-
PDF
- Split View
-
Views
-
Cite
Cite
Paolo Federico, David F. Abbott, Regula S. Briellmann, A. Simon Harvey, Graeme D. Jackson, Functional MRI of the pre-ictal state, Brain, Volume 128, Issue 8, August 2005, Pages 1811–1817, https://doi.org/10.1093/brain/awh533
Close - Share Icon Share
Abstract
The mechanisms underlying the transition from interictal to ictal states are poorly understood. Non-linear mathematical analysis of EEG frequency components has confirmed the presence of a pre-ictal state in focal epilepsy. We report on functional MRI (fMRI) analysis of the pre-ictal state in three patients with intractable focal epilepsy. Each subject had a typical partial seizure in the scanner while continuous blood oxygen level dependent (BOLD) fMRI images were acquired. The pre-ictal BOLD changes were first analysed by statistically comparing BOLD signals of two one-minute blocks. Further examination of the full time course was then performed. Each patient showed highly significant, focal BOLD signal changes. In Patient 1, a striking pre-ictal BOLD signal increase was seen over the region of the seizure focus identified on complementary epilepsy investigations. No significant BOLD signal decreases were observed. Patient 2 showed widespread pre-ictal BOLD increase contralateral to the presumed seizure focus, as well as a focal BOLD decrease near the presumed seizure focus. In Patient 3, pre-ictal BOLD increase was co-localized with the site of hyperperfusion seen on ictal single photon emission computed tomography (SPECT). However, this was contralateral to the seizure focus localization based on seizure symptomatology. No significant BOLD decreases were seen. The time course data in each patient studied showed change of the BOLD signal several minutes before the onset of the seizure. Highly significant BOLD fMRI signal changes occur before the onset of seizures, supporting the presence of a pre-ictal state. These changes can be localized to the site of the presumed seizure focus, as well as to other brain regions, suggesting that the pre-ictal BOLD signal changes and their underlying mechanisms are complex.
Introduction
The mechanisms underlying the generation of seizures are still not well understood. One of the most promising approaches to study the generation of seizures is the investigation of neurophysiological changes immediately before the onset of a seizure. Such a pre-ictal state can be separated from an interictal state without symptoms and from an ictal state with overt clinical symptoms. The pre-ictal state may result in physiological phenomena such as prodromal symptoms which occur minutes to days prior to seizure onset (Delamont et al., 1999).
Traditional analysis of surface and intracranial EEG signals have shown no obvious change preceding seizures (Rogowski et al., 1981; Katz et al., 1991). Non-linear mathematical analysis of EEG frequency components has been developed to describe the dynamics of the EEG signal prior to seizures (Lehnertz and Elger, 1995; Elger and Lehnertz, 1998). These studies have all shown pre-ictal changes in neuronal complexity and network activity that range from minutes to hours prior to seizure onset. More recently, the use of linear techniques such as changes in EEG signal energy were reported to occur hours prior to seizure onset (Litt et al., 2001).
Thermal measurement of cerebral blood flow in patients with temporal lobe epilepsy has shown significant increases in cerebral blood flow in the region of the seizure focus 10–12 min prior to ictal onset (Weinand et al., 1997). In addition, pre-ictal hyperperfusion at the seizure focus was observed in single photon emission computed tomography (SPECT) scans obtained fortuitously minutes prior to seizure onset under video-EEG monitoring (VEM) (Baumgartner et al., 1998). Thus, physiological and electrophysiological evidence supports the presence of a pre-ictal state. However, these studies have provided limited information about the localization of these changes. One technique that could address this issue is functional MRI (fMRI), which assesses cerebral activity non-invasively by detecting signal changes related to focal alterations of deoxyhaemoglobin concentration (Ogawa et al., 1990). This so-called blood oxygenation level dependent (BOLD) signal is, in turn, related to localized neuronal activity [in particular local field potentials (Logothetis et al., 2001)] that can be altered with epileptiform discharges.
We report on fMRI analysis of a pre-ictal state in three patients with drug refractory partial epilepsy who had a habitual seizure during scanning. In particular, we examined the spatial and temporal distribution of fMRI signal change in the pre-ictal period.
Patients and methods
Patients
Three consecutive patients who had seizures during fMRI scanning were studied. Two patients (Patients 2 and 3) were recruited for the study as they had predictable seizures, often within an hour of falling asleep. One patient (Patient 1) had a seizure while undergoing an EEG-fMRI study for the purpose of assessing the BOLD response to interictal discharges. The patients were identified via the comprehensive epilepsy program at Austin Health, a tertiary centre for the investigation of patients with epilepsy, in Heidelberg West, Victoria, Australia. All patients were evaluated for possible epilepsy surgery and had seizure characterization by VEM, routine 1.5 T MRI study using optimized epilepsy protocols, [2-18F]fluoro-2-deoxyglucose positron emission tomography (FDG PET), [(99m)Tc]technetium-ethylene cysteine dimer (ECD) SPECT, and neuropsychological investigations. Patient consent was obtained; parental consent was obtained for patients under the age of 16 years. The study protocol was approved by the Austin Health Human Research Ethics Committee.
Epilepsy characterization
Patient 1 (Fig. 1) was a 22-year-old right-handed man who had had frequent simple partial seizures since the age of 5 years. His stereotyped seizures started with tingling sensations in the right lower jaw, followed by drooling, facial twitching and bilateral dystonic posturing. Interictal EEG showed frequent, low amplitude spike or polyspike, and wave discharges with peak negativity at TP7, CP5 and T5. Ictal onset was at the same location. No lesion was seen on structural MRI. An interictal PET showed focal hypometabolism, and an ictal ECD SPECT showing focal hypermetabolism in the left low-central opercular region. Clinical localization was to the (sensory and motor) face area of the left post- and pre-central gyrus. The patient underwent two surgical resections of this area, guided by intra-operative electrocorticography (first operation) and subdural monitoring (second operation). Histological examination of the tissue showed cortical dysplasia with balloon cells. Seizures returned 9 months after the initial resection, but he remained seizure-free for two months following the second resection.
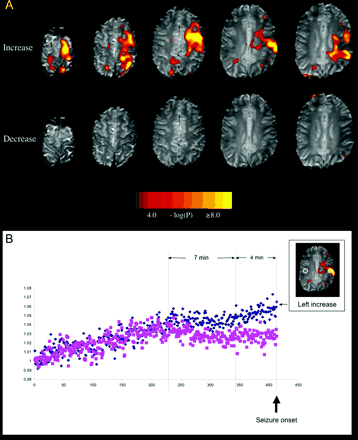
(A) Pre-ictal BOLD signal changes in Patient 1. Top row. BOLD signal increases were seen in the left frontal lobe and post-central gyrus. Bottom row. No significant BOLD signal decreases were seen. (B) The time course of the BOLD signal for 25 min prior to a seizure. This was measured in a 25 voxel ROI in the area of maximal BOLD signal. Increase (small image) is shown as diamonds and the BOLD signal in the homologous contralateral region is shown as squares. Image intensity in both regions was normalized to 1 in the first volume (y-axis) and plotted as a function of volume number (one volume every 3.6 s, x-axis). Note the signal fluctuates in a similar way in both regions for the first few minutes, followed by a divergence of BOLD signal 11 min prior to the seizure event, with relatively increased signal in the ROI ipsilateral to the seizure focus. This increase is biphasic, with an initial change maintained for ∼4 min, followed by a progressive increase until the seizure (vertical lines).
Patient 2 (Fig. 2) was a 15-year-old right-handed girl with a 3-year history of refractory nocturnal seizures. Seizures were characterized by sudden awakening, blank staring, confusion, axial flexion and bipedal automatisms. Interictal EEG showed rare right temporal sharp waves maximal at F8 and T4. Ictal EEG showed bifrontal rhythmic theta evolving to bifrontal spike and wave discharges. Structural MRI showed a small area of abnormal grey matter in the right frontal lobe anteriomedially (Fig. 2A), with corresponding increased signal on FLAIR (fluid attenuated inversion recovery) and T2-weighted images. An ictal ECD SPECT showed hyperperfusion in the area of the MRI abnormality, as well as in the thalamus and basal ganglia (Fig. 2A). Interictal ECD SPECT showed hypoperfusion to the right anterior temporal and left temporo-parietal cortex. An interictal PET showed mild hypometabolism in the right anterior mesial temporal lobe. The MRI lesion was interpreted as a small area of focal cortical dysplasia, and this lesion was seen as the most likely seizure focus. This patient did not have surgical resection as a change in medication was followed by a marked reduction in seizure frequency.
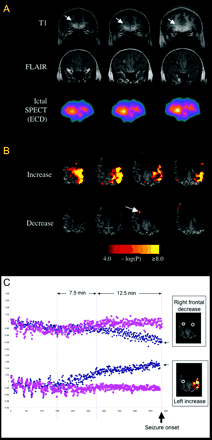
(A) MRI and ictal SPECT results in Patient 2. Top row. Coronal T1-weighted images showing a small area of abnormally thickened grey matter in the anterior right frontal lobe (arrow). Middle row. Coronal FLAIR images showing signal hyperintensity at the area of abnormal grey matter. Bottom row. Coronal ictal SPECT with ECD injection (17 s after seizure onset) showing anterior right frontal hyperperfusion. (B) Pre-ictal BOLD signal changes in Patient 2. Top row. A striking BOLD signal increase over the entire left frontal lobe and posterior aspect of the superior temporal lobe. Bottom row. A focal area of BOLD signal decrease was seen in the right anterior frontal region (arrow), near the MRI lesion. The loss of MRI signal in the frontopolar regions is due to a susceptibility artefact related to the patient's dental braces. (C) The time course is displayed in a similar way as for Patient 1, with the BOLD signal time course shown for 29 min prior to the seizure. The ipsilateral region is shown as diamonds and the homologous contralateral region as squares (similar to Fig. 1B). The time course is shown for the areas of BOLD signal decrease (top graph) and increase (bottom graph). In the first 9 min studied, there are fluctuations in the signal on both sides. This effect disappears and is followed by a divergence of the BOLD signal on the left side, contralateral to the presumed seizure focus (bottom row) 20 min prior to the seizure. About 7.5 min later (∼12.5 min prior to the seizure), divergence of BOLD signal with decrease in the area of the seizure focus is seen (top graph).
Patient 3 (Fig. 3) was an 11-year-old right-handed girl who had a 5-year history of frequent nocturnal complex partial seizures (up to 10 per night). Her seizures began with left arm tingling, followed by left hand and arm dystonia, head and trunk turning to left, and bipedal cycling automatisms. Interictal EEG showed epileptiform discharges at Cz and C4. Ictal EEG was non-lateralizing or localizing. Structural MRI showed no definite abnormalities. A late ictal ECD SPECT showed relative hyperperfusion in the left perisylvian and central region, and thalamus. Interictal ECD SPECT showed mild hypoperfusion in the left superior precentral gyrus area and left temporo-parietal junction. FDG PET showed nonspecific bilateral medial temporal hypometabolism. Seizure focus localization based on clinical and other investigations was uncertain. Based on the unusual sensation in the left hand, it was felt to involve a right hemisphere sensory area [supplementary sensorimotor area (SSMA), primary or secondary sensory area]. No epilepsy surgery has been offered.
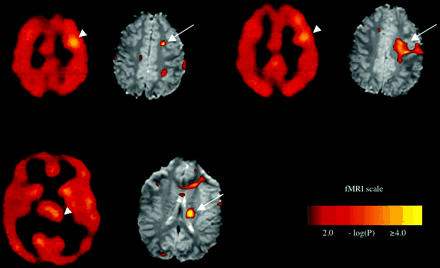
Ictal SPECT and pre-ictal BOLD signal increases in Patient 3 shown at three different levels, with corresponding SPECT images on the left and fMRI images on the right. Top row. Pre-ictal BOLD increases in the left premotor/prefrontal area at the grey–white junction (arrows) correspond with ictal hyperperfusion near these sites with ECD SPECT (arrowheads). Bottom row. Pre-ictal BOLD signal increases (arrow) in the left caudate nucleus, have a similar location to ictal ECD SPECT hyperperfusion (arrowhead).
Functional MRI
Image acquisition
Functional MRI was performed using a 3.0 T GE Signa LX whole body scanner (General Electric, Milwaukee, WI, USA). Subjects were fixed using a Velcro strap over the forehead. Preliminary imaging consisted of acquiring a T1-weighted 2D spin-echo sequence and angiographic series, with the same geometric orientation and voxel size as the subsequent functional images. The functional images were acquired as a series of single shot gradient-recalled echo-echo planar imaging (GRE-EPI) volumes providing T2*-weighted BOLD contrast [TR (repetition time)/TE (echo time) = 3600/40 ms; flip angle = 40°; 22 axial oblique slices 4 mm thick + 1 mm gap; FOV (field of view) = 24 cm; 128 × 128 matrix; 1.87 × 1.87 mm2 in-plane]. One imaging volume was acquired every 3.0 s. The first four images in each run were automatically discarded to allow the magnetization to reach a steady state.
Patient 1 was undergoing an event-related fMRI study of interictal discharges. This involved the use of a magnetic resonance compatible EEG-fMRI system developed and built in-house. The system is an improved version of that described previously (Archer et al., 2003), allowing continuous simultaneous acquisition of EEG and fMRI. Patients 2 and 3 were sleep deprived the night before their study, which took place in the morning. Simultaneous EEG-fMRI was not employed because previous VEM showed that interictal discharges were rare and ictal EEG was unhelpful. It was also felt that the presence of the EEG electrodes and cap would reduce patient comfort and the likelihood of attaining sleep, and therefore seizures. These patients were placed in the bore of the magnet and allowed to sleep. In all three patients, continuous whole brain EPI-BOLD volumes were acquired until a seizure was observed. Heart rate and oxygen saturation were monitored continuously. Seizure onset was determined clinically by a neurologist (P.F.) present in the scanner room or by the electroencephalographer. The seizures were recorded by video camera; this allowed us to verify that the seizures were typical for the patient and compatible with seizures captured during VEM. Once the seizure was recognized, image acquisition was stopped and the patients were moved rapidly out of the magnet bore.
Image processing and analysis
Images were transferred to a Linux workstation for preprocessing and analysis using MATLAB (Mathworks, Inc., Natick, MA, USA) and SPM99 (Wellcome Department of Cognitive Neurology, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Motion correction involved obtaining a rigid body transformation set of six parameters (three translations and three rotations) that minimized the squared difference between each image and the median image. Subsequent image analysis used iBrain™ (Abbott and Jackson, 2001), a program developed in-house, to perform a slice-by-slice analysis. A motion rejection step determined the intensity ‘centre-of-mass’ of each slice and rejected those with more than one third of a voxel of apparent motion from further analysis. A Gaussian smooth of full-width-at-half-maximum (FWHM) of six voxels was applied.
Block design analysis was carried out by comparing BOLD signals of a 1-min block immediately prior to seizure onset (‘task’) to another 1-min block (‘rest’) beginning 5 min prior to seizure onset in Patients 1 and 2, and beginning 3 min prior to seizure onset in Patient 3 (this patient had a more rapid seizure onset after the start of the scanning). The timing was chosen a priori: we hoped it would be a reasonably safe balance between the likelihood of detecting an effect while avoiding overly long intervals that might cause an unacceptable artefact (due to factors such as scanner drift and subject motion).
Statistical analysis involved the calculation of a Student's unpaired t-test of difference between the task and rest images on a voxel-by-voxel basis. The t-statistic map was overlaid onto the mean EPI-BOLD image, with a colour scale indicating statistical significance of the activations seen. This method of analysis and display permitted ready visualization of active brain regions without the risk of co-registration error. In the two cases where sufficient data were available, further examination of the time course was undertaken by measuring the BOLD signal in a circular region of interest (ROI) placed in an area of significant BOLD change on the statistical maps and in a homologous region in the contralateral hemisphere. This was measured for each volume acquired. Image intensity of the time course in each region was normalized to be 1 in the first volume (y-axis) and plotted as a function of volume number (x-axis), beginning from the start of imaging and ending at seizure onset. A similar time-course analysis was performed in two healthy adult control subjects in whom we performed 20 min resting-state FMRI.
Results
Patient 1
After 21 minutes of continuous scanning, the patient had a complex partial seizure that was typical for him. After the session, the patient indicated he may have had up to three auras prior to the seizure while in the scanner, although he could not be more precise with the details or timing of these events. It was not possible to discern this patient's low amplitude interictal discharges in the EEG recorded in the scanner.
Figure 1A shows the results of the analysis of pre-ictal BOLD signal change. A striking pre-ictal BOLD increase (top row; note the threshold and maximum values in the scale bar) was seen across the ipsilateral frontal lobe and post-central gyrus, where the signal was the greatest. In contrast, no significant BOLD signal decreases were seen prior to seizure onset (Fig. 1A, bottom row). The time course of the BOLD signal change is shown in Fig. 1B. The area of maximal BOLD signal increase and the homologous area on the contralateral side diverge in their BOLD signal response 11 min before the clinical seizure. There may be an increase in this divergence ∼4 min before the seizure (Fig. 1B). We also examined similar regions in two healthy control subjects in whom we performed 20-min resting-state fMRI. While some signal changes were also observed, the changes were more global, and divergences of this duration and magnitude were not apparent.
Patient 2
The patient fell asleep during the fMRI study and had a typical complex partial seizure after 25 min of continuous imaging. The first clinical sign of the seizure was head rising from the scanner table. The seizure lasted 35 s.
There was a striking pre-ictal BOLD increase across the left frontal lobe and posterior aspect of the superior temporal lobe (Fig. 2B, top row). A focal, highly significant pre-ictal BOLD signal decrease was seen in the right frontal region (Fig. 2B, bottom row). This region was posterior to the suspected focal cortical dysplasia in the right frontal pole. Susceptibility artefacts from braces affected the front polar region. The time course of the BOLD signal change is shown in Fig. 2C. There is divergence of the BOLD signal in the left (contralateral) hemisphere 20 min prior to the seizure (Fig. 2C, lower traces), associated with decreased variance in the signal on both the left and homologous right sides. In the presumed seizure focus (Fig. 2C, upper traces), there is divergence of the BOLD signal in the region of deactivation 12.5 min prior to the seizure.
Patient 3
The patient had a typical complex partial seizure in the scanner lasting 25 s. The seizure occurred 177 s after functional imaging (BOLD) was commenced and the first sign of it was left hand dystonic posturing.
Figure 3 shows the results of the analysis of pre-ictal BOLD signal change in Patient 3. There were several areas of pre-ictal BOLD increases. Prominent among these was a signal increase in the left (contralateral to presumed seizure focus) premotor/prefrontal area and in the left caudate (Fig. 3, arrows). These areas corresponded to sites where hyperperfusion was seen on ictal SPECT with ECD (Fig. 3, arrowheads). No significant pre-ictal BOLD decreases were seen.
Table 1 and the figures summarize the pre-ictal BOLD signal changes seen in the three patients.
Summary of clinical, SPECT and BOLD signal change locations for the three patients
| Patient . | Electroclinical and structural MRI localization . | Ictal SPECT hyperperfusion (cortical sites) . | Pre-ictal BOLD increase . | Pre-ictal BOLD decrease . |
|---|---|---|---|---|
| 1 | Highly consistent localization to left low central region | Ipsilateral IFG, insula, frontal pole | Ipsilateral frontal lobe | None |
| 2 | Most likely right frontal pole | Ipsilateral frontal pole | Contralateral frontal lobe and temporal lobe | Ipsilateral anterior frontal lobe |
| 3 | Suggestion for localization to right central region or SSMA | Contralateral perisylvian | Contralateral frontal lobe and caudate | None |
| Patient . | Electroclinical and structural MRI localization . | Ictal SPECT hyperperfusion (cortical sites) . | Pre-ictal BOLD increase . | Pre-ictal BOLD decrease . |
|---|---|---|---|---|
| 1 | Highly consistent localization to left low central region | Ipsilateral IFG, insula, frontal pole | Ipsilateral frontal lobe | None |
| 2 | Most likely right frontal pole | Ipsilateral frontal pole | Contralateral frontal lobe and temporal lobe | Ipsilateral anterior frontal lobe |
| 3 | Suggestion for localization to right central region or SSMA | Contralateral perisylvian | Contralateral frontal lobe and caudate | None |
IFG = inferior frontal gyrus.
Summary of clinical, SPECT and BOLD signal change locations for the three patients
| Patient . | Electroclinical and structural MRI localization . | Ictal SPECT hyperperfusion (cortical sites) . | Pre-ictal BOLD increase . | Pre-ictal BOLD decrease . |
|---|---|---|---|---|
| 1 | Highly consistent localization to left low central region | Ipsilateral IFG, insula, frontal pole | Ipsilateral frontal lobe | None |
| 2 | Most likely right frontal pole | Ipsilateral frontal pole | Contralateral frontal lobe and temporal lobe | Ipsilateral anterior frontal lobe |
| 3 | Suggestion for localization to right central region or SSMA | Contralateral perisylvian | Contralateral frontal lobe and caudate | None |
| Patient . | Electroclinical and structural MRI localization . | Ictal SPECT hyperperfusion (cortical sites) . | Pre-ictal BOLD increase . | Pre-ictal BOLD decrease . |
|---|---|---|---|---|
| 1 | Highly consistent localization to left low central region | Ipsilateral IFG, insula, frontal pole | Ipsilateral frontal lobe | None |
| 2 | Most likely right frontal pole | Ipsilateral frontal pole | Contralateral frontal lobe and temporal lobe | Ipsilateral anterior frontal lobe |
| 3 | Suggestion for localization to right central region or SSMA | Contralateral perisylvian | Contralateral frontal lobe and caudate | None |
IFG = inferior frontal gyrus.
Discussion
We have shown that highly significant BOLD signal changes occur prior to seizure onset in three patients with drug-refractory partial epilepsy. This supports the concept of a pre-ictal state.
In Patient 1, a striking pre-ictal BOLD increase was seen in the hemisphere ipsilateral to the seizure focus. The site of the BOLD increase was congruent with the seizure focus localization based on the other epilepsy investigations performed in this patient. Intra-operative findings, as well as the presence of balloon cell focal cortical dysplasia on histological examination, further indicate that the area of BOLD signal increase reflects the seizure focus. This observation is consistent with two previous studies showing increases in cerebral blood flow ipsilateral to the seizure focus prior to seizure onset measured by SPECT (Baumgartner et al., 1998) or bitemporal surface cerebral blood flow (Weinand et al., 1997). Our findings are also consistent with our previous observation of a boy with Rasmussen's encephalitis (Jackson et al., 1994) where BOLD signal increases in the affected hemisphere occurred up to 120 s before clinical seizure phenomena. It is likely that, in this case, the BOLD changes occurred long before the electroclinical onset, as previous VEM showed that identifiable discharges occurred <30 s before seizures. Our study is also consistent with a previous study of an adult female with a small right central malignant glioma, where a localized BOLD signal increase was seen in the edge of the lesion 65 s before foot movements indicating seizure activity (Krings et al., 2000). A BOLD signal decrease was seen 35 s later in cortex between the lesion and the foot motor area. Thus, both of these studies are consistent with our observation of significant BOLD signal changes occurring before clinical seizure onset.
Patient 2 had widespread pre-ictal BOLD signal increases contralateral to the presumed seizure focus, which the time course analysis indicates were present long before the seizure onset (Fig. 2C). One possible explanation of the pattern of changes is that the BOLD increase represents an increase in overall synaptic activity at sites that have inhibitory projections to the seizure focus itself (i.e. contralateral inhibition via callosal projections). While the large BOLD signal increase in the hemisphere contralateral to the presumed seizure focus may reflect areas involved in seizure generation, we suggest that the activation of the contralateral side may alternatively represent an active attempt to impede the transition from interictal to ictal activity at the seizure focus. The concept of distant inhibition is known in visual and other systems (Angelucci et al., 2002). The fact that the pre-ictal BOLD signal decreases near the presumed seizure focus supports the idea of suppressed pre-ictal neuronal activity in this area, which presumably requires an active inhibitory process in other brain regions. BOLD signal and its relationship to excitatory and inhibitory processes is known to be complex (Logothetis et al., 2001; Arthurs and Boniface, 2002; Laurienti, 2004).
Although Patient 3 was felt to have focal epilepsy, localization of the seizure focus sufficient to proceed with surgical evaluation was not possible. The best evidence of localization was the left hand sensory change at the seizure onset with later left hand dystonia and right paracentral interictal discharges. The pre-ictal fMRI suggested a BOLD signal increase involving mainly the left frontal lobe and subcortical areas including the left caudate. Interestingly, these pre-ictal fMRI changes, as well as ictal SPECT (Fig. 3) and PET changes, are compatible with a left frontal abnormality, which would be contralateral to the focus localization based on seizure symptomatology. We speculate that the clinical features at seizure onset may relate to the BOLD signal changes in the caudate. The caudate nucleus has strong connections with the SSMA, where seizures often produce ipsilateral sensory symptoms and dystonic posturing. It is therefore tempting to speculate that these left frontal or subcortical areas do actually represent the epilepsy network in this complex and unsolved patient.
The observed pre-ictal BOLD signal changes are assumed to reflect neuronal changes. However, it is possible in the context of human epilepsy that other processes such as glial, ionic or neurovascular processes may be regionally altered in a way that helps to initiate or at least facilitate the process of the transition to seizures. Furthermore, seizure generation is likely to take place over minutes to hours. The fact that different approaches to observing a pre-ictal state show different times of changes (minutes to hours) suggests multiple mechanisms occurring over different stages in ictogenesis and that no specific method depicts the timing of all events. It has been suggested that seizure precursors may begin locally as a series of rhythmic discharges. As the discharges become more frequent, adjacent or distant projection sites (cortical or subcortical) become recruited, increasing the amount of energy (Litt et al., 2001) and heralding oncoming seizures.
We have shown that BOLD fMRI signal changes can occur many minutes before the onset of seizures. This supports the presence of a pre-ictal state. The three cases presented suggest that the fMRI signals, and presumably the underlying neuronal changes that occur pre-ictally, are complex. We suggest that they are likely to reflect a combination of excitation and inhibition. BOLD fMRI signal change may reflect both brain regions involved pathologically in the generation of the seizure, as well as healthy brain regions that suppress the biochemical and electrical cascade that leads to seizures. Our data demonstrate that there are major BOLD signal changes in the lead up to a seizure event in areas that include, but are not limited to, the seizure focus. The relationship of these BOLD signal changes to seizure generation, and how these data might help to localize the ‘seizure focus’ for surgical treatment is clearly complex, yet these data offer exciting new insights into the pre-ictal events that lead to a seizure.
We wish to thank the patients and their families for participating in the study. We also wish to thank the EEG technologists at Austin Health for assistance with the EEG aspects of this study. We are grateful to Neurosciences Victoria (NSV), the National Health and Medical Research Council (NHMRC) and the Brain Imaging Research Foundation, Australia for financial support. P.F. held a Clinician Scientist Award from the Canadian Institutes of Health Research and a Clinical Fellowship from the Alberta Heritage Foundation for Medical Research while involved in the study at the Brain Research Institute, Australia.
References
Abbott DA, Jackson GD. iBrain—software for analysis of visualization of functional MR images.
Angelucci A, Levitt JB, Lund JS. Anatomical origins of the classical receptive field and modulatory surround field of single neurons in macaque visual cortical area V1.
Archer JS, Briellman RS, Abbott DF, Syngeniotis A, Wellard RM, Jackson GD. Benign epilepsy with centro-temporal spikes: spike triggered fMRI shows somato-sensory cortex activity.
Arthurs OJ, Boniface S. How well do we understand the neural origins of the fMRI BOLD signal?
Baumgartner C, Serles W, Leutmezer F, Pataraia E, Aull S, Czeck T, et al. Preictal SPECT in temporal lobe epilepsy: regional cerebral blood flow is increased prior to electroencephalography-seizure onset.
Delamont RS, Julu PO, Jamal GA. Changes in a measure of cardiac vagal activity before and after epileptic seizures.
Elger CE, Lehnertz K. Seizure prediction by non-linear time series analysis of brain electrical activity.
Jackson GD, Connelly A, Cross JH, Gordon I, Gadian DG. Functional magnetic resonance imaging of focal seizures.
Katz A, Marks DA, McCarthy G, Spencer SS. Does interictal spiking change prior to seizures?
Krings T, Topper R, Reinges MD, Foltys H, Spetzger U, Chiappa KH, et al. Hemodynamic changes in simple partial epilepsy: a functional MRI study.
Laurienti PJ. Deactivations, global signal and the default mode of brain function.
Lehnertz K, Elger CE. Spatio-temporal dynamics of the primary epileptogenic area in temporal lobe epilepsy characterized by neuronal complexity loss.
Litt B, Esteller R, Echauz J, D'Alessandro M, Shor R, Henry T, et al. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients.
Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal.
Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation.
Author notes
1Brain Research Institute, Heidelberg West, Departments of 2Medicine and 3Radiology, The University of Melbourne and 4Department of Neurology, Royal Children's Hospital, Parkville, Victoria, Australia and 5Department of Clinical Neurosciences, University of Calgary, Alberta, Canada