-
PDF
- Split View
-
Views
-
Cite
Cite
Eun Yeon Joo, Seung Bong Hong, Hyun Jung Han, Woo Suk Tae, Jee Hyun Kim, Sun Jung Han, Dae Won Seo, Kyung-Han Lee, Seung-Chyul Hong, Munhyang Lee, Seunghwan Kim, Byung Tae Kim, Postoperative alteration of cerebral glucose metabolism in mesial temporal lobe epilepsy, Brain, Volume 128, Issue 8, August 2005, Pages 1802–1810, https://doi.org/10.1093/brain/awh534
Close - Share Icon Share
Abstract
To investigate postoperative changes in the cerebral glucose metabolism of patients with mesial temporal lobe epilepsy (MTLE), statistical parametric mapping (SPM) analysis was performed on pre- and postoperative 18F-fluorodeoxyglucose PET (FDG-PET) images. We included 28 patients with MTLE who had undergone surgery and had been seizure-free postoperatively (16 had left MTLE and 12 right MTLE). All patients showed hippocampal sclerosis by pathology or brain MRI. FDG-PET images of the 12 right temporal lobe epilepsy patients were reversed to lateralize the epileptogenic zone to the left side in all patients. Application of the paired t-test in SPM to pre- and postoperative FDG-PETs showed that postoperative glucose metabolism decreased in the caudate nucleus, the pulvinar of the thalamus, fusiform gyrus, lingual gyrus and the posterior region of the insular cortex in the hemisphere ipsilateral to resection, whereas postoperative glucose metabolism increased in the anterior region of the insular cortex, temporal stem white matter, midbrain, inferior precentral gyrus, anterior cingulate gyrus and supramarginal gyrus in the hemisphere ipsilateral to resection. No significant postsurgical changes in cerebral glucose metabolism occurred in the contralateral hemisphere. Subtraction between pre- and postoperative FDG-PET images in individual patients produced similar findings to the SPM results, and additionally showed that postoperative glucose metabolism increased in the anterior thalamus in 12/28 patients (42.8%). SISCOM (subtraction ictal–interictal SPECT co-registered to MRI) performed in 17 patients showed ictal hyperperfusion in the ipsilateral temporal lobe, including the temporal stem white matter, midbrain, insular cortex and cingulate gyrus, bilateral basal ganglia and thalami, and multiple small regions in the frontoparietal lobes during seizures. This study suggests that brain regions showing a postoperative increase in glucose metabolism appear to represent the propagation pathways of ictal and interictal epileptic discharges in MTLE, whereas the postoperative decrease in glucose metabolism may be related to a permanent loss of afferents from resected anterior–mesial temporal structures.
Introduction
The distribution of hypometabolism by preoperative 18F-fluorodeoxyglucose PET (FDG-PET) in mesial temporal lobe epilepsy (MTLE) usually includes the ipsilateral mesial temporal region and frequently extends beyond the epileptic focus (Theodore et al., 1992; Hong et al., 1999). The mechanism of hypometabolism in brain regions (temporal or extratemporal) beyond the epileptic focus has not been elucidated. Postoperative study of cerebral glucose metabolism may be helpful in understanding this phenomenon. Patients with mesiobasal limbic temporal lobe epilepsy (TLE) showed increased glucose metabolism in both ipsilateral and contralateral hemispheres postoperatively, but reduced metabolism in the contralateral mesiobasal temporal lobe, which supports the presence of strong interhemispheric connections between right and left mesial temporal structures (Hajek et al., 1994). Moreover, a FDG-PET study using the region-of-interest (ROI) method demonstrated a postoperative increase in preoperatively reduced glucose metabolism in the inferior frontal and thalamus ipsilateral to the epileptic focus in TLE patients, which suggests a reversible effect of seizures on brain areas connected with, but remote from, the epileptogenic cortex (Spanaki et al., 2000). In patients with selective amygdalohippocampectomy, an ROI-based comparison of pre- and postoperative FDG-PET showed significant postoperative worsening of the hypometabolism in the ipsilateral temporal pole, but a postoperative increase in the metabolic activity in the contralateral anterior hippocampus and the orbitofrontal cortex bilaterally (Dupont et al., 2001). Inconsistencies among previous results are probably due to differences in methods and patient selection, as the majority of FDG-PET studies on postoperative changes in cerebral metabolism have used different ROI methods (Hajek et al., 1994; Newberg et al., 2000; Spanaki et al., 2000; Dupont et al., 2001). The ROI technique has advantages in terms of comparing the glucose metabolisms of homologous regions with those of the contralateral hemisphere or with the same regions in normal controls, but the shapes and sizes of defined ROIs vary between studies. Because of the different gyral and sulcal morphologies of the right and left hemispheres and individual variability, it is difficult to determine the exact location of homologous regions in the contralateral hemisphere or in normal controls. Moreover, ROIs include only predefined parts of the brain, not the whole brain. Therefore, we compared pre- and postoperative FDG-PET images using statistical parametric mapping (SPM), which is a proven, effective method for the voxel-by-voxel analysis of functional images (Ashburner and Friston, 2000), to investigate postsurgical changes in glucose metabolism throughout the whole brain in MTLE patients. The advantage of SPM is that it provides a fully automated form of neurophysiological imaging analysis throughout the whole brain. Using a variety of statistical approaches, SPM enables the analysis of data derived from groups of patients, and thus allows common metabolic patterns to be identified (Signorini et al., 1999). Additionally, subtraction between post- and preoperative FDG-PET images and SISCOM (subtraction ictal–interictal SPECT co-registered to MRI) were performed to investigate postoperative metabolism changes and ictal perfusion changes in individual patients.
Patients and methods
Patients
We recruited consecutively 28 patients with medically refractory MTLE who had a preoperative FDG-PET and had been seizure-free after anterior temporal lobectomy with amgydalohippocampectomy. All patients underwent a comprehensive presurgical evaluation, including long-term video-EEG monitoring, interictal and ictal single photon emission computed tomography (SPECT), interictal FDG-PET, a neuropsychological test and the Wada test. The location of the epileptic focus was determined by ictal EEG onset zone in conjunction with interictal spikes recorded at the ipsilateral temporal lobe and the presence of hippocampal atrophy or sclerosis on a brain MRI. If scalp ictal EEG was non-lateralized or showed bilateral epileptic origins, bilateral strips or depth electrodes were implanted for lateralizing the epileptic focus by intracranial EEG recording.
All patients met the following inclusion criteria for mesial TLE: (i) a unilateral temporal epileptic focus with typical complex partial seizures of temporal lobe origin confirmed by long-term video-EEG monitoring; (ii) hippocampal sclerosis by pathology or brain MRI; (iii) no extratemporal structural abnormality on brain MRI. The patients consisted of 15 males and 13 females, aged from 15 to 42 years (mean 31 years). Age at epilepsy onset ranged from 1 to 26 years (mean 12 years) and epilepsy duration ranged from 5 to 28 years (mean 15 years).
Study design
All 28 patients had an interictal FDG-PET 2–5 months before surgery. The postoperative FDG-PET was performed 12 months after surgery. All patients had been seizure-free until the postoperative FDG-PET was performed.
Informed consent was obtained from all patients and the institutional review board of our hospital authorized the study protocol, which included the administration of a radioactive substance and PET scanning.
Surgery and pathology
All patients had undergone an anterior temporal lobectomy with amygdalohippocampectomy for the curative treatment of epilepsy. In consideration of brain MRI findings, the Wada test and electrocorticography, the extents of resection of the hippocampus and lateral temporal cortex were determined.
Hippocampal sclerosis was confirmed by pathological examination in 27 of 28 patients. One patient had a few dead neurons in Sommer's sector, although she had a definite hippocampal sclerosis in preoperative MRI [increased signal and smaller size in unilateral hippocampus on fluid-attenuated inversion recovery (FLAIR) and T2-weighted image].
Two of 28 patients had dual pathology (hippocampal sclerosis plus another lesion in the mesial temporal lobe; one left, one right). Other lesions were a small oligodendroglioma in the uncus and a calcification in the parahippocampal gyrus in the ipsilateral hemisphere.
Medication during FDG-PET
To exclude the effect of drug changes on postoperative cerebral glucose metabolism, the combinations of preoperative antiepileptic drugs (AEDs) remained unchanged until the postoperative FDG-PET was done.
Sixteen of the 28 patients (57.1%) were on three AEDs before and after surgery. Thirteen of them had taken carbamazepine and valproic acid with one of the following drugs: vigabatrin, topiramate, phenobarbital or clonazepam. The remaining three patients were on carbamazepine, phenytoin and phenobarbital. Only two patients in the group that was taking three AEDs reduced the dose of carbamazepine slightly (from 1000 to 800 mg/day and from 1200 to 1000 mg/day, respectively).
Twelve patients (42.9%, 12/28) were on two AEDs. They had taken carbamazepine with one of valproic acid, phenytoin or clonazepam.
18F-FDG-PET imaging procedure
Preoperative PET images were obtained using a GE Advance PET scanner (GE Medical Systems, Milwaukee, WI, USA). Patients fasted for ≥4 h and were then given an intravenous injection of 260–370 MBq (7–10 mCi) FDG. EEG monitoring during the FDG uptake period (0 to 30 min after injection) demonstrated no EEG seizure activity and confirmed wakefulness in each subject. PET images were reconstructed using a Hanning filter (cut-off frequency = 4.5 mm) and displayed as a 128 × 128 matrix (pixel size = 1.95 × 1.95 mm with 35 slices of thickness 4.25 mm). Attenuation correction was performed using a standard calculation method using a series of ellipses. All studies were conducted in a quiet, dimly lit environment with minimal background noise. The subjects were studied in an awake, resting state with eyes closed and ears unplugged. Postoperative FDG-PET images were acquired 12 months after surgery using the same scanner and an identical protocol.
SPM analysis of 18F-FDG-PET images
Pre- and postoperative FDG-PET images were manipulated using MATLAB 5.2 (Mathworks) incorporated into SPM-99 (Wellcome Department of Cognitive Neurology, Institute of Neurology, University of London, UK) software. As patients had either left (n = 16) or right (n = 12) MTLE, brain FDG-PET images of the 12 right-TLE patients were reversed to lateralize the epileptogenic zone to the left side in all patients to increase the statistical value of the group analysis. Before the spatial normalization of pre- and postoperative FDG-PET images to a standard FDG-PET template, the postoperative FDG-PET image was linearly transformed to match the preoperative FDG-PET image. Through this linear registration, the postoperative FDG-PET could be registered to the preoperative FDG-PET correctly without the influence of postoperative tissue loss. Thereafter, the preoperative FDG-PET image was normalized to the standard FDG-PET template provided in SPM-99, using a 12-parameter affine and a non-linear transformation, and then the transformation matrix of the preoperative FDG-PET was adjusted to the postoperative FDG-PET of the same subject. The accuracy of the spatial normalization between postoperative FDG-PET and the standard PET template was checked using a cross-registration function. Spatially normalized images were then smoothed by convolution by using an isotopic Gaussian kernel with a 14 mm full width at half maximum to increase the signal-to-noise ratio. After spatial and global count normalization with proportional scaling, a paired t-test was used to perform group comparisons on pre- and postoperative PET images. The significance level was set to a familywise error corrected at P < 0.05 (Perneger, 1998), and the extended threshold was set to KE > 50. The results were displayed on the 2D planes of a normal subject's MRI template after spatial normalization.
Subtraction analysis between pre- and postoperative FDG-PET and SISCOM in individual patients
Subtraction between pre- and postoperative FDG-PET images was performed to map the postoperative changes in cerebral glucose metabolism in individual patients and to compare them with the results of SPM analysis.
SISCOM was performed in 17 patients who had both interictal and ictal SPECTs in order to map brain regions showing ictal hyperperfusion during TLE seizures. Brain SPECT scanning was performed 30–60 min after the injection of 25 mCi 99mTc-ethylcysteinate dimer using a three-headed Triad XLT system (Trionix Research Laboratory). Interictal SPECT studies were performed when the patients had no documented seizure activity for ≥24 h. For ictal studies, patients received the radiotracer injection during seizures. The mean time of the radiotracer injection after seizure onset was 30.5 ± 18.4 s and the mean duration of seizure during ictal SPECT was 93.9 ± 42.1 s. The subtraction techniques of FDG-PET and SPECT images were described previously (Jeong et al., 2004; Joo et al., 2004).
Results
SPM analysis of pre- and postoperative 18F-FDG-PET images (Table 1)
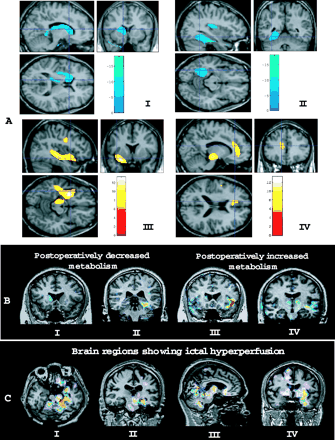
Postoperative glucose metabolism was reduced in the subcortical nuclei (caudate nucleus, thalamus) (Fig. 1A, I), fusiform gyrus, lingual gyrus and the posterior region of the insular cortex (Fig. 1A, II) of the hemisphere ipsilateral to resection.
Brain regions showing postoperative decrease or increase in glucose metabolism by paired t-test of SPM applied to pre- and postoperative FDG-PET images in mesial TLE patients
| Location . | Side . | Brodmann area . | Talairach coordinates (mm) . | . | . | . | Corrected P value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | x . | y . | z . | t . | . | |||||||
| Brain regions showing postoperative decrease in glucose metabolism | ||||||||||||||
| Caudate nucleus | I | – | −12 | 18 | 8 | −11.17 | <0.001 | |||||||
| Fusiform gyrus | I | 37 | −29 | −40 | −13 | −8.79 | <0.001 | |||||||
| Lingual gyrus | I | 37 | −24 | −46 | −11 | −7.60 | <0.005 | |||||||
| Posterior insula | I | 13 | −34 | −22 | 18 | −9.22 | <0.001 | |||||||
| Thalamus, pulvinar | I | – | −14 | −26 | 12 | −7.49 | <0.005 | |||||||
| Brain regions showing postoperative increase in glucose metabolism | ||||||||||||||
| Anterior insula | I | – | −30 | 6 | −14 | 13.27 | <0.001 | |||||||
| Temporal stem white matter | I | – | −42 | −22 | −10 | 12.10 | <0.001 | |||||||
| Midbrain | I | – | −14 | −20 | −8 | 11.15 | <0.001 | |||||||
| Inferior precentral gyrus | I | 6 | −50 | 0 | 26 | 7.86 | 0.001 | |||||||
| Anterior cingulate gyrus | I | 32 | −14 | 42 | −2 | 7.79 | 0.001 | |||||||
| Supramarginal gyrus | I | 40 | −52 | −38 | 34 | 7.39 | 0.003 | |||||||
| Location . | Side . | Brodmann area . | Talairach coordinates (mm) . | . | . | . | Corrected P value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | x . | y . | z . | t . | . | |||||||
| Brain regions showing postoperative decrease in glucose metabolism | ||||||||||||||
| Caudate nucleus | I | – | −12 | 18 | 8 | −11.17 | <0.001 | |||||||
| Fusiform gyrus | I | 37 | −29 | −40 | −13 | −8.79 | <0.001 | |||||||
| Lingual gyrus | I | 37 | −24 | −46 | −11 | −7.60 | <0.005 | |||||||
| Posterior insula | I | 13 | −34 | −22 | 18 | −9.22 | <0.001 | |||||||
| Thalamus, pulvinar | I | – | −14 | −26 | 12 | −7.49 | <0.005 | |||||||
| Brain regions showing postoperative increase in glucose metabolism | ||||||||||||||
| Anterior insula | I | – | −30 | 6 | −14 | 13.27 | <0.001 | |||||||
| Temporal stem white matter | I | – | −42 | −22 | −10 | 12.10 | <0.001 | |||||||
| Midbrain | I | – | −14 | −20 | −8 | 11.15 | <0.001 | |||||||
| Inferior precentral gyrus | I | 6 | −50 | 0 | 26 | 7.86 | 0.001 | |||||||
| Anterior cingulate gyrus | I | 32 | −14 | 42 | −2 | 7.79 | 0.001 | |||||||
| Supramarginal gyrus | I | 40 | −52 | −38 | 34 | 7.39 | 0.003 | |||||||
Height of threshold: familywise error corrected P < 0.05; extent threshold KE > 50. I = side ipsilateral to the resected temporal lobe.
Brain regions showing postoperative decrease or increase in glucose metabolism by paired t-test of SPM applied to pre- and postoperative FDG-PET images in mesial TLE patients
| Location . | Side . | Brodmann area . | Talairach coordinates (mm) . | . | . | . | Corrected P value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | x . | y . | z . | t . | . | |||||||
| Brain regions showing postoperative decrease in glucose metabolism | ||||||||||||||
| Caudate nucleus | I | – | −12 | 18 | 8 | −11.17 | <0.001 | |||||||
| Fusiform gyrus | I | 37 | −29 | −40 | −13 | −8.79 | <0.001 | |||||||
| Lingual gyrus | I | 37 | −24 | −46 | −11 | −7.60 | <0.005 | |||||||
| Posterior insula | I | 13 | −34 | −22 | 18 | −9.22 | <0.001 | |||||||
| Thalamus, pulvinar | I | – | −14 | −26 | 12 | −7.49 | <0.005 | |||||||
| Brain regions showing postoperative increase in glucose metabolism | ||||||||||||||
| Anterior insula | I | – | −30 | 6 | −14 | 13.27 | <0.001 | |||||||
| Temporal stem white matter | I | – | −42 | −22 | −10 | 12.10 | <0.001 | |||||||
| Midbrain | I | – | −14 | −20 | −8 | 11.15 | <0.001 | |||||||
| Inferior precentral gyrus | I | 6 | −50 | 0 | 26 | 7.86 | 0.001 | |||||||
| Anterior cingulate gyrus | I | 32 | −14 | 42 | −2 | 7.79 | 0.001 | |||||||
| Supramarginal gyrus | I | 40 | −52 | −38 | 34 | 7.39 | 0.003 | |||||||
| Location . | Side . | Brodmann area . | Talairach coordinates (mm) . | . | . | . | Corrected P value . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | . | . | x . | y . | z . | t . | . | |||||||
| Brain regions showing postoperative decrease in glucose metabolism | ||||||||||||||
| Caudate nucleus | I | – | −12 | 18 | 8 | −11.17 | <0.001 | |||||||
| Fusiform gyrus | I | 37 | −29 | −40 | −13 | −8.79 | <0.001 | |||||||
| Lingual gyrus | I | 37 | −24 | −46 | −11 | −7.60 | <0.005 | |||||||
| Posterior insula | I | 13 | −34 | −22 | 18 | −9.22 | <0.001 | |||||||
| Thalamus, pulvinar | I | – | −14 | −26 | 12 | −7.49 | <0.005 | |||||||
| Brain regions showing postoperative increase in glucose metabolism | ||||||||||||||
| Anterior insula | I | – | −30 | 6 | −14 | 13.27 | <0.001 | |||||||
| Temporal stem white matter | I | – | −42 | −22 | −10 | 12.10 | <0.001 | |||||||
| Midbrain | I | – | −14 | −20 | −8 | 11.15 | <0.001 | |||||||
| Inferior precentral gyrus | I | 6 | −50 | 0 | 26 | 7.86 | 0.001 | |||||||
| Anterior cingulate gyrus | I | 32 | −14 | 42 | −2 | 7.79 | 0.001 | |||||||
| Supramarginal gyrus | I | 40 | −52 | −38 | 34 | 7.39 | 0.003 | |||||||
Height of threshold: familywise error corrected P < 0.05; extent threshold KE > 50. I = side ipsilateral to the resected temporal lobe.
(A) SPM results of pre- and postoperative FDG-PET images. A postsurgical decrease in glucose metabolism was observed in the ipsilateral caudate nucleus, thalamus (I), fusiform gyrus and the posterior region of the insula (II), whereas a postsurgical increase in glucose metabolism was observed in the anterior region of the insula, temporal stem white matter, inferior precentral gyrus, ipsilateral midbrain (III) and anterior cingulate gyrus (IV) ipsilateral to resection (familywise error-corrected, P < 0.05). SPM results were overlaid on the brain MRI of a single normal control. Red-yellow colour means increased metabolism; blue colour indicates decreased metabolism. (B) Subtraction between pre- and postsurgical FDG-PET images in individual patients. I and II show postoperatively decreased glucose metabolism in the caudate nucleus after right temporal lobectomy (I) and in the fusiform gyrus after left temporal lobectomy (II). III and IV show postoperatively increased glucose metabolism in the left anterior insular cortex and small areas of the right anterior temporal region after left temporal lobectomy (III) and in the left temporal stem white matter and midbrain and in bilateral thalami after left temporal lobectomy (IV). (C) SISCOM (subtraction ictal–interictal SPECT co-registered to MRI) result of a patient with left mesial TLE. Ictal hyperperfusion was observed in the left mesial temporal region (amygdala–hippocampus), left midbrain and cerebellum (I∼III), and in bilateral basal ganglia, left anterior insula and left temporal stem white matter (IV).
Postoperative glucose metabolism increased in the anterior region of the insular cortex, midbrain, inferior precentral gyrus, temporal stem white matter (Fig. 1A, III), anterior cingulate gyrus (Fig. 1A, IV) and supramarginal gyrus of the hemisphere ipsilateral to resection. The ‘anterior insular cortex’ refers to the anterior part of the insula to the verticofrontal plane (perpendicular to the anterior–posterior bicommissural horizontal plane) passing through the anterior commissure, and the ‘posterior insular cortex’ means the posterior part of the insula to the verticofrontal plane (Bouilleret et al., 2002). No significant postoperative change in cerebral metabolism was observed in the hemisphere contralateral to resection.
Subtraction between the pre- and postoperative FDG-PET images in individual patients
Table 2 summarizes brain regions showing decreased or increased glucose metabolism postoperatively, by FDG-PET subtraction. In most patients, postoperative glucose metabolism decreased in the caudate nucleus, fusiform gyrus and lingual gyrus ipsilateral to the epileptic focus. On the other hand, postoperatively increased metabolism was observed frequently in the ipsilateral anterior insular cortex, temporal stem white matter and midbrain. The overall distribution of brain areas having significant postoperative metabolic changes was similar to subtraction and SPM analyses of pre- and postoperative FDG-PET images. In the majority of patients, postoperative metabolic changes were confined to the hemisphere ipsilateral to the resection. But in a small number of patients the postoperative increase in glucose metabolism was observed in the frontal lobe, thalamus, basal ganglia, cingulate gyrus or parietal lobe of the contralateral hemisphere. Additionally, FDG-PET subtraction showed increased postoperative metabolism in the ipsilateral anterior thalamus in 12/28 patients (42.8%), whereas postoperative metabolism decreased in the anterior, posterior or whole thalamus in six patients (21.4%).
Brain regions showing a postoperative decrease or increase in glucose metabolism in a subtraction analysis between pre- and postoperative FDG-PET images in individual patients
| . | Ipsilateral . | Contralateral . | ||
|---|---|---|---|---|
| Brain regions showing postoperative decrease in glucose metabolism | ||||
| Fusiform gyrus | 24 (85.7%) | 0 | ||
| Lingual gyrus | 27 (96.4%) | 0 | ||
| Basal ganglia | ||||
| Caudate nucleus | 19 (67.8%) | 0 | ||
| Putamen | 7 (25%) | 1 (3.6%) | ||
| Frontal lobe | 7 (25%) | 1 (3.6%) | ||
| Insular cortex | 7 (25%) | 0 | ||
| Thalamus | ||||
| Anterior | 2 (7.1%) | 0 | ||
| Posterior | 1 (10.7%) | 0 | ||
| Whole | 3 (3.5%) | 0 | ||
| Brain regions showing postoperative increase in glucose metabolism | ||||
| Temporal lobe | ||||
| Temporal stem white matter | 27 (96.4%) | 0 | ||
| Superior temporal gyrus | 4 (14.2%) | 0 | ||
| Anterior insular cortex | 25 (89.2%) | 0 | ||
| Frontal lobe | 21 (75%) | 5 (17.8%) | ||
| Midbrain | 19 (67.8%) | 0 | ||
| Anterior thalamus | 12 (42.8%) | 5 (17.8%) | ||
| Basal ganglia | ||||
| Caudate nucleus | 11 (39.2%) | 4 (14.2%) | ||
| Putamen or globus pallidus | 3 (10.7%) | 2 (7.1%) | ||
| Cingulate gyrus | 11 (39.2%) | 2 (7.1%) | ||
| Parietal lobe | 4 (14.2%) | 2 (7.1%) | ||
| . | Ipsilateral . | Contralateral . | ||
|---|---|---|---|---|
| Brain regions showing postoperative decrease in glucose metabolism | ||||
| Fusiform gyrus | 24 (85.7%) | 0 | ||
| Lingual gyrus | 27 (96.4%) | 0 | ||
| Basal ganglia | ||||
| Caudate nucleus | 19 (67.8%) | 0 | ||
| Putamen | 7 (25%) | 1 (3.6%) | ||
| Frontal lobe | 7 (25%) | 1 (3.6%) | ||
| Insular cortex | 7 (25%) | 0 | ||
| Thalamus | ||||
| Anterior | 2 (7.1%) | 0 | ||
| Posterior | 1 (10.7%) | 0 | ||
| Whole | 3 (3.5%) | 0 | ||
| Brain regions showing postoperative increase in glucose metabolism | ||||
| Temporal lobe | ||||
| Temporal stem white matter | 27 (96.4%) | 0 | ||
| Superior temporal gyrus | 4 (14.2%) | 0 | ||
| Anterior insular cortex | 25 (89.2%) | 0 | ||
| Frontal lobe | 21 (75%) | 5 (17.8%) | ||
| Midbrain | 19 (67.8%) | 0 | ||
| Anterior thalamus | 12 (42.8%) | 5 (17.8%) | ||
| Basal ganglia | ||||
| Caudate nucleus | 11 (39.2%) | 4 (14.2%) | ||
| Putamen or globus pallidus | 3 (10.7%) | 2 (7.1%) | ||
| Cingulate gyrus | 11 (39.2%) | 2 (7.1%) | ||
| Parietal lobe | 4 (14.2%) | 2 (7.1%) | ||
Values are number of patients who showed a postoperative decrease or increase in glucose metabolism in each brain structure. Percentages values in parentheses are with respect to the total number (28) of patients. Ipsilateral, contralateral = ipsilateral, contralateral to epileptic focus.
Brain regions showing a postoperative decrease or increase in glucose metabolism in a subtraction analysis between pre- and postoperative FDG-PET images in individual patients
| . | Ipsilateral . | Contralateral . | ||
|---|---|---|---|---|
| Brain regions showing postoperative decrease in glucose metabolism | ||||
| Fusiform gyrus | 24 (85.7%) | 0 | ||
| Lingual gyrus | 27 (96.4%) | 0 | ||
| Basal ganglia | ||||
| Caudate nucleus | 19 (67.8%) | 0 | ||
| Putamen | 7 (25%) | 1 (3.6%) | ||
| Frontal lobe | 7 (25%) | 1 (3.6%) | ||
| Insular cortex | 7 (25%) | 0 | ||
| Thalamus | ||||
| Anterior | 2 (7.1%) | 0 | ||
| Posterior | 1 (10.7%) | 0 | ||
| Whole | 3 (3.5%) | 0 | ||
| Brain regions showing postoperative increase in glucose metabolism | ||||
| Temporal lobe | ||||
| Temporal stem white matter | 27 (96.4%) | 0 | ||
| Superior temporal gyrus | 4 (14.2%) | 0 | ||
| Anterior insular cortex | 25 (89.2%) | 0 | ||
| Frontal lobe | 21 (75%) | 5 (17.8%) | ||
| Midbrain | 19 (67.8%) | 0 | ||
| Anterior thalamus | 12 (42.8%) | 5 (17.8%) | ||
| Basal ganglia | ||||
| Caudate nucleus | 11 (39.2%) | 4 (14.2%) | ||
| Putamen or globus pallidus | 3 (10.7%) | 2 (7.1%) | ||
| Cingulate gyrus | 11 (39.2%) | 2 (7.1%) | ||
| Parietal lobe | 4 (14.2%) | 2 (7.1%) | ||
| . | Ipsilateral . | Contralateral . | ||
|---|---|---|---|---|
| Brain regions showing postoperative decrease in glucose metabolism | ||||
| Fusiform gyrus | 24 (85.7%) | 0 | ||
| Lingual gyrus | 27 (96.4%) | 0 | ||
| Basal ganglia | ||||
| Caudate nucleus | 19 (67.8%) | 0 | ||
| Putamen | 7 (25%) | 1 (3.6%) | ||
| Frontal lobe | 7 (25%) | 1 (3.6%) | ||
| Insular cortex | 7 (25%) | 0 | ||
| Thalamus | ||||
| Anterior | 2 (7.1%) | 0 | ||
| Posterior | 1 (10.7%) | 0 | ||
| Whole | 3 (3.5%) | 0 | ||
| Brain regions showing postoperative increase in glucose metabolism | ||||
| Temporal lobe | ||||
| Temporal stem white matter | 27 (96.4%) | 0 | ||
| Superior temporal gyrus | 4 (14.2%) | 0 | ||
| Anterior insular cortex | 25 (89.2%) | 0 | ||
| Frontal lobe | 21 (75%) | 5 (17.8%) | ||
| Midbrain | 19 (67.8%) | 0 | ||
| Anterior thalamus | 12 (42.8%) | 5 (17.8%) | ||
| Basal ganglia | ||||
| Caudate nucleus | 11 (39.2%) | 4 (14.2%) | ||
| Putamen or globus pallidus | 3 (10.7%) | 2 (7.1%) | ||
| Cingulate gyrus | 11 (39.2%) | 2 (7.1%) | ||
| Parietal lobe | 4 (14.2%) | 2 (7.1%) | ||
Values are number of patients who showed a postoperative decrease or increase in glucose metabolism in each brain structure. Percentages values in parentheses are with respect to the total number (28) of patients. Ipsilateral, contralateral = ipsilateral, contralateral to epileptic focus.
Subtraction ictal–interictal SPECT co-registered to MRI (SISCOM)
SISCOM was performed in 17 patients (60.7%, 17/28; nine in left TLE, eight in right TLE) who had both ictal and interictal brain SPECTs. Ten of them (58.8%, 10/17) showed ictal hyperperfusion in the unilateral temporal lobe ipsilateral to the epileptic focus while seven (41.2%, 7/17) had ictal hyperperfusion in bilateral temporal lobes with ipsilateral predominance. Ictal hyperperfusion of ipsilateral temporal stem white matter was noted in 12 patients (70.6%, 12/17). Most patients showed ictal hyperperfusion in the ipsilateral or bilateral basal ganglia (100%, 17/17), the thalami (94.1%, 16/17) and several small areas of the frontal lobe (100%, 17/17), parietal lobe (88.2%, 15/17) or occipital lobe (82.3%, 14/17). The cerebral perfusion was also increased during seizures in ipsilateral midbrain (58.8%, 10/17), anterior insular cortex (52.9%, 9/17), or cingulate gyrus (64.7%, 11/17).
Discussion
We sought to examine postoperative changes in glucose metabolism throughout the whole brain in MTLE patients who achieved a seizure-free postoperative outcome.
Postoperatively decreased glucose metabolism in caudate nucleus, pulvinar of thalamus, fusiform gyrus and lingual gyrus
Since the striatum and thalamus are richly innervated by fibres from the hippocampus and amygdala via the fornix and mammillary bodies (Nauta, 1986), hippocampal cell loss results in diminished outflow to the ipsilateral striatum and thalamus, with subsequent subcortical hypometabolism in TLE patients (Dlugos et al., 1999).
The significant postoperative decrease in ipsilateral striatal volume was observed after temporal lobectomy (Rektor et al., 2002). Ictal hyperperfusion is frequently observed in the caudate nucleus, putamen and thalamus during TLE seizures with ictal dystonic posturing (Joo et al., 2004). This observation and the postoperatively decreased metabolism in the ipsilateral caudate in the present study suggest a shared functional link between the temporal lobe and striatum.
There are some classical anatomical studies in monkeys showing that the medial pulvinar of the thalamus is connected to the parahippocampal gyrus (Baleydier and Mauguiere, 1985) and the entorhinal cortex (Insausti et al., 1987). We confirmed the resection of the entorhinal cortex and parahippocampal gyrus on the postoperative brain MRI of our patients, which may have contributed to the postoperatively decreased metabolism of the pulvinar of the thalamus in our study.
The fusiform gyrus is anatomically located just anterior to the retinotopic visual cortex in functional MRI (Halgren et al., 1998), and projects heavily to anteromedial temporal areas, including the entorhinal and perirhinal cortices (Felleman and VanEssen, 1991; Suzuki, 1996). During the complex visual encoding of scenes in a functional MRI study in MTLE patients, the activation of the fusiform gyrus as well as the hippocampus and parahippocampal gyrus was increased (Rabin et al., 2004), which suggests a functional connection between the fusiform gyrus and anteromedial temporal areas. Although the occipital cortex was not directly injured during temporal lobectomy, ipsilateral occipital hypometabolism has occasionally been observed in postoperative FDG-PET studies (Wong et al., 2004). A postoperative metabolic decrease in the fusiform gyrus of the temporal lobe and the lingual gyrus of the occipital lobe appears to be due to deafferentation by the resection of anteromedial temporal structures.
Postoperative metabolism decreased in posterior insula but increased in anterior insula
The implication of the insula in MTLE has been demonstrated by direct intracerebral recording during TLE seizures (Isnard et al., 2000, 2004) and by FDG-PET studies (Bouilleret et al., 2002; Chassoux et al., 2004). A functional mapping study based on intracortical stimulation found the existence of two different cortical networks within the insular cortex (Ostrowsky et al., 2000, 2002; Isnard et al., 2004). MTLE patients with emotional symptoms showed hypometabolism in the anterior insula and those who experienced visceral symptoms showed hypometabolism in the posterior insula by FDG-PET (Bouilleret et al., 2002).
A decrease in 5-HT1A receptor binding has been shown in the anterior insular cortex (Merlet et al., 2004a) and the relation between 5-hydroxytryptamine1A (5-HT1A) receptors and the epileptogenic zone has been demonstrated (Merlet et al., 2004b).
Animal studies showed different connections of the anterior and posterior insular cortices: the anterior insula connects mainly to the anterior cingulate, the entorhinal cortex and the periamygdaloid cortex, while the posterior insula connects mainly to the temporopolar cortex, superior temporal sulcus, dorsolateral striatum and ventral thalamus (Insausti et al., 1987; Vogt and Pandya, 1987; Chikama et al., 1997).
The different changes in postoperative glucose metabolism within the insular cortex observed in the present study may support the established concept whereby the anterior and posterior portions of the insula have different neuroanatomical pathways and functional roles.
Postoperatively increased glucose metabolism in the inferior precentral gyrus, anterior cingulate gyrus, supramarginal gyrus, ventral midbrain, temporal stem white matter and anterior thalamus
The hippocampal formation projects to the frontal cortex reciprocally (Cavada et al., 2000). Preoperative FDG-PET studies have consistently demonstrated hypometabolism in frontal regions ipsilateral to the epileptogenic hippocampus (Henry et al., 1993; Savic et al., 1997). A SPECT study using SPM analysis in TLE patients showed ictal hyperperfusion in the middle frontal and precentral gyrus ipsilateral to the seizure focus (Van Paesschen et al., 2003). Moreover, it was reported that epilepsy surgery led to increased metabolism, mainly in the ipsilateral frontal lobe (Akimura et al., 1999; Spanaki et al., 2000).
A functional MRI study showed that the anterior cingulate is involved in executive functions (Corbetta et al., 1998). Frontal lobe dysfunction, such as executive impairment, was not uncommon in TLE patients (Hermann et al., 1988; Trenerry and Jack, 1994). The fact that epileptic discharges from the amygdala and the hippocampus preferentially propagate to the orbitofrontal and prefrontal areas via the anterior cingulate gyrus (Lieb et al., 1991) in TLE seizures may explain the involvement of the cingulate gyrus in the frontal lobe dysfunction of TLE patients. Some authors reported that improvements in executive functions occurred after anterior temporal lobectomy in TLE patients, who became seizure-free (Hermann and Seidenberg, 1995).
Recently, a magnetic resonance spectroscopic imaging study demonstrated reduced N-acetylaspartate/(creatine + choline) [NAA/(Cr + Cho)]) in the ipsilateral parietal lobe of MTLE patients with hippocampal sclerosis (Mueller et al., 2004), which might have been due to neuronal loss and gliosis or neuronal dysfunction (Hugg et al., 1993). The parietal lobe is commonly involved in seizure spread from the mesial temporal lobe (Blumenfeld et al., 2004a). Our result may suggest the reversibility of preoperative parietal dysfunction. Neuroimaging evidence suggests that the midbrain is actively engaged in TLE seizures. A voxel-based morphometric study in patients with intractable MTLE demonstrated decreased grey matter concentrations in the midbrain and in subcortical nuclei, such as the thalamus and caudate, and in parieto-occipital regions (Bonilha et al., 2004). It suggested that brain areas functionally and anatomically related to the hippocampus undergo volume reduction in AED-refractory unilateral MTLE patients. SISCOM in our study showed ictal hyperperfusion in the ipsilateral midbrain (Fig. 1C) in 10 of 17 patients.
Furthermore, postoperatively increased glucose metabolism in the ipsilateral midbrain was confirmed in 19 of 28 patients by FDG-PET subtraction. Our results suggest that preoperative dysfunction of the ipsilateral midbrain may be related to frequent seizure spreading, and is reversible after surgery, with a seizure-free outcome.
The postoperative increase in glucose metabolism in temporal stem white matter by FDG-PET SPM analysis was confirmed in individual patients by FDG-PET subtraction, which gave the same finding in 27/28 patients (96.4%) (Fig. 1B, IV). The temporal stem white matter was located posterior to the resected temporal lobe. SISCOM in our study showed ictal hyperperfusion of temporal stem white matter in 12/17 patients (70.6%) (Fig. 1C, IV). These findings suggest that the temporal stem white matter had been affected by frequent seizure spreading. Glucose utilization in the grey matter, which contains the cell bodies and dendrites of neurons, is greater than that in the white matter, which contains the myelinated axons (Phelps et al., 1982), and cerebral perfusion is much more profuse in the grey matter than in the white matter. It is not clear whether the postoperatively increased metabolism of white matter might have been the result of recovery from the cessation of seizures, or of another, unknown aetiology. Heterotopic neurons in temporal lobe white matter may contribute to ictal hyperperfusion (Tae et al., 2005) and postoperative metabolism changes in temporal stem white matter.
FDG-PET subtraction additionally showed that postoperative glucose metabolism increased in the anterior thalamus in 12 patients (42.8%). The fornix, mammillary bodies, and thalamic nuclei have been reported to show abnormalities in MRI and PET studies of TLE patients (Juhasz et al., 1999; Deasy et al., 2000; Oikawa et al., 2001). Concomitant interictal hypometabolism or hypoperfusion of the ipsilateral thalamus and epileptic temporal lobe was frequently observed in TLE patients (Yune et al., 1998; Choi et al., 2003), suggesting a close anatomical connection between those structures. The hippocampal formation is connected indirectly to the anterior thalamic nucleus via the mammillary body and reaches the cingulate cortex (Aggleton and Brown, 1999), and MTLE seizures frequently propagate to the thalamus (Blumenfeld et al., 2004b; Joo et al., 2004). SISCOM in our study showed ictal hyperperfusion in the thalamus in 16/17 patients. Thus, it is plausible that the postoperative increase in glucose metabolism in the anterior thalamus may be a functional recovery, due to the cessation of spreading of ictal and interictal discharges after surgery.
AED effects on postoperative glucose metabolism
AED medications could influence global and regional cerebral glucose metabolism (Theodore, 1988; Gaillard et al., 1996; Spanaki et al., 1999). Because all patients were receiving the same dose of AED or a slightly smaller dose of the AED at the time of postoperative FDG-PET scanning compared with the preoperative state, the effect of AED changes on postsurgical changes of cerebral glucose metabolism should have been negligible in our study. Furthermore, we compared globally normalized metabolic rates rather than absolute values in pre- and postoperative FDG-PET images. This procedure may adjust regional values in the whole brain, and thus remove global drug effects.
Conclusion
Using SPM, we performed a voxel-by-voxel analysis of whole-brain glucose metabolism and compared pre- and postoperative cerebral glucose metabolism. SPM analysis of whole-brain metabolism was able to identify postoperative metabolic changes not only in the cerebral cortex but also in the subcortical structures and brainstem. A subtraction study between pre- and postoperative PET images in individual patients confirmed the results of SPM analysis, with some additional findings. A SISCOM study revealed brain structures to which TLE seizures frequently spread. Suggested mechanisms underlying postoperative brain metabolism changes include the loss of deafferentation caused by the resection of mesial temporal structures, and the recovery of impaired brain activity after the cessation of interictal and ictal discharges by surgery.
This study was supported by a grant (no. HMP-03-PJ1-PG3-21300-0033) of the Good Health R&D Project, Ministry of Health and Welfare, Republic of Korea.
References
Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis.
Akimura T, Yeh HS, Mantil JC, Privitera MD, Gartner M, Tomsick TA. Cerebral metabolism of the remote area after epilepsy surgery.
Baleydier C, Mauguiere F. Anatomical evidence for medial pulvinar connections with the posterior cingulate cortex, the retrosplenial area, and the posterior parahippocampal gyrus in monkeys.
Blumenfeld H, Rivera M, McNally KA, Davis K, Spencer DD, Spencer SS. Ictal neocortical slowing in temporal lobe epilepsy.
Blumenfeld H, McNally KA, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, et al. Positive and negative network correlations in temporal lobe epilepsy.
Bonilha L, Rorden C, Castellano G, Pereira F, Rio PA, Cendes F, et al. Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy.
Bouilleret V, Dupont S, Spelle L, Baulac M, Samson Y, Semah F. Insular cortex involvement in mesiotemporal lobe epilepsy: a positron emission tomography study.
Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex.
Chassoux F, Semah F, Bouilleret V, Landre E, Devaux B, Turak B, et al. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study.
Chikama M, McFarland NR, Amaral DG, Haber SN. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate.
Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, Kim SE. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy.
Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, et al. A common network of functional areas for attention and eye movements.
Deasy NP, Jarosz JM, Elwes RC, Polkey CE, Cox TC. Thalamic changes with mesial temporal sclerosis: MRI.
Dlugos DJ, Jaggi J, O'Connor WM, Ding XS, Reivich M, O'Connor MJ, et al. Hippocampal cell density and subcortical metabolism in temporal lobe epilepsy.
Dupont S, Croize AC, Semah F, Hasboun D, Samson Y, Clemenceau S, et al. Is amygdalohippocampectomy really selective in medial temporal lobe epilepsy? A study using positron emission tomography with (18)fluorodeoxyglucose.
Felleman DJ, VanEssen DC. Distributed hierarchical processing in the primate cerebral cortex.
Gaillard WD, Zeffiro T, Fazilat S, DeCarli C, Theodore WH. Effect of valproate on cerebral metabolism and blood flow: an 18F-2-deoxyglucose and 15O water positron emission tomography study.
Hajek M, Wieser HG, Khan N, Antonini A, Schrott PR, Maguire P, et al. Preoperative and postoperative glucose consumption in mesiobasal and lateral temporal lobe epilepsy.
Halgren E, Dale AM, Sereno MI, Tootell RB, Marinkovic K, Rosen BR. Location of human face-selective cortex with respect to retinotopic areas.
Henry TR, Mazziotta JC, Engel J. Interictal metabolic anatomy of mesial temporal lobe epilepsy.
Hermann B, Seidenberg M. Executive system dysfunction in temporal lobe epilepsy: effects of nociferous cortex versus hippocampal pathology.
Hermann BP, Wyler AR, Richey ET. Wisconsin Card Sorting Test performance in patients with complex partial seizures of temporal-lobe origin.
Hong SB, Han HJ, Rho SY, Seo DW. Correlation of FDG PET hypometabolism with interictal spike frequency during FDG distribution phase in temporal lobe epilepsy.
Hugg JW, Laxer KD, Matson GB, Maudsley AA, Weiner MW. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopic imaging.
Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents.
Isnard J, Guenot M, Ostrowsky K, Sindou M, Mauguiere F. The role of the insular cortex in temporal lobe epilepsy.
Isnard J, Guenot M, Sindou M, Mauguiere F. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study.
Jeong Y, Chin J, Tae WS, Hong SB, Kim SE, Suh YL, Na DL. Serial positron emission tomography findings in a patient with hydrocephalic dementia and Alzheimer's disease.
Joo EY, Hong SB, Lee EK, Tae WS, Kim JH, Seo DW, et al. Regional cerebral hyperperfusion with ictal dystonic posturing: ictal-interictal SPECT subtraction.
Juhasz C, Nagy F, Watson C, da Silva EA, Muzik O, Chugani DC, et al. Glucose and [11C]flumazenil positron emission tomography abnormalities of thalamic nuclei in temporal lobe epilepsy.
Lieb JP, Dasheiff RM, Engel J Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures.
Merlet I, Ryvlin P, Costes N, Dufournel D, Isnard J, Faillenot I, et al. Statistical parametric mapping of 5-HT1A receptor binding in temporal lobe epilepsy with hippocampal ictal onset on intracranial EEG.
Merlet I, Ostrowsky K, Costes N, Ryvlin P, Isnard J, Faillenot I, et al. 5-HT1A receptor binding and intracerebral activity in temporal lobe epilepsy: an [18F]MPPF-PET study.
Mueller SG, Laxer KD, Cashdollar N, Flenniken DL, Matson GB, Weiner MW. Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy.
Nauta WJH. Circuitous connections linking cerebral cortex, limbic system, and corpus striatum. In: Doane BK, Livingston KE, editors. The limbic system: functional organization and clinical disorders. New York: Raven Press;
Newberg AB, Alavi A, Berlin J, Mozley PD, O'Connor M, Sperling M. Ipsilateral and contralateral thalamic hypometabolism as a predictor of outcome after temporal lobectomy for seizures.
Oikawa H, Sasaki M, Tamakawa Y, Kamei A. The circuit of Papez in mesial temporal sclerosis: MRI.
Ostrowsky K, Isnard J, Ryvlin P, Guenot M, Fischer C, Mauguiere F. Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy.
Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation.
Phelps ME, Mazziotta JC, Huang SC. Study of cerebral function with positron computed tomography.
Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, et al. Functional MRI predicts post-surgical memory following temporal lobectomy.
Rektor I, Kuba R, Brazdil M. Interictal and ictal EEG activity in the basal ganglia: an SEEG study in patients with temporal lobe epilepsy.
Savic I, Altshuler L, Baxter L, Engel J. Pattern of interictal hypometabolism in PET scans with fludeoxyglucose F 18 reflects prior seizure types in patients with mesial temporal lobe seizures.
Signorini M, Paulesu E, Friston K, Perani D, Colleluori A, Lucignani G. Rapid assessment of regional cerebral metabolic abnormalities in single subjects with quantitative and nonquantitative [18F]FDG PET: a clinical validation of statistical parametric mapping.
Spanaki MV, Siegel H, Kopylev L, Fazilat S, Dean A, Liow K. The effect of vigabatrin (gamma-vinyl GABA) on cerebral blood flow and metabolism.
Spanaki MV, Kopylev L, DeCarli C, Gaillard WD, Liow K, Fazilat S. Postoperative changes in cerebral metabolism in temporal lobe epilepsy.
Suzuki WA. The anatomy, physiology and functions of the perirhinal cortex.
Tae WS, Joo EY, Kim JH, Han SJ, Suh YL, Kim BT, et al. Cerebral perfusion changes in mesial temporal lobe epilepsy: SPM analysis of ictal and interictal SPECT.
Theodore WH, Sato S, Kufta C, Balish MB, Bromfield EB, Leiderman DB. Temporal lobectomy for uncontrolled seizures: the role of positron emission tomography.
Trenerry M, Jack CR. Wisconsin Card Sorting Test performance before and after temporal lobectomy.
Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis.
Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents.
Wong FC, Swartz BE, Gee M, Mandelkern M. Occipital hypometabolism demonstrated by positron emission tomography after temporal lobectomy for refractory epilepsy.
Author notes
Departments of 1Neurology, 2Neurosurgery, 3Nuclear Medicine and 4Pediatrics, Samsung Medical Center and Center for Clinical Medicine, SBRI, Sungkyunkwan University School of Medicine, Seoul, 5Department of Neurology, Myongji Hospital, Kwandong University, Goyang City and 6APCTP/NCSL, Department of Physics, POSTECH, Pohang, Korea
- magnetic resonance imaging
- fluorodeoxyglucose f18
- epilepsy
- glucose metabolism
- seizures
- single photon emission computed tomography
- anterior nuclear group
- caudate nucleus
- epilepsy, temporal lobe
- midbrain
- microscopy, scanning probe
- pulvinar
- surgical procedures, operative
- thalamus
- brain
- pathology
- temporal lobe
- tongue
- fluorodeoxyglucose positron emission tomography
- fusiform gyrus
- white matter
- insula of reil
- anterior cingulate gyrus
- precentral gyrus