of electron beam computed tomography and coronary angiography.
Lee TH, Boucher CA. Clinical practice. Noninvasive tests in patients with stable coronary artery disease.
Gerber TC, Kuzo RS, Lane GE, O'Brien PC, Karstaedt N, Morin RL, Safford RE, Blackshear JL, Pietan JH. Image quality in a standardized algorithm for minimally invasive coronary angiography with multislice spiral computed tomography.
Achenbach S, Ulzheimer S, Baum U, Kachelriess M, Ropers D, Giesler T, Bautz W, Daniel WG, Kalender WA, Moshage W. Noninvasive coronary angiography by retrospectively ECG-gated multislice spiral CT.
Schroeder S, Kopp AF, Kuettner A, Burgstahler C, Herdeg C, Heuschmid M, Baumbach A, Claussen CD, Karsch KR, Seipel L. Influence of heart rate on vessel visibility in noninvasive coronary angiography using new multislice computed tomography: experience in 94 patients.
Giesler T, Baum U, Ropers D, Ulzheimer S, Wenkel E, Mennicke M, Bautz W, Kalender WA, Daniel WG, Achenbach S. Noninvasive visualisation of coronary arteries using contrast-enhanced multidetector CT: influence of heart rate on image quality and stenosis detection.
Nieman K, Rensing BJ, van Geuns RJ, Vos J, Pattynama PM, Krestin GP, Serruys PW, de Feyter PJ. Non-invasive coronary angiography with multislice spiral computed tomography: impact of heart rate.