-
PDF
- Split View
-
Views
-
Cite
Cite
Rena Yadlapati, Alexander Kaizer, Madeline Greytak, Eze Ezekewe, Violette Simon, Sachin Wani, Diagnostic performance of salivary pepsin for gastroesophageal reflux disease, Diseases of the Esophagus, Volume 34, Issue 4, April 2021, doaa117, https://doi.org/10.1093/dote/doaa117
Close - Share Icon Share
Summary
Uncertain diagnostic performance has limited clinical adoption of salivary pepsin, a noninvasive diagnostic tool for gastroesophageal reflux disease (GERD). This study aimed to assess diagnostic performance of salivary pepsin, and test validity of thresholds in an external cohort of patients with or without GERD. This two-phase prospective study conducted at two centers enrolled adult asymptomatic volunteers, patients with symptoms of GERD undergoing reflux monitoring, and patients with Barrett’s esophagus (BE). Fasting saliva samples were processed for pepsin concentration using Peptest. Phase 1 compared pepsin concentration between No GERD (volunteers/functional heartburn) and GERD (erosive reflux disease/nonerosive reflux disease (NERD)/BE). Phase 2 tested validity of the diagnostic thresholds identified from Phase 1 among external functional heartburn and NERD cohorts. Of 243 enrolled subjects, 156 met inclusion criteria. Phase 1 (n = 114): Pepsin concentrations were significantly higher in GERD (n = 84) versus No GERD (n = 30) (73.8 ng/mL vs. 21.1 ng/mL; P < 0.001). Area under the curve for pepsin concentration was 0.74 (95% CI 0.65, 0.83). A salivary pepsin threshold of 24.9 ng/mL optimized the true negative rate and 100.0 ng/mL optimized the true positive rate. Phase 2 (n = 42): Pepsin concentrations were significantly higher in NERD (n = 22) versus Functional Heartburn (n = 20) (176.0 ng/mL vs. 53.3 ng/mL, P < 0.001). Applying Phase 1 thresholds in this external cohort, salivary pepsin 24.9 ng/mL was 86% sensitive (64%, 97%) and 100.0 ng/mL was 72% specific for distinguishing NERD from functional heartburn. Given modest sensitivity and specificity for GERD, salivary pepsin may have clinical utility as a noninvasive office based diagnostic screening tool for GERD.
INTRODUCTION
Gastroesophageal reflux disease (GERD) affects up to 30% of the adult US population and is the most frequent gastrointestinal diagnosis in primary care and subspecialty settings.1–3 Currently, GERD is clinically diagnosed based on patient report of troublesome esophageal symptoms such as heartburn, regurgitation, and chest pain.2 First-line management for patients with symptoms of GERD relies on empiric trials of proton pump inhibitor (PPI) therapy.4 However, up to 50% of patients with symptoms suggestive of GERD do not derive adequate symptom relief. Ambulatory reflux monitoring ultimately uncovers normal findings, or absence of GERD, in a majority of PPI nonresponders.3,5–7 Thus, reliable, minimally invasive and cost-effective validated approaches to diagnose GERD are urgently needed.
Measurement of pepsin in the saliva has been proposed as a noninvasive method to diagnose GERD.8,9 Pepsin, secreted by gastric chief cells as pepsinogen and activated in an acidic environment, is one of the primary constituents in gastro-esophageal refluxate. Presence of pepsin in the saliva may indicate reflux of fluids from the stomach to the oral cavity.10 Peptest (RD Biomed, Cottingham, UK) is a lateral flow device (LFD) which contains two antibodies to human pepsin and can rapidly detect the presence and quantify the concentration of pepsin in the saliva. Peptest is registered with the US Food and Drug Administration as a saliva-based GERD test. Recent studies support the utility of salivary pepsin for identifying GERD, though have been limited by small sample sizes and lack of comparison to objective data.11,12 Other studies have questioned the diagnostic reliability and appropriate threshold of salivary pepsin.13,14 Further, US-based data for performance of salivary pepsin among well-characterized patient groups are not available. To address these knowledge gaps we aimed to examine diagnostic thresholds of salivary pepsin across healthy volunteers and objectively defined GERD subjects, and subsequently assess the validity of salivary pepsin thresholds among an external cohort of objectively defined functional heartburn and nonerosive reflux disease (NERD) subjects.
METHODS
Study design and setting
This two-phase prospective study enrolled subjects over 35 months (August 2017 to June 2020) at two tertiary care centers (University of Colorado, Aurora, CO; University of California San Diego (UCSD), La Jolla, CA). The study was approved by the institutional review board at each site. This study was conducted in two phases. Phase 1 of the study recruited healthy volunteers and patients between August 2017 and March 2020 at University of Colorado. Phase 2, the validation phase, of the study recruited patients between October 2019 and June 2020 at UCSD.
Subjects
Phase 1
Phase 1 of the study enrolled healthy volunteers and symptomatic or disease patients. The goal in Phase 1 was to examine the performance of salivary pepsin across a broad spectrum of patients in order to assess diagnostic consistency and potential thresholds to distinguish between GERD and no GERD.
Inclusion criteria for healthy volunteers were adults without symptoms of dysphagia, heartburn, regurgitation, chest pain, sore throat, voice hoarseness, cough, or globus, with no prior history of GERD or Barrett’s esophagus (BE), no acid suppressive therapy use, and normal findings on endoscopy performed on the same day as saliva collection.
The symptomatic patients included adults with at least 8 weeks of troublesome symptoms of GERD including heartburn, regurgitation, noncardiac chest pain, with or without extra-esophageal symptoms, that underwent upper endoscopy and 96 hour wireless pH monitoring off acid suppression, if no findings of severe erosive reflux disease (Los Angeles C or D) were seen on endoscopy.2 Disease patients also included adults with BE with or without BE-related neoplasia.
Phase 1 subjects were separated into two overall groups: No GERD (healthy volunteers and subjects with functional heartburn), and GERD (subjects with NERD, erosive reflux disease (ERD), and BE) (Table 1). NERD was defined as an acid exposure time (% time spent below pH of 4.0) >6.0% based on the updated Porto consensus and the Lyon consensus.15,16 Functional heartburn was defined as an acid exposure time <4.0% and symptom association probability <95%.16
Subject groups and definitions
| Cohorts . | Definition . |
|---|---|
| No GERD | • Asymptomatic healthy volunteers |
| • Functional heartburn (reflux symptoms with acid exposure time <4.0% and symptom association probability <95% on ambulatory reflux monitoring)* | |
| GERD | • Erosive GERD (erosive esophagitis on upper endoscopy classified using the Los Angeles Classification System) |
| • Nonerosive GERD (reflux symptoms with acid exposure time >6.0% on ambulatory reflux monitoring)* | |
| • Histologic diagnosis of Barrett’s esophagus and Barrett’s esophagus-related neoplasia |
| Cohorts . | Definition . |
|---|---|
| No GERD | • Asymptomatic healthy volunteers |
| • Functional heartburn (reflux symptoms with acid exposure time <4.0% and symptom association probability <95% on ambulatory reflux monitoring)* | |
| GERD | • Erosive GERD (erosive esophagitis on upper endoscopy classified using the Los Angeles Classification System) |
| • Nonerosive GERD (reflux symptoms with acid exposure time >6.0% on ambulatory reflux monitoring)* | |
| • Histologic diagnosis of Barrett’s esophagus and Barrett’s esophagus-related neoplasia |
*Phase 2 only enrolled functional heartburn and nonerosive GERD subjects. GERD, gastroesophageal reflux disease
Subject groups and definitions
| Cohorts . | Definition . |
|---|---|
| No GERD | • Asymptomatic healthy volunteers |
| • Functional heartburn (reflux symptoms with acid exposure time <4.0% and symptom association probability <95% on ambulatory reflux monitoring)* | |
| GERD | • Erosive GERD (erosive esophagitis on upper endoscopy classified using the Los Angeles Classification System) |
| • Nonerosive GERD (reflux symptoms with acid exposure time >6.0% on ambulatory reflux monitoring)* | |
| • Histologic diagnosis of Barrett’s esophagus and Barrett’s esophagus-related neoplasia |
| Cohorts . | Definition . |
|---|---|
| No GERD | • Asymptomatic healthy volunteers |
| • Functional heartburn (reflux symptoms with acid exposure time <4.0% and symptom association probability <95% on ambulatory reflux monitoring)* | |
| GERD | • Erosive GERD (erosive esophagitis on upper endoscopy classified using the Los Angeles Classification System) |
| • Nonerosive GERD (reflux symptoms with acid exposure time >6.0% on ambulatory reflux monitoring)* | |
| • Histologic diagnosis of Barrett’s esophagus and Barrett’s esophagus-related neoplasia |
*Phase 2 only enrolled functional heartburn and nonerosive GERD subjects. GERD, gastroesophageal reflux disease
Phase 2 (validation phase)
Enrollment for Phase 2 of the study was limited to symptomatic patients without ERD in order to simulate the patient population that represents the diagnostic clinical challenge, typically characterized by patients with functional heartburn or NERD. Thus, Phase 2 subjects were comprised of adults with at least 8 weeks of troublesome symptoms of GERD including heartburn, regurgitation, noncardiac chest pain, with or without extra-esophageal symptoms, and no prior diagnosis of GERD. These subjects did not have findings of severe ERD (Los Angeles C or D esophagitis) on upper endoscopy. All subjects underwent 96 hour wireless pH monitoring off acid suppression and were separated into two groups: Functional heartburn (acid exposure time <4.0% and symptom association probability <95%), and NERD (acid exposure time >6.0%) (Table 1).
In both phases, subjects with reflux hypersensitivity (acid exposure time <4.0% and symptom association probability >95%) or those with inconclusive acid exposure time (4.0% to 6.0%) were excluded. Additionally, exclusion criteria included pregnancy, breastfeeding, non-English speaking, mentally disabled, imprisoned, unable to consent, or unable to produce 2 mL saliva.
Study protocol and data management
During both study phases fasting unstimulated active saliva samples were collected from all enrolled subjects and processed for pepsin analysis. Subjects completed two validated instruments, the GerdQ and Reflux Symptom Index (RSI) questionnaire. The six-item GerdQ evaluates reflux symptoms over a 7-day period, with higher scores on the range from 0 to 18 indicating more severe symptoms.17 The nine-item RSI evaluates laryngeal symptom burden, with higher scores on the range from 0 to 45 indicating more severe symptoms.18
Data collected for subjects included demographics, primary symptom, use of PPI (type and dose), endoscopic findings (presence of erosive esophagitis, length of BE segment according to the Prague classification,19 and hiatal hernia size), dysplasia severity on histology for BE subjects, GerdQ and RSI scores, and salivary pepsin concentration. Ambulatory reflux monitoring tracings were manually interpreted in a blinded fashion and data collected included total acid exposure time, daily acid exposure time, number of reflux events, symptom index, and symptom association probability. Data for all subjects were collected in de-identified datasets on institutional Research Electronic Data Capture databases at both sites.
Sample collection and analysis
One fasting saliva sample was collected for all subjects prior to endoscopic procedure. Unstimulated expectorated saliva samples were collected from subjects and placed into 15-mL sterile plastic tubes containing 0.5 mL of 0.01 mol/L citric acid at pH 2.5. The samples were promptly transferred to the refrigerator at 4°C. Pepsin was measured using the Peptest LFD (RD Biomed Ltd). Within seven days of collection, samples were centrifuged for 5 minutes at 4,000 rpm in a bench top centrifuge, and the supernatants were collected. A total of 80 μL of the supernatants layer was then mixed with 240 μL of migration buffer solution and vortexed for 10 seconds. Then 80 μL of the mixture was added to the well of the LFD. The LFD was transferred to the Peptest recorder which provided a quantified concentration of pepsin in ng/mL. Peptest has the ability to detect pepsin concentrations of 16 ng/mL or greater. Concentrations between 16 and 24.9 ng/mL are quantified as 16 < 25 ng/mL by the recorder.
Outcomes and sample size calculation
The primary outcome was salivary pepsin concentration. Predetermined target sample size for Phase 1 was 110 which conservatively assumes an allocation of 25% non-GERD and 75% GERD subjects which achieves a 95% CI for an estimated AUC of 0.70 of (0.58,0.82), which excludes 0.50 suggesting pepsin would have some benefit in predicting GERD. The sample size for Phase 2 was based on data from Phase 1 in which 18 subjects per group (functional heartburn and NERD) would achieve 80% power with an alpha of 0.05. Thus, the target sample size for Phase 2 was 36, 18 in each group.
Statistical analysis
The primary analysis was to identify the optimal threshold for pepsin that achieves desired sensitivity and specificity for classifying GERD versus no GERD as evaluated by receiver operating characteristic curves. Adjusted analyses considered age, body mass index, and sex in addition to pepsin in a logistic regression model to evaluate potential improvements in performance when using the predicted probability of being a case from the model. Models were fit comparing all GERD to no GERD, as well as a separate sensitivity model for comparing no GERD to NERD alone. The area under the curve (AUC) and 95% confidence interval was calculated for each model. The optimal threshold identified for pepsin (unadjusted) and the predicted probability (adjusted) were identified from the Phase 1 data, and then applied to the external Phase 2 validation data and summarized using sensitivity and specificity. Pepsin values measured as 16 to 24.9 ng/mL were imputed as 20.5 ng/mL, with sensitivity analyses to evaluate model performance if values were imputed as 16 or 24.9 ng/mL. The Phase 2 study also included a secondary analysis to assess quality control of device. This secondary analysis calculated the intraclass correlation coefficient assuming a two-way mixed-effects model for single measure absolute agreement since three pepsin values were calculated for all Phase 2 subjects. Comparisons of continuous measures between different groupings used a two-sample t-test. All figures created and analyses were conducted using R v3.6.3.
RESULTS
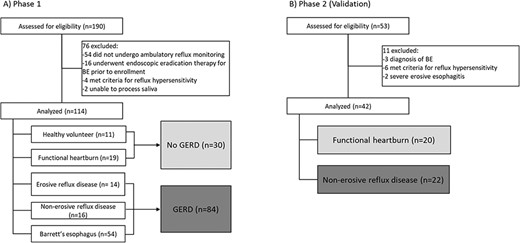
In total 243 subjects were recruited. Phase 1 recruited 190 subjects of which 76 were excluded and a total of 114 subjects were included. Phase 2 recruited 53 subjects of which 11 were excluded and a total of 42 subjects were included (Fig. 1).
Phase 1
Baseline characteristics of subjects
The 114 subjects in phase 1 included 30 (26%) no GERD (11 healthy volunteers and 19 functional heartburn), and 84 (74%) GERD (16 NERD, 14 ERD, 54 BE) with a mean age of 61.1 years (SD 13.8), 45 (40%) female, and mean body mass index of 27.8 kg/m2 (SD 6.2) (Table 2). Of those with BE, mean BE segment length was 5.2 cm (SD 4.5).
Baseline characteristics of subjects in Phase 1
| . | No GERD . | GERD . | |||
|---|---|---|---|---|---|
| Variable . | Healthy controls . | Functional HB . | NERD . | ERD . | BE . |
| (N = 11) | (N = 19) | (N = 16) | (N = 14) | (N = 54) | |
| Age, years | 45.3 (15.4) | 58.7 (14.9) | 61.4 (12.2) | 64.7 (10.7) | 64.2 (12.2) |
| Female | 10 (90.9%) | 13 (68.4%) | 10 (62.5%) | 5 (35.7%) | 7 (13.0%) |
| Body mass index, kg/m2 | 29.4 (6.1) | 28.3 (8.1) | 27.7 (6.8) | 27.5 (4.7) | 27.3 (5.8) |
| Acid exposure time, % | NA | 0.9 (1.3) | 9.29 (4.6) | 13.9 (1.1) | NA |
| Hiatal hernia present | 1 (9.1%) | 1 (5.3%) | 10 (62.5%) | 13 (92.9%) | 36 (66.7%) |
| Hiatal hernia size, cm | 2.00 (0.0) | 2.8 (2.2) | 3.6 (1.8) | 3.5 (1.7) | |
| GERDQ score | 5.33 (1.7) | 10.5 (3.7) | 7.62 (3.4) | 7.89 (2.3) | 8.08 (2.6) |
| Reflux symptom index score | 5.44 (8.0) | 24.6 (12.7) | 15.5 (14.1) | 6.4 (9.0) | 13.0 (10.2) |
| Pepsin concentration, ng/mL | 17.9 (31.4) | 22.9 (31.7) | 53.7 (51.3) | 101.0 (80.6) | 72.7 (70.2) |
| . | No GERD . | GERD . | |||
|---|---|---|---|---|---|
| Variable . | Healthy controls . | Functional HB . | NERD . | ERD . | BE . |
| (N = 11) | (N = 19) | (N = 16) | (N = 14) | (N = 54) | |
| Age, years | 45.3 (15.4) | 58.7 (14.9) | 61.4 (12.2) | 64.7 (10.7) | 64.2 (12.2) |
| Female | 10 (90.9%) | 13 (68.4%) | 10 (62.5%) | 5 (35.7%) | 7 (13.0%) |
| Body mass index, kg/m2 | 29.4 (6.1) | 28.3 (8.1) | 27.7 (6.8) | 27.5 (4.7) | 27.3 (5.8) |
| Acid exposure time, % | NA | 0.9 (1.3) | 9.29 (4.6) | 13.9 (1.1) | NA |
| Hiatal hernia present | 1 (9.1%) | 1 (5.3%) | 10 (62.5%) | 13 (92.9%) | 36 (66.7%) |
| Hiatal hernia size, cm | 2.00 (0.0) | 2.8 (2.2) | 3.6 (1.8) | 3.5 (1.7) | |
| GERDQ score | 5.33 (1.7) | 10.5 (3.7) | 7.62 (3.4) | 7.89 (2.3) | 8.08 (2.6) |
| Reflux symptom index score | 5.44 (8.0) | 24.6 (12.7) | 15.5 (14.1) | 6.4 (9.0) | 13.0 (10.2) |
| Pepsin concentration, ng/mL | 17.9 (31.4) | 22.9 (31.7) | 53.7 (51.3) | 101.0 (80.6) | 72.7 (70.2) |
NERD, nonerosive reflux disease; ERD, erosive reflux disease; BE, Barrett’s esophagus
Baseline characteristics of subjects in Phase 1
| . | No GERD . | GERD . | |||
|---|---|---|---|---|---|
| Variable . | Healthy controls . | Functional HB . | NERD . | ERD . | BE . |
| (N = 11) | (N = 19) | (N = 16) | (N = 14) | (N = 54) | |
| Age, years | 45.3 (15.4) | 58.7 (14.9) | 61.4 (12.2) | 64.7 (10.7) | 64.2 (12.2) |
| Female | 10 (90.9%) | 13 (68.4%) | 10 (62.5%) | 5 (35.7%) | 7 (13.0%) |
| Body mass index, kg/m2 | 29.4 (6.1) | 28.3 (8.1) | 27.7 (6.8) | 27.5 (4.7) | 27.3 (5.8) |
| Acid exposure time, % | NA | 0.9 (1.3) | 9.29 (4.6) | 13.9 (1.1) | NA |
| Hiatal hernia present | 1 (9.1%) | 1 (5.3%) | 10 (62.5%) | 13 (92.9%) | 36 (66.7%) |
| Hiatal hernia size, cm | 2.00 (0.0) | 2.8 (2.2) | 3.6 (1.8) | 3.5 (1.7) | |
| GERDQ score | 5.33 (1.7) | 10.5 (3.7) | 7.62 (3.4) | 7.89 (2.3) | 8.08 (2.6) |
| Reflux symptom index score | 5.44 (8.0) | 24.6 (12.7) | 15.5 (14.1) | 6.4 (9.0) | 13.0 (10.2) |
| Pepsin concentration, ng/mL | 17.9 (31.4) | 22.9 (31.7) | 53.7 (51.3) | 101.0 (80.6) | 72.7 (70.2) |
| . | No GERD . | GERD . | |||
|---|---|---|---|---|---|
| Variable . | Healthy controls . | Functional HB . | NERD . | ERD . | BE . |
| (N = 11) | (N = 19) | (N = 16) | (N = 14) | (N = 54) | |
| Age, years | 45.3 (15.4) | 58.7 (14.9) | 61.4 (12.2) | 64.7 (10.7) | 64.2 (12.2) |
| Female | 10 (90.9%) | 13 (68.4%) | 10 (62.5%) | 5 (35.7%) | 7 (13.0%) |
| Body mass index, kg/m2 | 29.4 (6.1) | 28.3 (8.1) | 27.7 (6.8) | 27.5 (4.7) | 27.3 (5.8) |
| Acid exposure time, % | NA | 0.9 (1.3) | 9.29 (4.6) | 13.9 (1.1) | NA |
| Hiatal hernia present | 1 (9.1%) | 1 (5.3%) | 10 (62.5%) | 13 (92.9%) | 36 (66.7%) |
| Hiatal hernia size, cm | 2.00 (0.0) | 2.8 (2.2) | 3.6 (1.8) | 3.5 (1.7) | |
| GERDQ score | 5.33 (1.7) | 10.5 (3.7) | 7.62 (3.4) | 7.89 (2.3) | 8.08 (2.6) |
| Reflux symptom index score | 5.44 (8.0) | 24.6 (12.7) | 15.5 (14.1) | 6.4 (9.0) | 13.0 (10.2) |
| Pepsin concentration, ng/mL | 17.9 (31.4) | 22.9 (31.7) | 53.7 (51.3) | 101.0 (80.6) | 72.7 (70.2) |
NERD, nonerosive reflux disease; ERD, erosive reflux disease; BE, Barrett’s esophagus
Salivary pepsin concentration
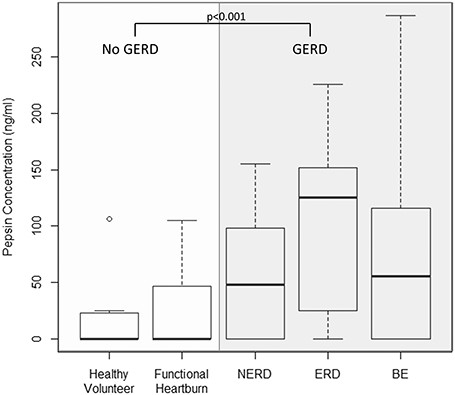
Mean salivary pepsin concentrations were significantly higher for GERD compared to No GERD subjects (73.8 ng/mL (SD 69.6) vs. 21.1 ng/mL (SD 31.1); P < 0.001). Among the No GERD subjects, mean pepsin concentrations were similar between healthy volunteers and functional heartburn (17.9 ng/mL (SD 31.4) and 22.9 (SD 31.7)). Among the GERD subjects, mean pepsin concentrations were similar between ERD (101.0 ng/mL (SD 80.6)), NERD (53.7 (SD 51.3)), and BE (72.7 (SD 70.2)) (Fig. 2). Supplemental Table 1 describes the pair-wise comparisons of pepsin concentration between subgroups. Among BE subjects, mean pepsin concentrations were similar across dysplasia severity (NDBE 66.0 (82.3), LGD 58.1 (73.8), HGD 87.7 (81.4), EAC 68.6 (63.0)).
Box plot of salivary pepsin concentration in Phase 1 groups. Concentration of salivary pepsin significantly differs between the No GERD versus GERD groups.
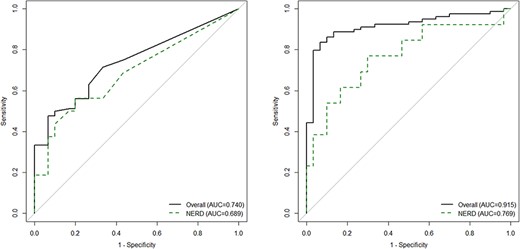
Receiver operating characteristics of pepsin concentrations
AUC of salivary pepsin for No GERD subjects versus GERD subjects was 0.74 (95% CI 0.65, 0.83), and increased to 0.92 (0.86, 0.97) when adjusted for age, sex, and body mass index (Fig. 3). A salivary pepsin of 24.9 ng/mL optimized the true negative rate of GERD with a sensitivity of 71% (95% CI 61%, 81%) and specificity of 67% (47%, 83%). Salivary pepsin levels greater than 100 ng/mL optimized the true positive rate of GERD with 93% specificity (95% CI 78%, 99%) (Supplementary Table 1).
Receiver operating characteristic curves for salivary pepsin in Phase 1. (A) Unadjusted model. (B) Adjusted model.
Sensitivity analyses
AUC of salivary pepsin for No GERD subjects versus NERD, excluding those with BE or ERD, was 0.69 (95% CI 0.52, 0.86), and when adjusted for age, sex, and body mass index increased to 0.77 (0.60, 0.94) (Fig. 3). Sensitivity analyses considering the quantification of 16 to 25 ng/mL as 16 ng/mL or as 24.9 ng/mL instead of 20.5 ng/mL did not result in any changes to the selected threshold since all imputed values were less than 25 ng/mL.
Phase 2 (validation phase)
Baseline characteristics of subjects
The validation phase enrolled 42 subjects: 20 (48%) Functional heartburn and 22 (52%) NERD with a mean age of 46 years (SD 13.9), 31 (74%) female, and mean body mass index of 26.2 kg/m2 (SD 6.0) (Table 3).
Baseline characteristics of subjects in Phase 2, validation phase
| Variable . | Non-GERD . | GERD . |
|---|---|---|
| (N = 20) | (N = 22) | |
| Age, years | 45.4 (16.5) | 46.0 (11.4) |
| Female | 15 (75.0%) | 16 (72.7%) |
| Body mass index, kg/m2 | 24.1 (4.56) | 28.1 (6.56) |
| Acid exposure time, % | 1.66 (1.28) | 10.8 (12.5) |
| GerdQ score | 7.24 (3.61) | 9.0 (3.42) |
| Reflux symptom index score | 17.3 (9.8) | 19.7 (11.5) |
| Pepsin concentration, ng/mL | 53.3 (57.0) | 176.0 (141.6) |
| Proportion with pepsin >24.95 ng/mL | 10 (50%) | 18 (82%) |
| Proportion with pepsin >100 ng/mL | 5 (25%) | 15 (68%) |
| Variable . | Non-GERD . | GERD . |
|---|---|---|
| (N = 20) | (N = 22) | |
| Age, years | 45.4 (16.5) | 46.0 (11.4) |
| Female | 15 (75.0%) | 16 (72.7%) |
| Body mass index, kg/m2 | 24.1 (4.56) | 28.1 (6.56) |
| Acid exposure time, % | 1.66 (1.28) | 10.8 (12.5) |
| GerdQ score | 7.24 (3.61) | 9.0 (3.42) |
| Reflux symptom index score | 17.3 (9.8) | 19.7 (11.5) |
| Pepsin concentration, ng/mL | 53.3 (57.0) | 176.0 (141.6) |
| Proportion with pepsin >24.95 ng/mL | 10 (50%) | 18 (82%) |
| Proportion with pepsin >100 ng/mL | 5 (25%) | 15 (68%) |
Baseline characteristics of subjects in Phase 2, validation phase
| Variable . | Non-GERD . | GERD . |
|---|---|---|
| (N = 20) | (N = 22) | |
| Age, years | 45.4 (16.5) | 46.0 (11.4) |
| Female | 15 (75.0%) | 16 (72.7%) |
| Body mass index, kg/m2 | 24.1 (4.56) | 28.1 (6.56) |
| Acid exposure time, % | 1.66 (1.28) | 10.8 (12.5) |
| GerdQ score | 7.24 (3.61) | 9.0 (3.42) |
| Reflux symptom index score | 17.3 (9.8) | 19.7 (11.5) |
| Pepsin concentration, ng/mL | 53.3 (57.0) | 176.0 (141.6) |
| Proportion with pepsin >24.95 ng/mL | 10 (50%) | 18 (82%) |
| Proportion with pepsin >100 ng/mL | 5 (25%) | 15 (68%) |
| Variable . | Non-GERD . | GERD . |
|---|---|---|
| (N = 20) | (N = 22) | |
| Age, years | 45.4 (16.5) | 46.0 (11.4) |
| Female | 15 (75.0%) | 16 (72.7%) |
| Body mass index, kg/m2 | 24.1 (4.56) | 28.1 (6.56) |
| Acid exposure time, % | 1.66 (1.28) | 10.8 (12.5) |
| GerdQ score | 7.24 (3.61) | 9.0 (3.42) |
| Reflux symptom index score | 17.3 (9.8) | 19.7 (11.5) |
| Pepsin concentration, ng/mL | 53.3 (57.0) | 176.0 (141.6) |
| Proportion with pepsin >24.95 ng/mL | 10 (50%) | 18 (82%) |
| Proportion with pepsin >100 ng/mL | 5 (25%) | 15 (68%) |
Pepsin concentrations in the saliva
A salivary pepsin greater than 24.9 ng/mL was 86% sensitive (95% CI 64%, 97%) and 44% specific (95% CI 22%, 69%) for diagnosing NERD from functional heartburn, and a salivary pepsin greater than 100 ng/mL was 71% sensitive (95% CI 48%, 89%) and 72% specific (95% CI 47%, 90%) for diagnosing NERD from functional heartburn. Mean salivary pepsin was significantly higher for the NERD compared to functional heartburn cohort (176.0 (SD 141.6) vs. 53.3 ng/mL (SD 57.0); P < 0.001).
Secondary analysis
Each saliva sample was processed and tested on three separate Peptest LFDs to assess quality control of device. Intra-class correlation between three repeated measurements was 0.998 (95% CI 0.995, 0.999), where values near 1.0 indicate near perfect agreement.20 The intra-test coefficient of variation was 4.39%, where values below 10% suggest generally good agreement between measurements.21
DISCUSSION
Noninvasive, accessible, and cost-effective diagnostic tools are critically needed to avoid overdiagnosis of GERD and inappropriate PPI use. This prospective two-phased US-based study examined the performance of salivary pepsin for objective GERD using well-defined groups (Phase 1), and validated diagnostic performance among an external cohort of functional heartburn and NERD patients (Phase 2). This study demonstrated that fasting salivary pepsin using Peptest has modest diagnostic performance for patients with objective GERD, with a salivary pepsin of 24.9 ng/mL optimizing the true negative rate with 86% sensitivity and salivary pepsin of 100.0 ng/mL optimizing the true positive rate with 72% specificity for distinguishing NERD from functional heartburn.
In our study, fasting salivary pepsin of 24.9 ng/mL performed better than reported performance of standard tools commonly used in clinical practice to diagnose GERD and guide therapeutic decisions (validated questionnaire based assessments: 65% sensitive and 65% specific; response to PPI trial: 78% sensitive and 54% specific).17,22–24 A recent network meta-analysis by Zhang et al.24 similarly suggested that the diagnostic performance of salivary pepsin was comparable in specificity to ambulatory reflux monitoring and endoscopy, and superior to patient reported symptoms and the PPI test. In our study receiver operating characteristics also highlight 93% specificity of fasting salivary pepsin for GERD, where patients with salivary pepsin >100 ng/mL have a 5.0 times higher likelihood of objective GERD than no GERD. Prior studies from the UK and Japan have similarly reported high specificity for objective GERD.10,25,26 While there is potential for patients to have a salivary pepsin concentration between 25 ng/mL and 100 ng/mL, a minority of subjects in the validation phase (5/20 (25%) No GERD and 3/22 (14%) GERD subjects) had salivary pepsin concentrations within this range (Table 3).
It is important to address the lessons learnt from the existing mixed literature regarding the diagnostic characteristics and thresholds for salivary pepsin. While elevated salivary pepsin concentrations of over 500 ng/mL were reported in two prior studies,13,27 our experience mirrors that of others where none of the controls, or even disease subjects, had salivary pepsin levels >500 ng/mL. The possibility of use of a faulty lot was raised for some of the prior results. Through our own experience with salivary pepsin we also have recognized the importance of adhering to step by step specimen processing (as described in the methods), as missteps can generate false positive results. It is possible that missteps in processing are also an etiology of findings of very high salivary pepsin levels by other investigators. In 2016 we studied salivary pepsin among a smaller group of subjects that was not objectively characterized, and detected a trend of higher salivary pepsin concentrations in patients with laryngeal and reflux symptoms compared to those without reflux symptoms.14 Thus, based on our own and others’ prior experiences we ensured adequate sample size, limited inclusion to objectively defined groups of subjects, ensured we did not have any old Peptest lots at the onset of the study, and continued to adhere to stringent sample processing in this study.
In this study diagnostic performance of salivary pepsin for GERD further improved when the model factored a priori selected variables that are readily availability in a clinical setting and established risk factors for GERD and BE.28–31 Our study generates the hypothesis that a risk prediction model inclusive of salivary pepsin and clinical data could predict GERD with high diagnostic performance, and this is a ripe area for future investigation. In Europe, and more recently in the USA, Peptest is available as a direct to consumer tool in which customers independently collect saliva in tubes and mail the samples to a central lab, and receive a report for the consumer and health care provider at an approximate cost of $125. The comparative performance of this seemingly attractive direct to consumer model compared to a research arena needs to be examined.
There are limitations to this study. This study was designed to include an objectively characterized group of no GERD and GERD subjects, with an initial intent to enroll predominantly ERD or NERD. While a similar number of symptomatic and BE patients were initially enrolled, 54 symptomatic patients were excluded for not undergoing ambulatory reflux monitoring. Although this was not the intended ratio of GERD: BE, BE represents an important objective phenotype of GERD, and we felt it was a strength to study salivary pepsin across objectively defined cohorts given the well-established pitfalls of diagnosis based on symptom presentation alone. To address this potential limitation a sensitivity analyses excluding BE and ERD subjects identified similar performance characteristics of salivary pepsin for NERD. This study excluded patients with inconclusive acid exposure times (4.0 to 6.0%) and reflux hypersensitivity as it is unclear whether these patients represent GERD or non-GERD phenotypes. This study did not aim to assess salivary pepsin thresholds in patients with isolated extra-esophageal symptoms or suspected laryngopharyngeal reflux, and, thus, these results cannot be translated to that group. Our study protocol collected one fasting sample per patient and there is potential for variability in salivary pepsin concentrations across days or at different times within the day. However, our prior research and research by others has highlighted reproducibility across three consecutive days.10,14 Further, studies show that pepsin can be found in up to one-third of healthy asymptomatic subjects during postprandial periods.10 Therefore, we elected to apply a clinically practical one fasting sample collection method that is supported by prior studies.
In conclusion, measurement of salivary pepsin using Peptest has several attributes of an optimal diagnostic tool.32 It is already well known to be affordable, rapid in time to diagnosis, noninvasive, and easy to administer. Our prospective study importantly highlights and validates the performance characteristics of salivary pepsin, particularly the modest sensitivity of a threshold of 24.9 ng/mL and modestspecificity of a threshold of 100.0 ng/mL for GERD. Implications of salivary pepsin measurement have potential to reduce time to diagnosis, minimize empiric and often ineffective PPI therapy, and streamline care for patients with GERD symptoms. With further research clarifying the clinical role of salivary pepsin, one can envision a screening role of salivary pepsin using Peptest in a primary care or specialty care setting for patients presenting with suspected GERD in which patients with low salivary pepsin levels may represent a group with low likelihood of GERD. For these patients a PPI trial may not be needed, and rather a focus on alternative etiologies of symptoms could be considered.
Specific author contributions
Study concept and design: Rena Yadlapati, Sachin Wani; Study oversight: Rena Yadlapati, Sachin Wani;. Acquisition of data: Rena Yadlapati, Eze Ezekewe, Madeline Greytak, Violette Simon, Sachin Wani; Analysis and interpretation of data: Rena Yadlapati, Sachin Wani, Madeline Greytak, Alexander Kaizer; Drafting of manuscript: Rena Yadlapati, Eze Ezekewe, Alexander Kaizer, Madeline Greytak, Violette Simon, Sachin Wani; Critical Revision of the manuscript for important intellectual content: Rena Yadlapati, Eze Ezekewe, Alexander Kaizer, Madeline Greytak, Violette Simon, Sachin Wani; Finalization of manuscript: Rena Yadlapati, Eze Ezekewe, Alexander Kaizer, Madeline Greytak, Violette Simon, Sachin Wani.
Writing Assistance
None.
ACKNOWLEDGMENTS
Conflicts of interest
Eze Ezekewe, Alexander Kaizer, Madeline Greytak, Violette Simon: None.
Rena Yadlapati: Consultant for Medtronic, Ironwood Pharmaceuticals, Diversatek. Advisory board: Phathom Pharmaceuticals. Research funding: Ironwood Pharmaceuticals.
Sachin Wani: Consultant for Boston Scientific, Medtronic, Cernostics and Interpace.
Research Funding Support
This study was supported by the ACG Junior Faculty Development Award (PI Yadlapati) and University of Colorado Department of Medicine Outstanding Early Scholars Program (PI Wani). Peptest supplies were provided by RD BioMed.