-
PDF
- Split View
-
Views
-
Cite
Cite
Mariusz Stasiolek, Antonios Bayas, Niels Kruse, Anja Wieczarkowiecz, Klaus V. Toyka, Ralf Gold, Krzysztof Selmaj, Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis, Brain, Volume 129, Issue 5, May 2006, Pages 1293–1305, https://doi.org/10.1093/brain/awl043
Close - Share Icon Share
Abstract
Plasmacytoid dendritic cells (pDCs) represent a DC subtype that exerts divergent functions in innate and adoptive immunity including the immediate reaction to microbial factors and the induction of immunoregulatory responses. It is thought that different DC subtypes may be critically involved in the pathogenesis of multiple sclerosis (MS). In our study we assessed the phenotype, maturation and functional properties of peripheral blood pDCs from 35 clinically stable, untreated multiple sclerosis patients, 30 healthy controls and 9 patients with pneumonia, which was used as a non-specific inflammatory condition (NIC). Ex vivo expression of CD86 and 4-1BBL was significantly lower on pDCs from multiple sclerosis patients than from controls and patients with NIC (22 versus 47 versus 41% and 12 versus 35 versus 32%, respectively). When stimulated with IL-3 and CD40L, pDCs of multiple sclerosis patients showed inefficient maturation as demonstrated by significantly lower or delayed upregulation of CD86, 4-1BBL, CD40 and CD83. Additionally, in multiple sclerosis, stimulation of pDCs by unmethylated cytosine-phosphate-guanosine oligodeoxynucleotides (CpG ODN) resulted in a significantly lower interferon (IFN) alpha secretion than in controls. In multiple sclerosis, but not in controls, pDCs failed to upregulate proliferative responses and IFN-gamma secretion of autologous peripheral blood mononuclear cells (PBMC) in a co-culture system. Moreover, depletion of pDCs in multiple sclerosis patients, but not in controls, had no effect on generation of CD4+Foxp3+ regulatory T cells. We also provide data showing that glatiramer acetate (GA) treatment partially restores phenotype and function of pDCs in multiple sclerosis patients. These findings suggest functional abnormalities of pDCs in these patients, which might be of importance in the understanding of the development of immune dysregulation in this disease.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the CNS. Target-specific T cells, which are normally controlled by tolerance mechanisms, are assumed to initiate and perpetuate an autoimmune reaction that leads to severe CNS damage (Martin et al., 1992; Steinman, 1996). The mechanism of the initial T-cell activation is not known but several lines of evidence suggest their activation by microbial factors with subsequent development of autoimmune reactions (Sriram et al., 1999; Haahr et al., 2004; Rotola et al., 2004 ). Dendritic cells (DCs) are critically involved in the primary induction of T-cell activation as part of the mounting immune responses. In humans there are at least two main subsets of peripheral blood DCs: myeloid (mDCs) and plasmacytoid (pDCs) (Lipscomb and Masten, 2002; Shortman and Liu, 2002). mDCs and pDCs differ functionally in many aspects including antigen uptake, cytokine secretion profiles and priming of an immune response (Shortman and Liu, 2002). The most striking feature of pDCs distinguishing this subset from other DCs is a vigorous reaction to various pathogens with high secretion of type I interferons (IFNs) (Cella et al., 1999; Siegal et al., 1999). In the adoptive arm of the immune response, pDCs demonstrate a pronounced ability to prime Th1 (Cella et al., 2000) and Th2 cells (Rissoan et al., 1999), as well as regulatory T cells associated with immunoregulation (Pulendran et al., 2000; Gilliet and Liu, 2002; Moseman et al., 2004). Thus, the insufficiency of pDC function might lead to inappropriate immune reactions and contribute to the impairment of immunoregulatory mechanisms, which in turn contributes to the development of autoimmune disorders.
It has been shown that the state of maturity (Dhodapkar et al., 2001; Janjic et al., 2002) and, in particular, the expression of co-stimulatory molecules strongly influence the properties of DCs (de Jong et al., 2002; Hsu et al., 2002). The best-characterized co-stimulatory molecules belong to the B7 and the tumour necrosis factor (TNF)/TNF receptor (TNFR) families. B7.1/CD80 and B7.2/CD86 expressed on antigen-presenting cells (APCs) are considered as the predominant molecules delivering ‘second signals’ during T-cell activation (Chambers and Allison, 1997; Chambers, 2001). 4-1BBL/CD137L provides additional co-stimulatory signals that are at least partially independent from B.7 ligation (Kim et al., 1998; Gramaglia et al., 2000; Bertram et al., 2002), whereas the interaction of CD40, expressed on DCs, with CD40L, expressed on T cells, is crucial for final DC maturation (Cella et al., 1996; Bleharski et al., 2001). Signals provided by the pattern recognition receptors have a particularly strong influence on the maturation and function of DCs (Hertz et al., 2001; Biragyn et al., 2002). Within the Toll Like Receptor (TLR) family, TLR9 is of particular interest because it is expressed exclusively on pDCs and B cells and its ligands such as unmethylated cytosine-phosphate-guanosine oligodeoxynucleotides (CpG ODN) act as very effective direct activators of these cells (Rothenfusser et al., 2002).
Myeloid and plasmacytoid subsets of DCs are both present in the cerebrospinal fluid of multiple sclerosis patients (Pashenkov et al., 2001); however, their role in the pathogenesis of multiple sclerosis remains unknown. In the present study we focused our interest on pDCs from peripheral blood and investigated their surface expression of molecules involved in differentiation, antigen presentation, co-stimulatory signal delivery and maturation. Subsequently, we assessed their functional properties, including their effect on the generation of immunoregulatory cells (Treg cells).
Material and methods
Patients and controls
For this study only patients with a diagnosis of relapsing-remitting multiple sclerosis (RR multiple sclerosis) and with a clinically stable disease course were chosen. Patients had to be free of immunomodulatory treatment during the study period and at least for the preceding 6 months. Patients having suffered from a relapse and/or receiving glucocorticosteroid treatment for at least 90 days before cell collection were excluded from the study. Owing to these stringent selection criteria and the difficult demands to meet the leucapheresis procedure conditions, only few of over 300 patients screened in our multiple sclerosis centres entered the study. Thirty-five patients with a mean Expanded Disability Status Score (EDSS) of 2.7 (SD ± 1.6) were eventually included for the ex vivo analysis. Thirty healthy subjects, without any kind of permanent treatment, served as a control group. An additional group of patients (n = 9) with pneumonia was used as a non-specific inflammatory condition control (NIC). The groups were matched for sex and age. Blood withdrawal and leucapheresis were approved by the local ethics committees.
For the assessment of the effect of disease-modifying drugs (DMD) on the pDC function, an additional group of nine patients treated with glatiramer acetate (GA) was included in the study. The mean age and EDSS was comparable with the other multiple sclerosis patients. The treatment period with GA was, on average, 30 months. All patients were clinically stable during GA treatment and at the time of sampling for this study.
Leucapheresis
For pDC sorting experiments, large numbers of peripheral blood mononuclear cells (PBMC) had to be obtained, which was not feasible through venipuncture. Therefore, a leucapheresis procedure was needed to isolate sufficient numbers of PBMCs. All leucaphereses were performed at the Department for Transfusion Medicine of the University Hospital in Würzburg, Germany, and at the Blood Donation Center, Lodz, Poland, with automated continuous-flow blood cell separators (COBE Spectra, Gambro BCT, Inc., USA or Fenwal CS-3000, Baxter International Inc., USA). Typically, ∼2–4 × 109 mononuclear cells were collected from 4–6 l of peripheral blood over 1.5–2 h. After leucapheresis, PBMC suspensions were immediately used for the DC isolation procedures and culture experiments described below. None of the patients and controls suffered from major adverse events.
Fluorescence-activated cell sorting (FACS) analysis
PBMCs were isolated from peripheral blood samples obtained through venipuncture by centrifugation on a discontinuous density gradient (Histopaque 1077, Sigma-Aldrich, St Louis, USA). The mononuclear cell fraction was washed three times in phosphate buffered saline (PBS), counted and suspended in PBS for flow cytometry analysis. PBMCs from multiple sclerosis patients, healthy controls and NIC patients were assessed by three- or two-colour flow cytometry using a FACSCalibur® cytometer and CELLQuest®software (BD Biosciences, San Jose, CA, USA). The myeloid subset of DCs can be further divided into two populations expressing blood dendritic cell antigen-1 (BDCA1), which has been shown to be identical to CD1c, or BDCA3. In contrast, BDCA2 and BDCA4 are specific for blood pDCs (Dzionek et al., 2000, 2001, 2002). Monoclonal antibodies (mAb) specific for BDCA antigens were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). All remaining mAb and appropriate isotype controls were purchased from BD Biosciences Pharmingen (San Jose, CA, USA). The BDCA2+ plasmacytoid DC and the BDCA3+ mDC subtypes were recognized by staining with fluorescein isothiocyanate (FITC) conjugated antibodies specific for BDCA2 (AC144, mouse IgG1) and BDCA3 (AD5-14H12, mouse IgG1), respectively. The CD1c/BDCA1+ subtype was recognized as a population positive for anti-BDCA1 (AD5-8E7, mouse IgG2a) and negative for anti-CD19 (HIB19, mouse IgG1) staining. All DC populations were counterstained with phycoerythrin (PE) conjugated mAb: anti-BDCA4 (AD5-17F6, mouse IgG1), anti-HLA-DR [G46-6(L243), mouse IgG2a], anti-CD11c (B-ly6, mouse IgG1), anti-CD40 (5C3, mouse IgG1), anti-CD47 (B6H12, mouse IgG1), anti-CD80 (L307.4, mouse IgG1), anti-CD83 (HB15e, mouse IgG1), anti-CD86 [2331(FUN-1), mouse IgG1], anti-CD123 (9F5, mouse IgG1), anti-CD137L (C65-485, mouse IgG1), anti-CD137 (4B4-1, mouse IgG1), anti-CD209 (DCN46, mouse IgG2b).
Intracellular staining for Foxp3 was performed on freshly isolated and cultured PBMC with PE Anti-Human Foxp3 Staining Set (eBioscience, San Diego, CA, USA) according to manufacturer's protocol. Before fixation the cells were counterstained with FITC-conjugated mAb specific for CD4 (BD Biosciences Pharmingen, San Jose, CA, USA).
Isolation and culture of plasmacytoid DCs
pDCs were isolated from the PBMC suspensions obtained by leucapheresis of 18 multiple sclerosis patients and 12 healthy subjects. PBMCs were isolated by Histopaque 1077 gradient centrifugation (30 min, 300 g, 20°C) (Sigma-Aldrich). 2 × 109 cells were then sorted with BDCA4 isolation kit (Miltenyi Biotec) in the magnetic field of a MidiMACS® sorter (Miltenyi Biotec). Positive fractions routinely contained >95% of BDCA2+CD123+ pDCs. Cells from the pDC-negative fraction were subjected to an additional run on the sorting column in order to remove any residual pDCs and were afterwards used in co-culture experiments (pDC-negative fraction). The content of pDCs in depleted fraction was routinely <0.1%.
Isolated pDCs were plated for 96 h on 48-well culture plates (Nunc, Roskilde, Denmark) at a concentration of 5 × 105/ml in a culture medium containing RPMI 1640, streptomycin 100 μg/ml, penicillin 100 U/ml, 2 mM l-glutamine (Gibco, Life Technologies, Vienna, Austria) and 10% heat-inactivated fetal calf serum (FCS; Boehringer Mannheim, Germany) supplemented with 10 ng/ml of recombinant human interleukin-3 (IL-3) (R&D Systems). For the last 48 h of culture, 0.5 μg/ml of recombinant human soluble CD40 ligand (sCD40L, R&D Systems) was added. Every 24 h a sample of cells was harvested for flow cytometry analysis and culture supernatants were collected for measurement of IFN-alpha and IL-12 production.
Allogeneic proliferation assay
In co-culture experiments, 1 × 105 PBMCs depleted of pDCs that were obtained from multiple sclerosis patients (n = 8) and healthy subjects (n = 7) were cultured on a round bottom 96-well plate with 1 × 105 irradiated (15 Gy) allogeneic PBMCs as stimulators and graded numbers of freshly isolated autologous pDCs (pDCs to pDC-depleted PBMC ratio: 1 : 100, 1 : 20 and 1 : 10). In parallel, identical co-culture experiments were performed in a culture medium supplemented with 10 ng/ml IL-3. All experiments were done in triplicate. In each case, allogeneic stimulators were obtained from the same healthy donor. After 48 h supernatants were collected and 1 μCi of [3H]-thymidine (Amersham, United Kingdom) was added to each well for the next 16 h. At the end of the culture the cellular incorporation of [3H]-thymidine was determined. The outcome of co-culture proliferation assay was counted as a stimulation index (SI). Additionally, the secretion of IFN-gamma and IL-2 was measured in culture supernatants by enzyme-linked immunosorbent assay (ELISA).
CpG ODN stimulation
2 × 106 PBMC/ml from multiple sclerosis patients and healthy controls were cultured on a round bottom 96-well culture plate for 72 h in culture medium (see pDC culture, without IL-3 or sCD40L) or culture medium additionally supplemented with 0.5 or 1 μM of type A unmethylated CpG ODN (CpG 2216, 20mer; TIB MOLBIOL, Berlin, Germany) as a stimulator of TLR-9. Every 24 h culture supernatants were collected for measurement of IFN-alpha secretion by ELISA.
CD4+Foxp3+ cell analysis in culture
1 × 106 PBMC/ml from multiple sclerosis patients and healthy controls were cultured on a round bottom 96-well culture plate for 6 days in culture medium supplemented with 10 μg/ml human Myelin Basic Protein (MBP) (Chemicon, CA, USA). In parallel experiments, prior to culture, PBMC were depleted of pDCs with BDCA4+-specific magnetic beads. The content of pDCs in the depleted fraction was routinely <0.1%. At the end of the culture, cells were harvested and the percentage of Foxp3+CD4+ cells was assessed by flow cytometry.
Cytokine secretion
Cell culture supernatants were collected, aliquoted and stored at −20°C. Immediately before the measurement, aliquots were brought to room temperature and analysed for cytokines content (IFN-alpha, IL-12, IL-2, IFN-gamma) using sandwich ELISA kits (human IFN-alpha, Bender MedSystems; human IL-2, human IL-12 p70, BD Bioscience; human IFN-gamma, R&D Systems) according to manufacturers' protocols.
Statistical analysis
The statistical analyses were performed using STATGRAPHICS PLUS v. 5.0 software. All comparisons of group data were done with the non-parametric Mann–Whitney test, with P = 0.05 as the level of significance.
Results
The frequency of the blood DC subtypes in multiple sclerosis does not differ from healthy subjects
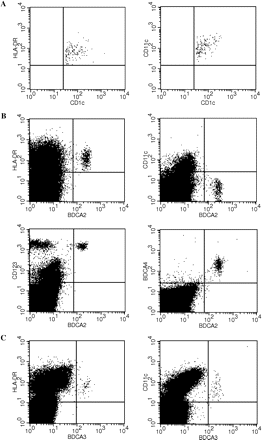
All BDCA1+CD19− and all BDCA3+ cells expressed CD11c and HLA-DR, whereas BDCA2+ cells were negative for CD11c and positive for CD123, HLA-DR and BDCA-4. This observation confirms the specificity of BDCAs for blood DC subtypes (Fig. 1).
Representative flow cytometry analysis of blood DC subpopulations. Multicolour staining for mDC subtypes and for pDCs is shown. (A) PBMCs were gated on CD19-negative and CD1c/BDCA1-positive cells to eliminate B cells. mDCs were identified on the basis of CD1c/BDCA1 expression. These cells were HLA-DR-positive (left) and CD11c-positive (right). (B) PBMCs from the same donor: pDCs were recognized as the population positive for BDCA2 staining. These cells were positive for HLA-DR (upper left), CD123 (lower left), BDCA4 (lower right) and negative for CD11c (upper right). (C) PBMCs from the same donor: mDCs were identified on the basis of high BDCA3 expression. These cells were positive for HLA-DR (left) and CD11c (right).
There was no significant difference in the frequency of DC subsets in the blood between clinically stable multiple sclerosis patients (n = 35) and control subjects (n = 30), and the DC level remained <1% of total PBMC population. Also the ratios between DC subsets were not significantly different between multiple sclerosis and controls (data not shown).
Blood pDCs in multiple sclerosis present an immature expression profile of co-stimulatory molecules
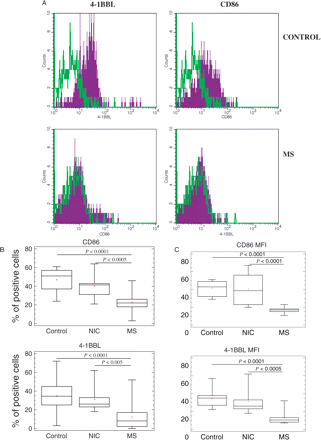
To assess the maturational state of DC subsets in the blood of multiple sclerosis patients and controls, we performed phenotypic analyses evaluating expression of several co-stimulatory molecules. CD40, CD80, CD83 were all expressed at negligible levels and there was no difference between multiple sclerosis and controls (data not shown). In contrast, the expression of CD86 and 4-1BBL was significantly lower on multiple sclerosis-derived BDCA2+ pDCs than in healthy controls (22 versus 47%, P < 0.0001 and 12 versus 35%, P < 0.0001, respectively) and patients with NIC (22 versus 41%, P < 0.0005 and 12 versus 32%, P < 0.005, respectively) (Fig. 2). As a control experiment, we assessed the expression of CD86 and 4-1BBL on the two mDC subsets—CD1c/BDCA1+ and BDCA3+—and found a similar expression pattern in multiple sclerosis and healthy subjects (data not shown).
Phenotypic differences of peripheral blood pDCs in untreated stable multiple sclerosis patients. PBMC freshly isolated from untreated multiple sclerosis patients, patients with NIC and healthy controls were stained and assessed by tri-colour FACS as described in Material and methods. pDCs were recognized on the basis of BDCA2 staining and forward–side scatter characteristic. (A) Histogram plots of FACS analysis of representative healthy control and multiple sclerosis patient. The staining with mAb specific for CD86 or 4-1BBL is demonstrated in purple histograms. Green histograms represent appropriate isotype controls. Because of the low frequency of pDCs within PBMC (although 2 × 105 PBMC were subjected to FACS analysis) the number of positive counts on histograms was relatively low (ca. 1 × 103 per histogram). (B) Box and whisker plots show the average percentage and (C) mean fluorescence intensity (MFI) of pDCs positive for CD86 and 4-1BBL staining in healthy control, NIC and multiple sclerosis group, respectively. The horizontal line represents the median value. Statistically significant differences are indicated (Mann–Whitney test).
These results suggest that the pDCs isolated freshly from the blood of multiple sclerosis patients have an immature profile of co-stimulatory molecule expression when compared with pDCs in healthy controls and patients with NIC; this differential profile was not observed in mDCs.
Blood pDCs in multiple sclerosis show impaired maturation in vitro
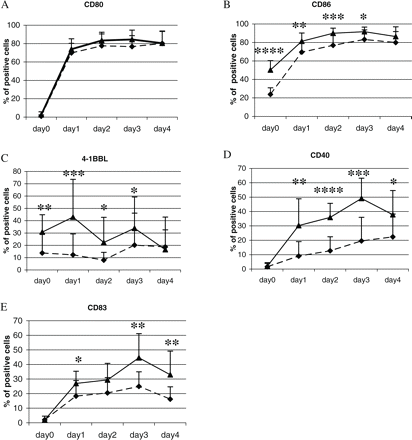
To assess whether the observed phenotypic abnormalities of multiple sclerosis-derived pDCs are meaningful and impose an altered functional pattern, we isolated peripheral blood pDCs to perform a series of in vitro experiments. In order to obtain sufficient numbers of pDCs for culture assays, we had to use cells isolated during leucapheresis. pDCs were cultured in the presence of IL-3 and sCD40L, cytokines which are widely used as maturation and activation factors for pDCs in culture (Grouard et al., 1997; Ito et al., 2001), for 96 h. Every 24 h, pDC expression of co-stimulatory molecules was evaluated by flow cytometry. These experiments showed that already after 24 h of culture the upregulation of CD86 was significantly lower in pDCs from multiple sclerosis patients and reached the level of the control group only after 96 h (Fig. 3B), in contrast to CD80 (Fig. 3A). Similarly, the expression of 4-1BBL remained significantly lower on multiple sclerosis-derived pDCs until the fourth day of culture (Fig. 3C). More importantly, in multiple sclerosis the upregulation of CD40 was impaired. The expression of this molecule, which is indispensable for DC maturation and activation, was significantly lower in multiple sclerosis during the whole culture period (Fig. 3D). The maturation defect of pDCs in multiple sclerosis was further confirmed by significantly lower upregulation of CD83, another molecule considered to be an important DC maturation marker (Fig. 3E). Taken together, these results demonstrate an impaired maturational process of pDCs in multiple sclerosis in response to IL-3 and CD40L.
Maturation of pDCs in vitro. pDCs were isolated from peripheral blood of untreated stable multiple sclerosis patients and healthy controls and cultured for 96 h with rhIL-3 (10 ng/ml). For the last 48 h soluble rhCD40L (0.5 μg/ml) was added to the culture medium. Every 24 h starting from the beginning of culture, a sample of cells was harvested and assessed by FACS. The graphs present changes in the expression of CD80 (A), CD86 (B), 4-1BBL (C), CD40 (D) and CD83 (E) on pDCs isolated from healthy controls (n = 12, closed triangles with solid lines) and multiple sclerosis patients (n = 18, closed diamonds with dashed lines). The mean percentages ± SD of pDCs positive for each antigen at each time point of culture are shown. Statistically significant differences are indicated (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; Mann–Whitney test).
In parallel to the phenotypic evaluation, we assessed the cytokine profile of pDCs cultured with IL-3 and sCD40L. Every 24 h of culture we collected supernatants and measured the secretion of IFN-alpha and IL-12 by ELISA. Under conditions used in this experiment, pDCs produced low amounts of IFN-alpha and IL-12 p70. In the multiple sclerosis group the secretion of both cytokines showed a wider range, and differences between groups were not statistically significant (data not shown).
Blood pDCs in multiple sclerosis show impaired allogeneic stimulation
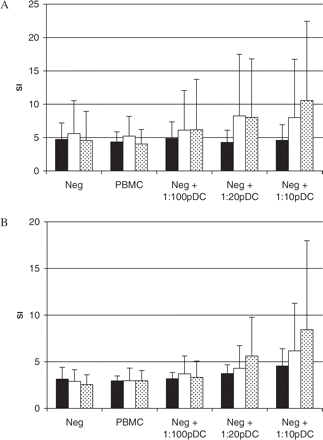
In order to assess pDC function in multiple sclerosis patients, we investigated the ability of pDCs to regulate the proliferation and cytokine secretion of autologous PBMCs when stimulated with allogeneic irradiated cells. For this purpose we cultured allogeneic PBMCs as stimulators with autologous PBMCs that had first been deprived of pDCs, and then we gradually supplemented PBMC preparations with increasing numbers of freshly isolated autologous pDCs. We observed that in both multiple sclerosis and controls, PBMCs depleted of pDCs proliferated in response to allogeneic stimulation. As expected, in the healthy control group, addition of pDCs to the co-culture clearly increased the proliferation of autologous PBMC in a dose-dependent manner (Fig. 4A). In striking contrast, in multiple sclerosis patients addition of pDCs to the allogeneic co-culture system had no effect on autologous PBMC proliferation even when pDCs were used in high numbers (Fig. 4A). Interestingly, when the medium in the co-culture system was supplemented with IL-3, the effect of pDCs on the autologous PBMC proliferation was slightly increased also in multiple sclerosis patients, although still at a much lower level than in healthy controls (Fig. 4B). These results indicate that the observed lower stimulatory activity of pDCs derived from multiple sclerosis patients is not related to an inappropriate IL-3 concentration but rather represents an intrinsic property of pDCs from multiple sclerosis patients.
Immunoregulatory effect of pDCs in allogeneic co-culture experiments. 105 PBMCs depleted of pDCs (pDC negative fraction = Neg) obtained from untreated multiple sclerosis patients (n = 8), GA-treated multiple sclerosis patients (n = 6) and healthy subjects (n = 7) were cultured with 105 irradiated allogeneic PBMC as stimulators and graded numbers of freshly isolated autologous pDCs for 64 h in culture medium (A) or in culture medium supplemented with rhIL-3 (10 ng/ml) (B). All results of co-culture proliferation assays were given as an SI ± SD (untreated multiple sclerosis patients = black bars, GA-treated multiple sclerosis patients = empty bars, healthy controls = dotted bars).
To confirm that the observed impairment of DC in allogeneic stimulation assays in multiple sclerosis is specific for pDCs rather than mDCs, we also added autologous mDCs to parallel allogeneic co-culture experiments. In contrast to the experiments with pDCs, the effects of mDCs on the allogeneic reaction were similar for both multiple sclerosis and control mDC (not shown).
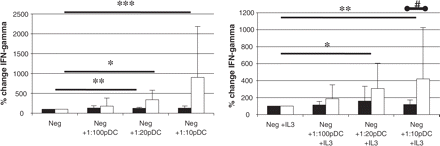
Supernatants collected in allogeneic co-culture experiments were used for the analysis of cytokine production. We found that in healthy subjects, pDCs induced a significantly higher increase in IFN-gamma production than in multiple sclerosis patients when added to the co-culture system (Fig. 5 left). Addition of IL-3 yielded comparable findings (Fig. 5 right). The IFN-gamma production was directly proportional to the SI of PBMC, as shown in Fig. 4. For IL2-secretion, addition of pDCs induced an increase in both multiple sclerosis and healthy controls, but there was only a trend towards higher IL-2 secretion in controls (not shown).
The effect of pDCs on cytokine secretion in allogeneic co-culture. Co-culture experiments were performed as described in Fig. 4. After 48 h co-culture supernatants were collected and the IFN-gamma content was measured by ELISA. Graphs present the percentage change of mean cytokine secretion ± SD (multiple sclerosis patients = black bars, healthy controls = empty bars) after addition of increasing numbers of pDC to co-culture. Please note significant increase in IFN-gamma secretion in control but not in multiple sclerosis over 2 days of pDCs in co-culture (*P < 0.05, **P < 0.01, ***P < 0.001; Mann–Whitney test) and significantly higher IFN-gamma secretion by control cells in IL-3-supplemented co-culture compared with multiple sclerosis (#P = 0.03; Mann–Whitney test).
pDCs from multiple sclerosis patients show impaired IFN-alpha secretion in response to CpG ODN stimulation
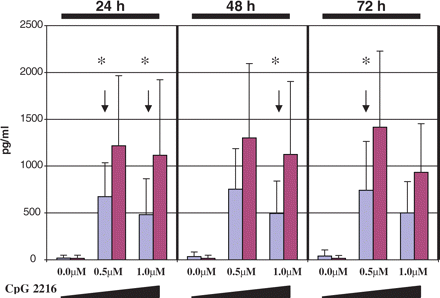
To assess the functional properties of pDCs with regard to their role in innate immunity we performed a separate set of experiments with CpG ODN mimicking microbial stimulation of TLR-9. We used CpG 2216, a type A CpG ODN that is known to selectively activate pDCs, which in turn do vigorously secrete IFN-alpha (Rothenfusser et al., 2002). Since the secretory response of pDCs to CpG 2216 stimulation is unique among human PBMCs, we have compared IFN-alpha production after CpG 2216 stimulation in the culture of PBMCs obtained from multiple sclerosis patients and control subjects. PBMCs were exposed to CpG 2216 at the concentration of 0.5 and 1 μM. Beginning from Day 1 post-exposure and persisting throughout 3 days of the culture period, IFN-alpha secretion by PBMCs in healthy controls was, on the average, twice as high when compared with multiple sclerosis patients (Fig. 6). There was no significant difference in the relative numbers of pDCs in PBMCs between multiple sclerosis and control PBMC samples used in this set of experiments, suggesting that the overall secretion of IFN-alpha in our PBMCs cultures reflects secretion by pDCs. We also assessed by FACS the expression of TLR-9 on PBMCs subjected to CpG stimulation. Intracellular staining showed no correlation between TLR-9 expression and IFN-alpha production in multiple sclerosis and control groups (n = 9 and n = 12, respectively) (data not shown).
IFN-alpha production in PBMCs after CpG 2216 stimulation. 2 × 106/ml PBMCs isolated from untreated stable multiple sclerosis patients (n = 9, blue bars) and healthy subjects (n = 12, red bars) were cultured for 72 h in culture medium or in culture medium supplemented with 0.5 or 1.0 μM of CpG 2216. Every 24 h culture supernatants were collected and IFN-alpha content was measured by ELISA. Graphs represent the mean IFN-alpha secretion ± SD for increasing doses of CpG 2216: 0.0, 0.5 and 1.0 μM, measured over three consecutive days. Note significantly higher IFN-alpha secretion by control PBMCs (containing pDCs) as compared with multiple sclerosis (*P < 0.05; Mann–Whitney test).
pDCs from multiple sclerosis patients lose their immunoregulatory function
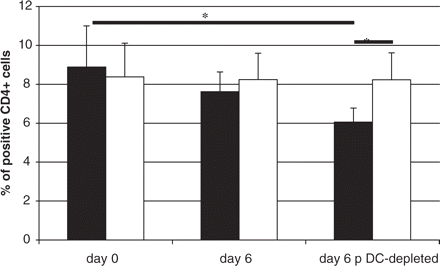
To assess the potential influence of pDCs on an immunoregulatory cell population (Treg cells) we measured CD4+Foxp3+ cells cultured in the presence or absence of pDCs in a medium supplemented with human MBP. In healthy control subjects, depletion of pDCs led to a decrease in the number of CD4+Foxp3+ Treg cells (Fig. 7), whereas in multiple sclerosis patients depletion of pDCs did not affect the number of CD4+Foxp3+ Treg cells. These results suggest the loss of regulatory function of pDCs in multiple sclerosis patients.
Depletion of pDCs has no effect on the number of CD4+Foxp3+ cells in multiple sclerosis patients. PBMC were assessed for CD4+Foxp3+ cells content ex vivo (Day 0) and then after 6 days in culture with MBP (10 mg/ml). In parallel experiments pDCs were depleted from PBMC as described in Material and methods. CD4+Foxp3+ cells were defined with double staining with anti-CD4 mAb and anti-Foxp3 mAb and by flow cytometry. The black and empty bars represent the average number ± SD of CD4+Foxp3+ cells from several experiments in healthy subjects (n = 7) and multiple sclerosis patients (n = 7), respectively. The number of CD4+Foxp3+ cells was significantly reduced (P < 0.05) after pDCs depletion in healthy controls but not in multiple sclerosis patients (*P < 0.05; Mann–Whitney test).
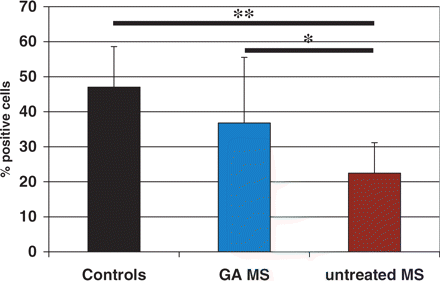
GA partially restores phenotype and function of pDC in multiple sclerosis
To address the functional significance of the observed disturbances in pDCs in multiple sclerosis patients, we performed a parallel set of experiments with pDCs obtained from multiple sclerosis patients treated with GA, an immunomodulatory compound approved for therapy in multiple sclerosis. All these patients had shown a positive clinical response to GA as measured by reduced relapse rate and slower disease progression. As shown in Fig. 8, the expression of CD86 on pDC derived from patients treated with GA was higher than in untreated multiple sclerosis patients (P < 0.02). The difference between GA-treated multiple sclerosis patients and healthy controls was not significant. In allogeneic stimulation in the presence of pDCs isolated from patients treated with GA, the proliferative response of PBMCs was partially restored as compared with untreated multiple sclerosis patients (empty bars in Fig. 4). These results indicate that the observed dysfunction of pDCs can be ameliorated with the improvement of the clinical course of the disease and indirectly support the concept of a regulatory role of pDCs in multiple sclerosis.
Expression of CD86 on pDCs from multiple sclerosis patients treated with GA. PBMC freshly isolated from untreated multiple sclerosis patients (n = 35), multiple sclerosis patients treated with GA (n = 9) and healthy controls (n = 30) were stained for CD86 and assessed by FACS as described in Material and methods. Bars represent mean percentage ± SD of pDCs positive for CD86 staining (*P < 0.02, **P < 0.0001; Mann–Whitney test).
Discussion
The main finding of this study is the demonstration of a maturational defect and altered immunoregulatory function of pDCs in multiple sclerosis patients as demonstrated by an impaired response to CpG ODN, allogeneic stimulation and generation of CD4+Foxp3+ Treg cells.
In our experiments we first analysed the phenotype of peripheral blood DCs in clinically stable, untreated RR multiple sclerosis patients, healthy subjects and patients with NIC and found an abnormally low CD86 and 4-1BBL expression in freshly isolated pDCs in multiple sclerosis. CD86 and 4-1BBL belong to the most important co-stimulatory molecules of DCs, and their activities seem to be mutually supportive (Cannons et al., 2001; Diehl et al., 2002). The low expression of these markers in multiple sclerosis might render the pDCs less efficient in mounting an immune response. While for pDCs there is no previous account in multiple sclerosis, it was recently reported for monocyte-derived (mo) DCs that the expression of CD86 is low in multiple sclerosis patients (Huang et al., 2001). These moDCs form a different DC population after several days of differentiation and maturation in culture, and, importantly, the phenotypic and functional properties of moDCs are strongly dependent on the culture conditions (Chang et al., 2000; Duperrier et al., 2000; Pietschmann et al., 2000). In general, moDCs represent cells of myeloid lineage but are different from blood mDC, which were used in our study (Pickl et al., 1996; Gieseler et al., 1998). This most probably explains why in our experiments we did not observe any phenotypic differences in mDCs between multiple sclerosis and healthy subjects. Until recently, there were very scarce data available regarding the expression of 4-1BBL on human DCs (Summers et al., 2001; Kim et al., 2002). Although the direct role of 4-1BBL in the differentiation of T-cell response is not fully understood (Chu et al., 1997; Cannons et al., 2001; Ye et al., 2002), it has been shown in an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE) induced with myelin oligodendrocyte protein (MOG), that 4-1BBL co-stimulation was beneficial for the course of the disease (Sun et al., 2002). The administration of a specific agonistic antibody to 4-1BB on the day of immunization caused enhanced apoptosis of encephalitogenic T cells resulting in disease amelioration. These results suggested a potential contribution of 4-1BBL to peripheral immune regulation in EAE.
In order to assess the potential functional implication of the phenotypic differences observed in this study, we isolated peripheral blood pDCs from large volumes of leucapheresis samples in multiple sclerosis patients and healthy subjects and investigated pDC maturation in vitro. Low CD86 and 4-1BBL expression in freshly isolated pDCs was followed by impaired upregulation of both these molecules in culture in response to IL-3 and sCD40L. Thus, in multiple sclerosis the defective co-stimulatory phenotype of pDCs seems to persist during their maturation. CD86 is considered a key co-stimulatory molecule at the most early phase of an immune reaction (Chambers, 2001). This is partly due to the kinetics of CD86 expression. The molecule is expressed at low levels even on unstimulated cells, and upon stimulation the level of expression increases rapidly. To the contrary, 4-1BBL has been shown to regulate development and persistence of the response (Bertram et al., 2002). Moreover, it has been shown that B7 molecules CD80 and CD86 transmit reverse signals to DC and in this way modulate their immune function to be more tolerogenic (Munn et al., 2004). Thus, the abnormal expression of both molecules on pDCs suggests that these cells may result in immune dysregulation upon interaction with other immune cells. Additionally, we observed in multiple sclerosis that, during maturation, pDCs failed to efficiently express CD40, a molecule of crucial relevance for DC-T cell interaction. It has been demonstrated recently that in patients with CD40 deficiency, the phenotype and immunological functions of DCs were strongly impaired although there were no differences in blood DC number (Fontana et al., 2003). The ineffective maturation process of pDCs in multiple sclerosis was further confirmed in this study by our finding of a lower and delayed expression of another DC maturation marker CD83.
pDCs provide a link between the innate and adaptive immunity (Kadowaki et al., 2000). As an example we have chosen the TLR-9 pathway of the innate immune system and assessed pDC dysfunction after stimulation with CpG ODN, a potent inducer of IFN-alpha secretion by pDCs (Vollmer et al., 2004). In humans, the expression of TLR-9 is restricted to B cells and pDCs. pDCs are characterized by the exclusive ability to rapidly synthesize large amounts of IFN-alpha in response to CpG ODN (Rothenfusser et al., 2002). As we show here, in multiple sclerosis pDCs have a significantly lower capacity to secrete IFN-alpha upon stimulation with CpG ODN, which corroborates our findings of functional impairment of these cells in multiple sclerosis. It has been suggested that the instantaneous/immediate secretion of IFN-alpha by pDCs shapes the immune response by influencing several cell types with regulatory properties including mDCs, NK cells, and gamma-delta T cells (Biron, 1998; Rothenfusser et al., 2001; Hornung et al., 2002). In turn, these cells and their products control at least partially the maturation process of pDCs and thus influence the pDC function in adoptive immunity (Kadowaki and Liu, 2002). We suggest that the lower capacity of multiple sclerosis-derived pDCs to produce IFN-alpha as observed here may indicate an impairment interfering with the normal immunoregulatory circuits. Similar dysfunction of pDCs was detected in neonatal pDCs, which displayed decreased upregulation of CD80, CD83 and CD86 and were intrinsically deficient in CpG ODN-induced IFN-alpha production at birth (De Wit et al., 2004).
The functional consequences of the observed pDC's maturational deficits in multiple sclerosis were assessed also in allogeneic co-culture experiments. PBMCs mixed with allogeneic target cells are known to respond by vigorous proliferation (McLellan et al., 1996). This response depends on the appropriate function of mDCs and pDCs (Tourkova et al., 2001; Klangsinsirikul et al., 2002) and particularly on their interaction with effector cells via the CD40-CD40L pathway (McLellan et al., 1996; Sun et al., 2003). We have shown here that the ability of the pDCs to induce autologous PBMC proliferation in response to allogeneic stimulation is lesser in multiple sclerosis compared with that in healthy subjects. In these experiments pDCs isolated from multiple sclerosis patients also failed to induce secretion of IFN-gamma by PBMCs. The observed functional impairment as seen with pDCs from multiple sclerosis patients was specific for pDCs, since blood mDCs used in parallel experiments showed similar potency to induce allogeneic stimulation both in multiple sclerosis and healthy subjects. It was shown that pDCs exposed to CpG ODN stimulated the development of CD4+CD25+ regulatory T cells from allogeneic naïve CD4+ T cells (Mossman et al., 2004; Sakaguchi, 2005). Also naïve CD8+ T cells have been demonstrated to acquire a regulatory function when stimulated with pDCs (Gilliet and Liu, 2002). Taking into consideration the nature of the DC-T cell interaction, the low level of co-stimulatory molecules expression, as shown in this study, may potentially influence the ability of pDCs in multiple sclerosis to interact with the immune system, and in particular to generate regulatory cells. In line with this hypothesis we have demonstrated that depletion of pDCs resulted in a decreased level of CD4+Foxp3+ regulatory cells in healthy controls but not in multiple sclerosis patients. These results correspond well with the findings that the Treg cells defined as CD4+CD25hi show dysfunction in multiple sclerosis patients (Viglietta et al., 2004; Huan et al., 2005).
In an additional attempt to link the observed dysfunction of pDCs with the generally accepted clinical efficacy of an approved immunomodulatory treatment for multiple sclerosis, we performed pDC phenotyping and functional assays in patients treated with GA. Indeed, in these patients pDC phenotype and the ability to induce allogeneic reaction were partially restored. We conclude that the GA-induced amelioration of clinical disease activity in multiple sclerosis correlated, at least partially, with improved properties of pDCs, suggesting a potential role of these cells in immune mechanisms of multiple sclerosis.
In summary, we provide evidence that pDCs isolated ex vivo from the blood of multiple sclerosis patients demonstrate both phenotypic and functional abnormalities. Although to date no single immune feature has been shown to be pivotal for the onset of multiple sclerosis or for defined disease characteristic in established multiple sclerosis, the dysfunction of pDCs at several levels described here may importantly contribute to the impairment of the immunoregulatory circuit in multiple sclerosis.
*Both authors contributed equally to the work.
Both senior authors contributed equally to the work.
This work was supported by the Polish-German Cooperation Grant in Neuroscience (PBZ-MIN-001/P05/26), a bi-national grant from the Bundesministerium für Bildung und Forschung, Germany (01GZ0303); the Centre of Excellence MolMed; the University Research Fund at the University of Würzburg; a visiting scientist scholarship to M.S. from the SFB 581 and a restricted research grant by Aventis Germany to K.S. and R.G. at Würzburg. We wish to thank Prof. H. Wagner, Institute of Medical Microbiology, Immunology and Hygiene, Munich, Germany, for very helpful discussions on DC and TLR biology and for a gift of CpG ODN; Prof. H. Wiendl for helpful discussion on the manuscript; Dr A. Opitz, Department for Transfusion Medicine of the University of Würzburg, Germany, and Dr E. Gauer, Blood Donation Center, Lodz, Poland, for performing leucaphereses. We thank our patients and the voluntary healthy control subjects for agreeing to participate in our study. The excellent technical assistance by B. Conrad and A. Schmitt is gratefully acknowledged.
References
Bertram EM, Peggy L, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following unmethylated cytosine-phosphate-guanosine oligodeoxynucleotides influenza infection.
Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2.
Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections.
Bleharski JR, Niazi KR, Sieling PA, Cheng G, Modlin RL. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines.
Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy.
Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization.
Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon.
Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation.
Chambers CA. The expanding world of co-stimulation: the two-signal model revisited.
Chang CC, Wright A, Punnonen J. Monocyte-derived CD1a+ and CD1a- dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation.
Chu NR, DeBenedette MA, Stiernholm BJ, Barber BH, Watts TH. Role of IL-12 and 4-1BB ligand in cytokine production by CD28+ and CD28- T cells.
De Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals.
De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, et al. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns.
Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells.
Diehl L, den Boer AT, van der Voort E, Fransen M, van Bostelen L, Krimpenfort P, et al. In vivo targeting through 4-1BB enables Th-independent priming of CTL in the presence of an intact CD28 costimulatory pathway.
Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Bardin C, Jacquet C, Yoneda K, et al. Distinct subsets of dendritic cells resembling dermal DCs can be generated in vitro from monocytes, in the presence of different serum supplements.
Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood.
Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, et al. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions.
Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction.
Fontana S, Moratto D, Mangal S, De Francesco M, Vermi W, Ferrari S, et al. Functional defects of dendritic cells in patients with CD40 deficiency.
Gieseler R, Heise D, Soruri A, Schwartz P, Peters JH. In-vitro differentiation of mature dendritic cells from human blood monocytes.
Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells.
Gramaglia I, Cooper D, Miner KT, Kwon BS, Croft M. Co-stimulation of antigen-specific CD4 T cells by 4-1BB ligand.
Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand.
Haahr S, Plesner AM, Vestergaard BF, Hollsberg P. A role of late Epstein-Barr virus infection in multiple sclerosis.
Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, et al. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2.
Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides.
Hsu TL, Chang YC, Chen SJ, Liu YJ, Chiu AW, Chio CC, et al. Modulation of dendritic cell differentiation and maturation by decoy receptor 3.
Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK, et al. Decreased FOXP3 levels in multiple sclerosis patients.
Huang YM, Stoyanova N, Jin YP, Teleshova N, Hussien Y, Xiao BG, et al. Altered phenotype and function of blood dendritic cells in multiple sclerosis are modulated by IFN-α and IL10.
Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs.
Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway.
Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity.
Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity.
Kim YJ, Kim SH, Mantel P, Kwon BS. Human 4-1BB regulates CD28 co-stimulation to promote Th1 cell responses.
Kim YJ, Li G, Broxmeyer HE. 4-1BB ligand stimulation enhances myeloid dendritic cell maturation from human umbilical cord blood CD34(+) progenitor cells.
Klangsinsirikul P, Russell NH. Peripheral blood stem cell harvests from G-CSF-stimulated donors contain a skewed Th2 CD4 phenotype and a predominance of type 2 dendritic cells.
Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease.
Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases.
McLellan AD, Sorg RV, Williams LA, Hart DN. Human dendritic cells activate T lymphocytes via a CD40 : CD40 ligand-dependent pathway.
Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells.
Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3–dioxygenase activity in dendritic cells.
Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid.
Pickl WF, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes.
Pietschmann P, Stockl J, Draxler S, Majdic O, Knapp W. Functional and phenotypic characteristics of dendritic cells generated in human plasma supplemented medium.
Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, et al. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo.
Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, et al. Reciprocal control of T helper cell and dendritic cell differentiation.
Rothenfusser S, Hornung V, Krug A, Towarowski A, Krieg AM, Endres S, et al. Distinct CpG oligonucleotide sequences activate human gamma delta T cells via interferon-alpha/beta.
Rothenfusser S, Tuma E, Endres S, Hartmann G. Plasmacytoid dendritic cells: the key to CpG.
Rotola A, Merlotti I, Caniatti L, Caselli E, Granieri E, Tola MR, et al. Human herpesvirus 6 infects the central nervous system of multiple sclerosis patients in the early stages of the disease.
Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self.
Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood.
Sriram S, Stratton CW, Yao S, Tharp A, Ding L, Bannan JD, et al. Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis.
Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system.
Summers KL, Hock BD, McKenzie JL, Hart DN. Phenotypic characterization of five dendritic cell subsets in human tonsils.
Sun W, Wang Q, Zhang L, Liu Y, Zhang M, Wang C, et al. Blockade of CD40 pathway enhances the induction of immune tolerance by immature dendritic cells genetically modified to express cytotoxic T lymphocyte antigen 4 immunoglobulin.
Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis.
Tourkova IL, Yurkovetsky ZR, Shurin MR, Shurin GV. Mechanisms of dendritic cell-induced T cell proliferation in the primary MLR assay.
Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis.
Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities.
Author notes
1Department of Neurology, Medical University of Lodz, Lodz, Poland, 2Department of Neurology, University of Würzburg, Würzburg and 3Institute for MS Research, Medical Faculty of the University and Gemeinnuetzige Hertie-Stiftung, Goettingen, 4Department of Neurology, Klinikum Augsburg, Germany