-
PDF
- Split View
-
Views
-
Cite
Cite
Michael A. Cant, Jeremy Field, Helping effort in a dominance hierarchy, Behavioral Ecology, Volume 16, Issue 4, July/August 2005, Pages 708–715, https://doi.org/10.1093/beheco/ari051
Close - Share Icon Share
Abstract
In many cooperatively breeding species, group members form a dominance hierarchy or queue to inherit the position of breeder. Models aimed at understanding individual variation in helping behavior, however, rarely take into account the effect of dominance rank on expected future reproductive success and thus the potential direct fitness costs of helping. Here we develop a kin-selection model of helping behavior in multimember groups in which only the highest ranking individual breeds. Each group member can invest in the dominant's offspring at a cost to its own survivorship. The model predicts that lower ranked subordinates, who have a smaller probability of inheriting the group, should work harder than higher ranked subordinates. This prediction holds regardless of whether the intrinsic mortality rate of subordinates increases or decreases with rank. The prediction does not necessarily hold, however, where the costs of helping are higher for lower ranked individuals: a situation that may be common in vertebrates. The model makes two further testable predictions: that the helping effort of an individual of given rank should be lower in larger groups, and the reproductive success of dominants should be greater where group members are more closely related. Empirical evidence for these predictions is discussed. We argue that the effects of rank on stable helping effort may explain why attempts to correlate individual helping effort with relatedness in cooperatively breeding species have met with limited success.
In eusocial and cooperatively breeding groups, reproduction at any moment in time is monopolized to some degree by one or several individuals. In many taxa, however, including all eusocial vertebrates and many insects, nonbreeding or subordinate group members are potentially capable of reproduction (Ross and Matthews, 1991; Stacey and Koenig, 1990). These subordinates may have a good chance of eventually inheriting a breeding position (Alexander et al., 1991; Bourke, 1999; Field et al., 1999), and recent theory has strongly emphasized the importance of these future direct benefits in maintaining helping (Clutton-Brock, 2002; Kokko and Johnstone, 1999; Kokko et al., 2001; Ragsdale, 1999; Shreeves and Field, 2002). Group members commonly form a dominance hierarchy or queue in which rank is based on size or fighting ability (Buston, 2003) or some convention such as age (Cant, 2000; Shreeves and Field, 2002), and subordinates can inherit the breeding position if they outlive those ahead of them in the queue (Field et al., 1999; Hughes and Strassmann, 1988; Samuel, 1987; Strassmann and Meyer, 1983, in Hymenoptera; and Creel and Waser, 1994; Emlen, 1991; Stacey and Koenig, 1990; Wiley and Rabenold, 1984, in vertebrates).
Despite the near-ubiquity of dominance hierarchies in cooperatively breeding groups, few models of social behavior take into account the effect of dominance rank on the costs and benefits of cooperative behavior. For example, a conspicuous and universal feature of these animal societies is the great variation that exists between individuals in helping effort. Most attempts to understand this variation start by assuming (implicitly or otherwise) that the costs and benefits of helping are the same for all individuals, in which case kin-selection theory predicts that individuals should work harder to rear more closely related offspring (Emlen, 1991; Grafen, 1984). This approach has produced mixed results in vertebrates (Clutton-Brock et al., 2000). In social insects, the relationship between relatedness and helping effort has rarely been examined (Queller et al., 2000), but helpers may often be unable to discriminate relatedness at the individual level (Keller, 1997; Queller et al., 1990; Strassmann et al., 1997). In general, it seems that factors other than relatedness must be invoked to explain the considerable variation in work rate that exists among helpers (Cant and Field, 2001; Clutton-Brock et al., 2000; Kokko et al., 2001).
The lack of a consistent correlation between relatedness and helping effort is perhaps not surprising when one considers that the presence of a dominance hierarchy erects systematic differences between group members in their potential for future reproduction, and thus in the amount of direct fitness they stand to lose through performing costly helping behavior. Recently, Cant and Field (2001) developed a multiplayer kin-selection model to explore how helping effort should vary with an individual's inheritance rank, that is, her position in the queue to inherit breeding status. Their model was based on the idea that subordinates face a trade-off because current investment in help reduces the subordinate's future reproductive success. The model made two predictions. First, helpers with a greater expectation of direct reproduction in the future, for example those of higher inheritance rank, should work less hard for the dominant. Second, where productivity increases with group size, subordinates of a given rank should work less hard in larger groups because the payoff from inheritance is greater in a larger, more productive group.
Here, we extend the approach of Cant and Field (2001) in several ways to examine the robustness of their model to more realistic assumptions and to generate new, testable predictions about the influence of dominance rank on helping effort.
(1) We define explicitly the form of costs. Cant and Field (2001) assumed that helping led to a decline in the future reproductive value of the helping individual, but the direct fitness of other subordinates was unaffected. This situation would arise, for example, where helping depleted personal resources that an individual could otherwise have saved for future reproduction. In many animals, however, the main cost of helping is probably reduced survivorship (O'Donnell and Jeanne, 1992; Schmid-Hempel, 1998; Schmid-Hempel and Wolf, 1988). Reduced survivorship not only decreases the chance that a helper will itself survive to inherit a breeding position but simultaneously increases the chance that other individuals further down the queue will inherit. This effect is explored in the current model.
(2) We incorporate a link between productivity and the total amount of help received. A previous model (Cant and Field, 2001) assumed that the breeder's payoff was a linear function of group size and did not depend on subordinate helping effort. Here we make the more plausible assumption that the fitness of the breeder depends on collective helping effort. Note, however, that our model considers only kin-selected benefits of helping and not potential future direct benefits such as those associated with “group augmentation” (Kokko et al., 2001).
(3) We allow the mortality rate of subordinates to vary between ranks. Where position in a social queue is based on age, older, higher ranked individuals may suffer elevated intrinsic mortality because of the effects of ageing (Rose, 1991). In other cases, the opposite pattern may hold, with high-ranked subordinates enjoying reduced mortality because they have better access to resources or safe refuges (Balshine-Earn et al., 1998). We explore both these possibilities.
(4) We allow for varying costs of help. Recently, several authors have suggested that individual variation in the costs of help may underlie individual variation in work rate among subordinates. In vertebrates, for example, and perhaps in the mother-offspring groups of some social insects, younger, smaller animals may be less able to bear the costs of a given level of help than their older group mates (Boland et al., 1997; Clutton-Brock et al., 2000; Heinsohn and Legge, 1999). This is potentially important because factors such as size or quality, which influence the ability of an individual to bear the costs of helping, may also determine its rank. We examine queues in which the higher ranked individuals suffer lower costs for a given level of help.
THE MODEL
We focus on a group of n individuals comprising a single dominant breeder (the rank 1 individual) and (n − 1) subordinate helpers who form a linear hierarchy or queue to inherit the position of dominant. Group members are symmetrically related by the coefficient of relatedness r. The hierarchy is a strict queue, meaning that members of the queue ascend in rank only when an individual in front of them dies. New individuals are recruited at the bottom of the queue whenever there is a death, so that group size remains constant over time.
All members of the group can invest in the dominant's offspring. Investment by the dominant is termed “parental investment” and is denoted by h1. Investment by the subordinates is termed “help” or “helping effort”: the helping effort of the ith-ranked subordinate is denoted hi. We assume that a dominant's direct fitness is proportional to her own investment plus the total investment that she receives from her subordinates. This establishes the coupling between the corporate level of investment and group productivity.
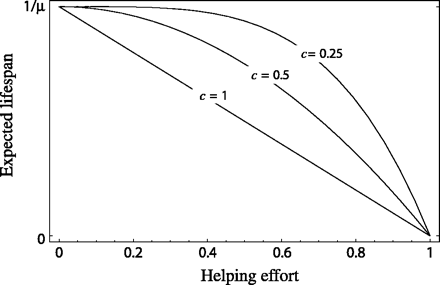
Here, the parameter μi denotes the mortality rate of an individual who provides zero help; this will be referred to as the “basal” mortality rate of an individual at that rank. Note that the expected lifespan of the subordinate at rank i is simply the reciprocal of its mortality rate, 1/mi. The parameter c is a measure of the survival cost of helping (0 < c ≤ 1 ). Low values of c indicate that helping is cheap, with mortality rate rising relatively slowly as helping effort increases. By contrast, high values of c indicate that helping is relatively costly, so that mortality rate increases rapidly as h increases. Function 1 implies that mortality rate approaches infinity (and thus expected lifespan, 1/m, approaches zero) as h approaches 1. The relationship between expected lifespan and helping effort is plotted in Figure 1 for three values of the parameter c.
Assumed function relating helping effort to expected lifespan (the inverse of instantaneous mortality rate m). The parameter c measures the costliness of helping.
We wish to determine the evolutionarily stable levels of effort invested by each group member. Consider, then, a population in which individuals invest effort levels ĥ1,ĥ2…ĥn, and suppose that a mutant strategy arises in this population which specifies some alternative effort level hi (≠ĥi) for individuals of rank i (i ≠ 1; the effort of the dominant is considered separately below). We use the logic of Frank's direct fitness method to take into account effects of kinship on the level of helping in the group (Frank, 1995, 1998; Taylor and Frank, 1996). The direct fitness of a focal mutant individual at rank i will differ from that of a typical ith-ranked subordinate in two ways. First, adopting the mutant strategy will change the probability that the mutant survives to inherit the breeding position. Second, because of relatedness, a focal mutant that inherits the breeding position will have an elevated probability of herself being helped by a mutant at rank i. Specifically, on accession there will be a probability r that our focal mutant receives help level hi rather than ĥi from her ith-ranked helper.
To obtain solutions for a group of a given size n we use Expressions 3 and 4 to derive analytical expressions for ∂wi/∂hi for i = 1 to n, set these equal to zero, and solve simultaneously using numerical methods in Mathematica 4.2 (Wolfram Research Inc., Champaign, IL). The graphical results for representative parameter values are presented below.
RESULTS
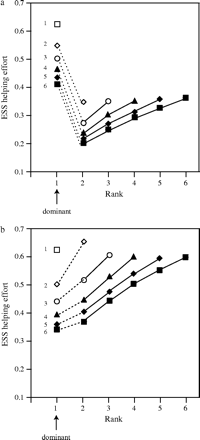
Figure 2 shows the results of the model for two values of relatedness among group members. A number of predictions are evident. First, the evolutionarily stable levels of helping effort are higher for subordinates of lower rank. This is because high-ranking subordinates have a relatively high chance of inheriting dominant status and, therefore, have more to lose by investing in helping effort, which reduces their survival. By contrast, low-ranked subordinates have relatively little chance of breeding in the future, and so their inclusive fitness is maximized by investing effort in helping the current dominant to rear offspring. Note that our model predicts that the effort of the dominant may be higher or lower than that of her subordinates, depending on relatedness and the rank of the subordinate.
Evolutionarily stable levels of investment at each rank for two values of relatedness: (a) r = .1; (b) r = .5. Numbers on the left of the plotted points denote group size. In each group size, investment levels for dominants (rank 1 individuals) are shown connected by a dotted line to those for subordinates (connected points) because the expression for the dominant's direct fitness differs from the general direct fitness expression used to solve for the subordinate's stable helping levels. Other parameters c = 0.5, μ = 0.1.
A second result is that the helping effort of individuals of a given rank is lower in larger groups. This is because a subordinate in a large group can expect greater investment (and greater overall productivity) on inheriting the breeding position, and so she should be less willing to invest in rearing the current dominant's offspring at the expense of her chances of surviving to realize this productivity. Third, comparison of Figure 2a,b shows that subordinates should invest at greater levels where relatedness is high because the inclusive fitness benefit of a given level of effort increases with relatedness between group members. Finally, the model predicts that the investment level of the dominant in multimember groups is inversely related to the degree of relatedness among group members. This is because the dominant can take advantage of the increased help in high-relatedness groups by reducing her own level of investment. This compensation is only partial, however, as total investment increases with relatedness for all group sizes (see below).
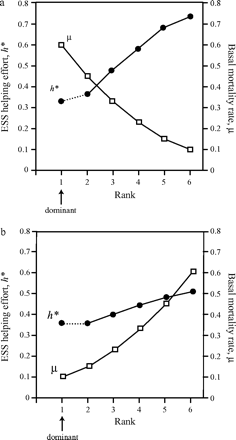
Figure 3a,b shows the effect of varying basal mortality rates (i.e., the inverse of basal lifespan) on the evolutionarily stable levels of help. In Figure 3a, basal mortality decreases down the hierarchy. This pattern might be expected, for example, where group members form an age-based queue to inherit dominance. Assuming senescence, older, higher ranked individuals will exhibit higher basal mortality rates by definition (Rose, 1991). In Figure 3b, basal mortality increases down the hierarchy, which may apply to situations in which high rank confers access to superior resources or a safer environment. It can be seen that helping effort among subordinates increases down the hierarchy whether basal mortality increases or decreases with rank. This result holds for all the relationships between basal mortality and rank that we tested.
Stable levels of investment in a group of size 6 where basal mortality rate μ varies with rank. (a) Basal mortality decreases down the hierarchy and (b) basal mortality increases down the hierarchy. Open squares show basal mortality rate at each rank; closed circles show stable helping effort at each rank. Other parameters r = .5, c = 0.5.
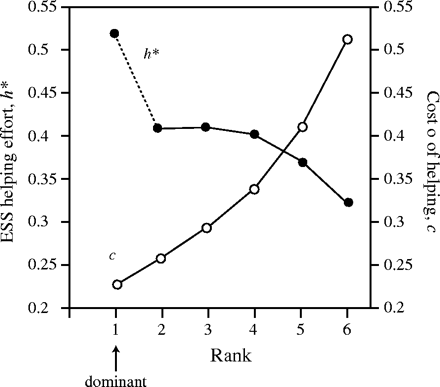
The above results are obtained assuming that the costs of helping are independent of rank. In many cases, however, the costs of helping may be higher for lower ranked individuals (Heinsohn and Legge, 1999). Figure 4 shows the evolutionarily stable levels of helping effort for individuals of different rank when the cost of helping increases down the hierarchy. Note that the effect of rank on helping effort has been reversed: stable helping effort is lower for individuals of lower rank. Whether this happens depends on how steeply the cost of helping increases with decreasing rank. This means that the prediction that lower ranked subordinates should work harder, while being robust to changes in the assumptions concerning group size, relatedness, and basal mortality rates, does not necessarily hold for systems in which higher ranked subordinates experience lower costs for a given level of help.
Stable levels of investment in a group of size 6 where the cost of helping c (=1/q) is higher for individuals of lower rank. Open squares show cost of helping at each rank; closed circles show stable helping effort at each rank. Other parameters r = .5, μ = 0.1.
Figure 5 shows the effect of these patterns of helping effort on the relationship between group size and productivity for three values of relatedness. Total productivity (measured as the sum of the evolutionarily stable strategy levels of investment) increases with group size, and the productivity of groups of a given size is higher for higher values of relatedness. This is because subordinates are selected to invest more when they are closely related to the dominant compared to when they are less closely related.
Total productivity (measured as
DISCUSSION
Below we summarize the main predictions of the model and briefly review relevant empirical data from cooperatively breeding vertebrates and social insects.
Prediction 1: lower ranked group members should work harder to rear the offspring of the dominant than higher ranked group members
This prediction arises because high-ranking subordinates, who have a relatively high expectation of inheriting the position of breeder, stand to lose more direct fitness through helping compared with low-ranking subordinates. The prediction holds across all values of relatedness for which subordinates are selected to provide at least some help. It also holds irrespective of the relationship between basal mortality and rank. The only parameter in the model that affects this relationship between rank and helping effort is the cost of helping: where higher ranked subordinates suffer lower costs for a given level of help, they may be selected to work at higher rates than those further down the queue.
Evidence from several eusocial hymenopterans supports the prediction that higher ranked subordinates should work less hard. Cant and Field (2001) measured foraging effort in Polistes dominulus and then removed successive dominants to determine directly the order in which subordinates inherited the vacant breeding position. As predicted, those wasps that helped least were most likely to inherit the breeding position after the removal of the dominant. In Ropalidia marginata, wasps that became dominant after queen removal had previously spent significantly less time away from the nest than other wasps (Chandrashekara and Gadagkar, 1992). In other Hymenoptera, individuals with higher social ranks, as deduced from behavioral interactions, also spend less time off the nest, but it is not known whether social ranks equate with inheritance ranks (references in Ratnieks and Reeve, 1992; but see Strassmann and Meyer, 1983, for a counterexample). These hymenopterans are good candidates to test predictions concerning the effect of rank on helping effort because individuals may be unable to discriminate degrees of relatedness among nest mates (Keller, 1997; Queller et al., 1990; Strassmann et al., 1997), and the costs of helping (primarily, risk of predation during foraging) are unlikely to vary with rank (Cant and Field, 2001). Note that our model predicts that where relatedness is high the level of investment of dominants will be lower than that of subordinates, which fits well with evidence from social wasps that dominants rarely forage to provision young (Cant and Field, 2001; Field et al., 1998, 2000; Reeve, 1991).
Among cooperatively breeding vertebrates, the picture is less clear. In naked mole rats (Heterocephalus glaber), higher ranked subordinates work less hard, in agreement with the model, but this result could also arise because these individuals tend to be less closely related to the dominant than lower ranked helpers (Clarke and Faulkes, 1997; Reeve, 1992). There is no correlation between the social rank of subordinates and helping effort in some bird species (bicolored wren: Rabenold, 1984; Arabian babblers: Wright, 1997), whereas in other species older and presumably higher ranked subordinates help more than their younger group mates (suricates: Clutton-Brock et al., 2001; white-winged choughs: Heinsohn and Cockburn, 1994; white-throated magpie-jays: Langen and Vehrencamp, 1999).
Our model suggests that these conflicting patterns in vertebrates are probably due to variation in the cost of helping among individuals of different rank rather than variation in the age of helpers. The importance of varying costs has been highlighted by several recent studies showing that experimental feeding of subordinates leads to an increase in helping effort (suricates: Clutton-Brock et al., 2000; white-winged choughs: Boland et al., 1997; Arabian babblers: Wright et al., 2001). In vertebrates, higher ranked animals are frequently larger, in better condition, or more experienced, and will suffer lower unit costs of helping compared to individuals of low rank (Heinsohn and Legge, 1999). In such circumstances, our model suggests that the effect of rank on helping effort can be counteracted by the effect of variation in costs. Attempts to test the predicted effect of rank on helping effort in vertebrates will first need to control for differences between ranks in the cost of help.
Prediction 2: helpers of a given rank should work less hard in larger groups
This prediction arises because subordinates in larger groups receive a larger direct fitness payoff on inheriting the position of breeder. In our models this result holds because individuals who die are replaced, and thus group size remains constant over time. The consequences of relaxing this assumption are discussed in a later section.
In their study of helping effort in P. dominulus, Cant and Field (2001) found that subordinates of a given inheritance rank worked significantly less hard in larger groups, as predicted. Similar data is lacking for other social insects. In cooperatively breeding vertebrates, individual helping effort is often lower in larger groups (Clutton-Brock et al., 2000, 2001; Gilchrist, 2001; Rabenold, 1984; Wright, 1997). It is possible, however, that this result reflects the greater need for help in small groups (Heinsohn and Legge, 1999). This effect is not addressed in the current model, in which the benefit of a given level of help is the same regardless of group size. To test whether subordinates help less in larger groups because of their greater expectation of direct fitness in the future, further studies might use manipulations of both brood size and group size in order to change the payoff of inheriting while keeping the need for help constant.
Prediction 3: total productivity will increase with relatedness between group members
Controlling for group size, groups of close relatives are predicted to be more productive than those comprising less closely related individuals. This is because total helping effort by subordinates is greater where relatedness is high. Surprisingly, few studies have examined the relationship between productivity and relatedness while controlling for group size. The best evidence comes from the study of Langer et al. (2004), who managed to create two-female groups of high and low relatedness in a social bee (Exoneura nigrescens). They found that total productivity was higher in groups of high relatedness and suggested that this was because related breeders invested less in a “tug-of-war” over reproduction. The current model suggests an alternative interpretation: high-relatedness groups are more productive because total investment is higher among closely related individuals. This highlights a key shortcoming of models of reproductive skew, namely that grouping benefits depend only on the presence or absence of subordinates, and not on their helping effort (Cant and Reeve, 2002).
In cofoundress associations of P. dominulus, Queller et al. (2000) found no clear relationship between relatedness and productivity, although other variables were apparently not controlled for. In vertebrates, a number of studies have found that helping effort is positively correlated with relatedness (Curry, 1988; Komdeur, 1994; Owens DD and Owens MJ, 1984; Reeve, 1992; Reyer, 1984; but see Clutton-Brock et al., 2000 for counterexamples), but none of these studies control for group size. Two studies that were able to control for group size found no association between helping effort and kinship (Clutton-Brock et al., 2000; Emlen and Wrege, 1988). Neither of these studies examined the relationship between kinship and productivity directly. Clearly, additional analyses and/or more data are required to test this prediction of the model.
Limitations of the model
In the interests of tractability we have made a number of simplifying assumptions. Perhaps the most unrealistic of these is that group size does not change over time. That is, as soon as an individual in the hierarchy dies, an individual of equal relatedness joins the bottom of the queue. In effect, we are assuming that there is an unlimited pool of equally related, “floating” group members, each of whom stands to benefit from the death of a group member as this will open up the opportunity to join the bottom of the queue. In our model, the magnitude of this potential indirect benefit of dying in the queue is independent of rank, and so we do not expect it to influence our main results. Our results would be affected, however, if relatedness varies systematically with rank, as occurs in some cooperatively breeding species (Creel and Waser, 1997). Relatedness may increase or decrease down the queue, depending on the pattern of dispersal. For example, where new recruits join from outside the group, a dominant may be most closely related to high-ranking subordinates. Conversely, where joiners are the dominant's offspring, relatedness may be highest with those at the bottom of the queue. We also ignore the population consequences of helping effort. For example, a single unit of helping effort is assumed to bring a fixed fitness benefit irrespective of group size, whereas in reality the rewards of helping will depend on the dispersal or recruitment options of the resulting offspring, which depends on current group size relative to that of other groups. To investigate the impact of group size on helping effort more fully, we would need to model density dependence and other population processes explicitly (Pen and Weissing, 2000).
One consequence of our assumption of constant group size is that subordinates cannot gain group augmentation benefits from helping (Kokko et al., 2001). These benefits arise when helping increases the probability of recruiting others into the group, thus ensuring that a subordinate inherits a large, productive group in future. Kokko et al. (2001) developed a dynamic model to evaluate the relative strength of group augmentation and kin selection as mechanisms promoting the evolution of helping behavior. Their results indicate that in the absence of group augmentation, helping effort will be higher in lower ranked subordinates, in agreement with our results. Given sufficient group augmentation benefits of helping, however, this result is reversed, with the helping effort of the rank 2 subordinate exceeding that of the rank 3. This is because a rank 2 subordinate, while more sensitive to the negative impact of helping effort on its probability of inheritance, is nevertheless more likely to reap the future benefits of current offspring production. Group augmentation benefits can, therefore, erode the differences in future direct fitness that drive our results. Such benefits will be most important for animals in long-lived stable breeding groups, such as those of many cooperatively breeding vertebrates, providing an alternative explanation for the absence of a clear relationship between rank and helping effort in these species.
Conclusion: dominance hierarchies and individual behavior
Dominance hierarchies have been studied in a wide range of social animals. This usually involves recording the directionality of threatening or aggressive behaviors, or the outcome of competition for resources, for each pair of individuals in the group. Group members are then ordered according to a criterion such as minimization of the number of reversals, and statistical tests can be used to determine whether directionality is more consistent than expected by chance (Appleby, 1983). The possibility of inheritance, however, suggests that an alternative method of ordering group members is according to their positions in the queue to inherit. These inheritance ranks are likely to be correlated with social ranks, but the correlation may not be perfect (Monnin and Peeters, 1999). Other things being equal, the ratio of expected direct to indirect fitness will be larger for higher ranked individuals. Moreover, our model indicates that differences in this ratio are more pronounced toward the front of the queue. These two features of social queues may have important implications for other forms of social behavior, such as aggression (Cant and Johnstone, 2000). For example, one might expect aggression between adjacent ranks to become more pronounced toward the front of a queue, where the payoff from a reversal in ranks is greatest. This topic could be addressed in future modeling efforts.
Studies of kin selection that focus exclusively on genetic relatedness offer little insight into why apparently equivalent individuals should vary so much in their propensity to aid relatives or, indeed, any other forms of social behavior (Cant and Field, 2001). We believe that a major cause of this variation between individuals is that in the vast majority of primitively eusocial or cooperatively breeding groups, animals are effectively queuing for the chance to reproduce directly. The presence of an inheritance hierarchy erects consistent, systematic differences between group mates in the direct fitness that they stand to gain, or lose, in their interactions with other members of the group, other conspecifics, and predators. These differences between ranks in future fitness may prove to be a more important influence on social behavior than the criteria, such as age, size, or fighting ability, by which a hierarchy is formed in the first place.
Financial support was provided by a Royal Commission for the Exhibition of 1851 Research Fellowship and a Royal Society University Research Fellowship (to M.A.C.) and a NERC grant (to J.F.). Thanks to Rufus Johnstone, David Queller, Dik Heg, and an anonymous referee for generous help and insightful comments.
References
Alexander RD, Noonan KM, Crespi BJ,
Balshine-Earn S, Neat FC, Reid H, Taborksy M,
Boland CRJ, Heinsohn R, Cockburn A,
Bourke AFG,
Cant MA, Field J,
Cant MA, Johnstone RA,
Chandrashekara K, Gadagkar R,
Clarke FM, Faulkes CG,
Clutton-Brock TH,
Clutton-Brock TH, Brotherton PNM, O'Riain MJ, Griffin AS,
Clutton-Brock TH, Brotherton PNM, O'Riain MJ, Griffin AS, Gaynor D, Kansky R, Sharpe L, McIlrath M,
Creel SR, Waser PM,
Creel SR, Waser PM,
Curry RL,
Emlen ST,
Emlen ST, Wrege PH,
Field J, Shreeves G, Sumner S,
Field J, Shreeves G, Sumner S, Casiraghi M,
Field J, Solis CR, Queller DC, Strassmann JE,
Frank SA,
Gilchrist JS,
Grafen A,
Heinsohn R, Cockburn A,
Hughes CR, Strassmann JE,
Keller L,
Kokko H, Johnstone RA,
Kokko H, Johnstone RA, Clutton-Brock TH,
Kokko H, Sutherland WJ,
Komdeur J,
Langen TA, Vehrencamp SL,
Langer P, Hogendoorn K, Keller L,
Monnin T, Peeters C,
O'Donnell S, Jeanne RL,
Queller DC, Zacchi F, Cervo R, Turillazzi S, Henshaw MT, Santorelli LA, Strassmann JE,
Pen I, Weissing FJ,
Rabenold KN,
Ragsdale JE,
Ratnieks FLW, Reeve HK,
Reeve HK,
Reeve HK,
Reyer HU,
Samuel CT,
Schmid-Hempel P, Wolf T,
Stacey PB, Koenig WD (eds),
Strassmann JE, Klingler CJ, Arevalo E, Zacchi F, Husain A, Williams J, Seppa P, Queller DC,
Strassmann JE, Meyer DC,
Wiley RH, Rabenold KN,
Wright J,
Author notes
aDepartment of Zoology, University of Cambridge, Downing Street, Cambridge CB2 3EJ, UK
bDepartment of Biology, University College London, Wolfson House, 4 Stephenson Way, London NW1 2HE, UK



![Total productivity (measured as \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \({\sum}_{i{=}1}^{n}{\hat{h}}_{i}\) \end{document}) versus group size, where group members adopt the evolutionarily stable levels of helping effort \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(h_{j}^{{\ast}},\) \end{document} for three values of relatedness. Other parameters c = 0.5, μ = 0.1.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/beheco/16/4/10.1093_beheco_ari051/2/m_behecoari051f05_lw.gif?Expires=1716341411&Signature=uR4fwQdiQcoM3HutHG3zJt2n9vxsCWuhOt--JOeVVTUnJIVK1zZI5dmQXGdLcg4JbgUs2-QZotsOOxlHbXmYLh-19agyciarhfvRVC6XRmDXQDxkNaXisvsT8q3nLWftIkdqxLxj0obziVTfAfZXR99KBNKKYRqyGUALqiHYNtwjnOs7l-Bwb8Iw5OD0tNZU0LPAszW8HsnXU8Q0dFJH3cBs5kamTWqr5iA7-UpEoze~C~I41OQ1W90THy6Xq4IveT~qnAClw9ElvBjysdUyUTw-8J1-IQfTTwT9eLlqHkPnkWCIEMEHhbz1O~EBrY2zvPqexPNhfBCE-tiRl-MaIA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
