-
PDF
- Split View
-
Views
-
Cite
Cite
Teruhiko Takahara, Yukihiro Kohmatsu, Atsushi Maruyama, Hideyuki Doi, Hiroki Yamanaka, Ryohei Yamaoka, Inducible defense behavior of an anuran tadpole: cue-detection range and cue types used against predator, Behavioral Ecology, Volume 23, Issue 4, July-August 2012, Pages 863–868, https://doi.org/10.1093/beheco/ars044
Close - Share Icon Share
Abstract
Inducible behavioral defense in response to predator cue detection is a key phenomenon in predator–prey interactions. The mechanisms by which prey use chemical/visual cues to avoid predation remain little known. We hypothesized that the distance at which prey species detect predator cues would be related to avoiding detection by the predator. To test this hypothesis, we performed laboratory experiments using an anuran tadpole (Hyla japonica) and a predatory dragonfly nymph (Anax parthenope julius). Tadpole activity level was reduced as a function of exposure to chemical cues from the dragonfly predator, but activity levels did not change when tadpoles were exposed to potential visual cues from the dragonfly. The distances over which tadpoles detected predator cues were longer than those over which the dragonfly predator detected tadpoles. The differences in cue-detection ranges between tadpoles and dragonfly predators are related both to predator avoidance by tadpoles and effective foraging strategies by dragonfly predators. Chemical cue detection as a trigger of inducible defense by prey species may shape predator–prey relationships in aquatic habitats.
INTRODUCTION
Predator–prey interactions have often led to the evolution of inducible defense systems in prey species, including changes in morphology, life history, and behavior (reviewed in Tollrian and Harvell 1999). Inducible defenses are triggered by tactile, visual, and chemical cues associated with predator presence (reviewed in Dodson et al. 1994). Chemical cues used to trigger these inducible defenses are derived from alarm signals or urine directly from the prey under predation risk, metabolites and feces of the predator, or metabolites passing through the predator but originating in the prey (Petranka et al. 1987; Kiesecker et al. 1999; LaFiandra and Babbitt 2004; Richardson 2006; Schoeppner and Relyea 2009). The ability to detect chemical cues confers obvious potential benefits on prey individuals and populations by providing early warning of predator presence (e.g., Kats and Dill 1998; Van Donk 2007; Kishida et al. 2010; Schulte et al. 2011), but inducible defense responses to predators can be costly in terms of growth and survivorship (Van Buskirk and Schmidt 2000). For example, anuran larvae that alter their behavior and morphology have a reduced competitive impact on other species (Werner and Anholt 1996; Peacor and Werner 2000). Thus, the ability to detect chemical cues would have evolved to maximize prey fitness taking into account the cost–benefit balance of inducible defense.
Characteristics of predator chemical cues, particularly chemical concentration, can provide reliable information about predators to diverse prey animals, such as fish, amphibian larvae, snails, and water fleas (Von Elert and Pohnert 2000; Van Buskirk and Arioli 2002; Turner and Montgomery 2003; Kesavaraju et al. 2007). For example, the defensive behavior of Rana lessonae tadpoles was most responsive to the number of conspecifics killed by the dragonfly predator Aeshna cyanea (Van Buskirk and Arioli 2002). This report suggests that cue concentration is used by prey as an indicator of predation risk; an increase in the number of eaten conspecifics around prey provides an indication of threat, and the concentration of chemical cues is expected to increase with number of predation events. Cue concentration may also be related to the distance between predator and prey (Petranka and Hayes 1998; Turner and Montgomery 2003). As the distance between predator and prey decreases, predation risk is expected to increase and the concentration of cues exuded from the predator and experienced by the prey is expected to be higher. However, the relationship between distance to predators and chemical cues has not been carefully investigated.
Hitherto, the cue concentration is focused to consider the prey response against predation risk, but we assumed that the distance is an ultimate factor to predict the prey response and consider the adaptation of prey response to predation. Our aim was to estimate the detection range of predator chemical and visual cues by prey and consider benefits and evolutionary background to determine the range and utilization of chemical and visual cues with reference to the attacking range of the predator. For this purpose, we conducted laboratory experiments using an anuran tadpole species (Hyla japonica) and the predatory dragonfly nymph (Anax parthenope julius). This tadpole species exhibits reduced activity levels with detecting the chemical cue from a predator, and this response is an effective defensive behavior, as it reduces the detection by dragonfly predator (Takahara et al. 2008).
Tadpoles may evaluate chemical cues associated with their distance from nymphs, which should be highly correlated with detection probability. This speculation is expected because tadpoles that notice predators too slowly would be captured. Conversely, when predation risk is low because the predator is a long distance away, reductions in activity levels may decrease the opportunities for food acquisition.
To understand the effects of predator detection distance via chemical cues on the defensive responses of tadpoles, we tested the following 2 working hypotheses: 1) tadpoles exhibit priority use of chemical over visual cues for predator detection. Chemical cue is favorable information for prey species to monitor predation risk than visual or tactile cues (e.g., Stauffer and Semlitsch 1993; Kiesecker et al. 1996; Mathis and Vincent 2000) because aquatic habitats often greatly decrease prey visibility due to turbidity of water and dense vegetation, and 2) the intensity of defensive activity of a tadpole in response to chemical cues changes along with the distance from the dragonfly nymph. That is, the tadpoles would exhibit a stronger defensive response with decreasing of the distance between them and dragonfly nymph. The distance at which the tadpoles detect dragonfly chemical cues may be related to avoiding detection by the predator. The possible linkages between the intensity of defensive activity of prey by detecting chemical cues and prey detection range by the predator has not been well understood. We evaluated how the behavioral responses of the H. japonica tadpole change in response to potential chemical or visual cues according to its distance from the predatory nymph of A. parthenope julius. We also evaluated the distance required for detection of tadpoles by the nymph. Last, we discussed why the cue types and the ranges utilized by both prey and predator are different.
MATERIALS AND METHODS
Study animals and husbandry
Adult H. japonica frogs were collected with a hand net from several paddy fields in Kyoto City (35°04´ N, 135°44´ E) in July 2005. Frogs were kept as single male–female pairs in plastic cages (30 × 20 × 17 cm) with aged tap water (5 cm in depth), prepared by allowing the water to aerate overnight, in a laboratory at the Kyoto Institute of Technology. Four clutches of eggs were obtained the next day from 4 mating pairs; all adult frogs were subsequently released to their exact capture sites. After hatching, tadpoles from a mixture of the 4 clutches were kept in a 50-l plastic tank (73 × 40 × 33 cm) prior to the experiments. Tadpoles were fed powdered TetraFin and tablet TetraCorydoras (Tetra Werke GmbH, Melle, Germany) daily. Dragonfly nymphs (body length > 40 mm) were collected with a hand net from a pond in Otsu City (34°58´ N, 135°57´ E), about 18 km southeast of the sampling fields in Kyoto City. They were kept individually in plastic cups (8.5 cm in diameter, 8.5 cm high) perforated with holes (diameter < 5 mm), which were placed in 5-l plastic tanks. Each nymph was fed 2 tadpoles twice a week. All animals were maintained under a 16:8-h light/dark photoperiod at 25 ± 2 °C.
Experiment 1: tadpole responses to chemical cues from dragonfly nymphs
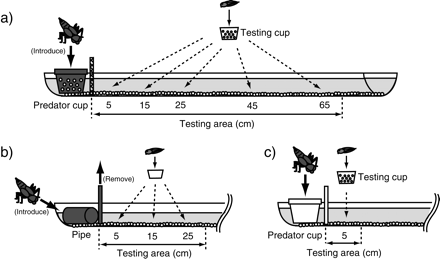
This experiment used an opaque plastic gutter (90 cm length, 10 cm width, and 5 cm height), containing 2 l of aged tap water and gravel substrate (depth: 5 mm) (Figure 1a). An opaque perforated plastic cup with a top cover (8.0 and 6.5 cm in diameter at the top and bottom, 4 cm in height, holes < 1 mm) was placed 10 cm from the side within the trough for introducing the dragonfly nymphs at the beginning of each trial (hereafter, “predator cup”). The testing area to which the tadpoles were introduced consisted of 5 sections at distances of 5, 15, 25, 45, and 65 cm from the predator cup. A transparent perforated plastic cup (8.0 and 6.5 cm in diameter at the top and bottom, respectively, 4 cm in height, holes < 1 mm; hereafter, “testing cup”) was placed at the center of each distance and served to contain the tadpole during the trials. The testing cup was utilized to orientate the tadpoles in each distance treatment. The areas to which the nymph and the tadpole were introduced were divided by a mesh plastic partition (mesh size < 0.5 mm; thickness = 1 mm), which allowed the tadpoles to be exposed to chemical cues without potential visual cues from the nymph.
Experimental setup: (a) tadpole responses to chemical cues from predators, (b) the responses of dragonfly nymphs to visual cues from tadpoles, and (c) tadpole responses to potential visual cues from predators.
At the beginning of each trial, a tadpole was introduced into the testing cup, to which 10 mg of powdered and 10 mg of tablet food were added. In preliminary observations, the size of the cup was sufficient to observe how the tadpoles responded to predator chemical cues. Five minutes after the tadpole had acclimated to the experimental conditions, the tadpole activity was recorded during 1-min observations with a digital video camera (DCR-HC40; Sony, Tokyo, Japan) from above. After 5 min, the dragonfly nymph was quietly introduced into the predator cup and the tadpoles were then exposed to predator chemical cues for 55 min. Both species had been fed their respective diets 1 day before the trials. The tadpole activity was recorded during 1-min observations at each 5-min interval throughout the 55-min trial period. To evaluate the behavioral response to dragonfly cues, the immobile time of tadpole activity was measured as the duration of stopping of both the tadpole body and tail movements. The control treatment was performed in the same manner at a 5-cm distance without introduction of a nymph. In preliminary observations, we had found that H. japonica tadpoles discriminate between chemical cues from predators and nonpredators, as reported in other amphibian species (e.g., Mathis et al. 2003). Trials for the five distance treatments and the control treatment were replicated independently 10 times. Tadpoles (body length = 7.0 ± 0.3 mm [mean ± 1 SD], developmental stage = 25–27; Iwasawa and Futagami 1992) were never used in more than one trial. Fifteen of the nymphs (body length > 40 mm) were used twice, but not more than once in each distance treatment.
Experiment 2: responses of the dragonfly nymphs to tadpoles
The experimental channel used in Experiment 1 was also deployed in this experiment (Figure 1b). An opaque plastic pipe (3 cm in diameter, 6 cm long), open at the top, was placed perpendicularly to the gutter 10 cm from the side within the trough as the position for dragonfly nymphs at the initiation of each trial. The testing area consisted of 3 sections at different distances from the pipe, 5, 15, and 25 cm, because in preliminary observations, dragonfly nymph responses were not observed from farther away than 25 cm. A transparent nonperforated plastic cup (6.0 and 4.5 cm in diameter at the top and bottom, respectively, and 3.5 cm in height) was placed at the center of each distance and served to contain the tadpole during the trials. The control treatment was performed in the same manner at a 5-cm distance without introduction of a tadpole. The areas for the nymph and the tadpole were divided by an opaque plastic partition (thickness = 1 mm), which prevented visual interaction between the 2 species.
At the beginning of each trial, the nymph was introduced into the pipe. The nymph was forced to stick tightly to the partition to keep the nymph orienting toward the center of the trough. One tadpole was introduced in the cup to which 10 mg of powdered and 10 mg of tablet food were added. Both species had been fed their respective diets 1 day before the trials. Five minutes after the acclimation of the nymph and tadpoles to experimental conditions, the partition was quietly removed to allow the nymph to advance in the trough and to detect visual cues from the tadpole in the transparent cup. Subsequently, we recorded whether the nymph detected, approached, and finally attacked the tadpole in the cup within each 20-min trial, and the trials were replicated independently 8 times in each treatment. Some dragonfly nymphs are known to detect prey using visual cues, including prey movement and size (Pritchard 1965; Rebora et al. 2004). Attack by the nymphs was defined as striking with the labial mask toward the tadpole in the cup. Tadpoles (body length = 6.0–7.0 mm) and nymphs (body length > 40 mm) were never used in more than one trial.
Experiment 3: tadpole responses to potential visual cues from dragonfly nymphs
The test for tadpole responses to potential visual cues from the nymph used the same methods as in Experiment 1, except that a transparent plastic partition (thickness = 1 mm) divided the tadpole and nymph areas, and a transparent nonperforated plastic cup was used to introduce the dragonfly nymph (Figure 1c). This setup allowed for potential visual cues without chemical cues derived from the nymph. Tadpole responses were examined at the 5-cm distance treatment, and the control treatment did not introduce the nymph (10 replicates each). Tadpoles (body length = 7.1 ± 0.1 mm, developmental stage = 25–27) and nymphs (body length > 40 mm) were never used in more than one trial.
Statistical analyses
We performed all statistical analyses and graphics using R ver. 2.13.0 software (R Development Core Team 2011). In Experiment 1 and 3, the values of control treatments were used to standardize long-term trends in values from other treatments during the experiments, which may have been affected by putting tadpoles into the cups. To quantify the effects of the treatments, we calculated the natural logarithm of the response ratios (LRRs) between the values after introducing the dragonfly nymph in the experiments and the values before nymph introduction (LRR = ln [Xafter/Xbefore]) (Hedges et al. 1999). The LRR of each experimental time represents the effect size of tadpole activity in response to introduction of the nymph, which is standardized by the activity before introducing the nymph. In this study, a higher LRR means that the tadpoles were less active after than before the introduction of dragonfly nymphs (i.e., no-predator cue). The LRR values of each distance treatment were averaged from 20 to 55 min based on the preliminary time-series analysis of the 5-cm distance treatment (see Figure 2). To estimate the differences in LRRs among the different distances from the dragonfly nymph, statistical significance was determined using the 99% confidence intervals of the LRR. The statistical test using effect size and 99% confidence interval is robust and reduce Type I error to estimate the behavioral differences (Colegrave and Ruxton 2003; Payton et al. 2003; Nakagawa and Cuthill 2007). The chi-square (χ2) test was used to evaluate the number of trials in which the nymphs attacked or did not attack in response to visual cues from the tadpoles for each treatment (3 distance sections and the control).
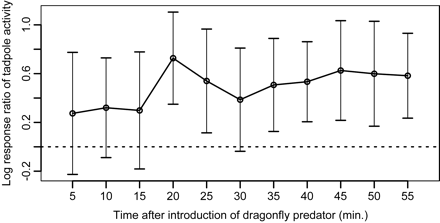
Time-series plot of the log response ratio (LRR) of tadpole activity in response to chemical cues from dragonfly nymphs at 5-cm distance treatment. The values of control treatment (i.e., at a 5-cm distance without introduction of a nymph) were used to standardize long-term trends in tadpole activity from the 5-cm distance treatment during the experiments. The LRR of each experimental time represents the effect size of tadpole activity in response to introduction of the nymph, which is standardized by the activity before introducing the nymph. Error bars indicate ±99% confidence intervals, and the horizontal dashed line denotes LRR = 0. Confidence intervals not including zero designate a significant effect of the treatment on tadpole activities.
RESULTS
Experiment 1: tadpole responses to chemical cues from dragonfly nymphs
The time-series plot of the LRRs of tadpole activity in response to chemical cues from dragonfly nymphs at the 5-cm distance treatment showed that tadpole responses to chemical cues started after around 20 min (Figure 2); thus, we compared the mean LRRs of the 5 different distances after 20 min. The time series of LRRs at the different distances were more varied, and at 45- and 65-cm distance treatments, the LRRs were almost not significantly different from 0. The mean LRRs of tadpole activity were different between distances from the dragonfly nymph (Figure 3). At 65-cm distance treatment, the LRR did not differ significantly from 0, and at 45-cm distance treatment, the LRR was lower than those at distances of 5–25 cm.
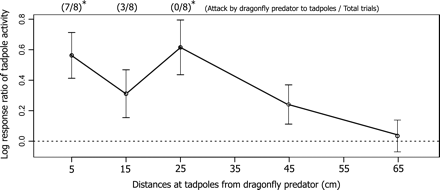
Mean log response ratios (LRRs) of tadpole activity in response to chemical cues from dragonfly nymphs at different distances. Each value of the experimental distance was averaged, the values from 20 to 55 min of each distance treatment based on the preliminary time-series analysis of the 5-cm distance treatment. Error bars indicate ±99% confidence intervals, and the horizontal dashed line designates LRR = 0. Confidence intervals not including zero indicate a significant effect of the treatment on tadpole activities. The ratios on the plot denote the frequencies of responses by dragonfly nymphs to visual cues from tadpoles at 3 different distances. Asterisks on the ratios at each distance treatment indicate that the number of trials including attack and nonattack observations differed significantly by a χ2 test (P < 0.05).
Experiment 2: responses of dragonfly nymphs to tadpoles
The number of trials resulting in attack and no-attack behavior differed significantly between 5-cm distance (7/8, attack trials/all trials) (χ2 test, χ12 = 4.5, P = 0.03), 25-cm distance (0/8) (χ12 = 8.0, P < 0.01), and control treatments (0/8) (χ12 = 8.0, P < 0.01) but was not significant for the 15-cm distance treatment (3/8) (χ12 = 0.5, P = 0.48) (Figure 3). The control treatment showed no attacks by the nymph on the cup without a tadpole. The attack rates were higher with shorter distances between the nymphs and the tadpoles (5 cm, 87.5%; 15 cm, 37.5%; 25 cm, 0%).
Experiment 3: tadpole responses to potential visual cues from dragonfly nymphs
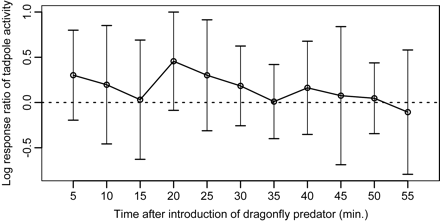
For potential visual cues, significant time-dependent changes in the LRR of tadpole activity were not detected based on mean and confidence interval plots (Figure 4), although the distance used in the experiment was close to the dragonfly predator.
Time-series plot of log response ratios (LRRs) of tadpole activity in response to potential visual cues from dragonfly nymphs at 5-cm distance treatment. The values of control treatment (i.e., at a 5-cm distance without introduction of a nymph) were used to standardize long-term trends in tadpole activity from the 5-cm distance treatment during the experiments. The LRR of each experimental time represents the effect size of tadpole activity in response to introduction of the nymph, which is standardized by the activity before introducing the nymph. Error bars indicate ±99% confidence intervals, and the horizontal dashed line denotes LRR = 0. Confidence intervals not including zero designate a significant effect of the treatment on tadpole activities.
DISCUSSION
Hyla japonica tadpoles responded to chemical cues from the dragonfly predator by reducing their activity levels, but did not respond to potential visual cues from the predator. The significant distance at which the tadpoles responded to chemical cues was at least 45 cm, which is farther than the attack distance of the predator.
Hyla japonica tadpoles exhibited the predicted reduction in activity when exposed to chemical cues from dragonfly predators, but not when exposed to potential visual cues. Considering that decreased activity is a defensive behavior (Chovanec 1992; Skelly 1994; Anholt and Werner 1995; Peacor 2006; Takahara et al. 2008), these results indicate that the tadpole preferentially utilizes chemical cues to detect the predator. Many studies on aquatic prey animals show that a variety of species utilize chemical cues exuded into the water from predators (Kats and Dill 1998; Mathis and Vincent 2000; Hickman et al. 2004; Lehtiniemi et al. 2005).
Nymphs of the dragonfly A. parthenope julius used in this study utilized visual cues to detect tadpoles. Various other dragonfly species also detect prey using visual cues, including prey movement and size, rather than using chemical cues (Pritchard 1965; Rebora et al. 2004). The number of nymph attacks increased as distance to the tadpole prey decreased; the distance required to detect the tadpole appeared to be in the range of approximately 15 cm. Because H. japonica tadpoles can use chemical cues to detect dragonfly predators at a distance of at least 45 cm, tadpoles can recognize predators before the predators can visually detect the tadpoles. A failure to detect predators such as dragonfly nymphs is often fatal for tadpoles (Relyea and Werner 1999; Teplitsky et al. 2004; Stav et al. 2007). The tadpoles sense chemical cues over longer distances than those at which they could use potential visual cues to avoid predators, even in clear water. Therefore, the preferential use of chemical cues by tadpoles before they are visually detected by the predator is an efficient predator-avoidance strategy. Under low predation risk, unnecessary predator-avoidance activities decrease the opportunities for prey to acquire food (Lima 1998). To maximize their fitness, tadpoles should recognize predation risk at the current position (i.e., distance from the predator) using predator chemical cues to avoid losing time and energy to unnecessary predator-avoidance activities. Consequently, the balance between such costs and risks would induce the evolution of the tadpoles to optimize the distance at which they are able to recognize the predator.
The intensity of tadpole response against chemical cues was different according to the distance from the nymph. When closer to the predator, the tadpoles decreased their activity more than them in the treatments at which the nymph was at distant distances. Because the nymphs can easily detect and capture tadpoles at shorter distances, the predation risk would increase along with decreasing of the distance from the nymph. It is also reported that the intensity of responses by prey species strengthens as concentration of chemical cues rises (Von Elert and Pohnert 2000; Van Buskirk and Arioli 2002; Mirza and Chivers 2003; Lönnstedt and McCormick 2011). We clearly found that the distance from the predator can also predict the responses of prey against chemical cues like the concentration of the chemicals. Thus, we suggest that the tadpoles can respond according to cue concentration associated with the distance from the predator with changing of magnitude of predation risk.
Aquatic environments restrict visibility because of water turbidity or dense vegetation, and this likely affects several predator–prey interactions (Lehtiniemi et al. 2005; Van De Meutter et al. 2005; Webster et al. 2007). For example, the distance at which smallmouth bass (Micropterus dolomieu) can recognize prey shortens as turbidity increases (Sweka and Hartman 2003). In such habitats, chemical cues may be more reliable than visual cues for tadpoles to obtain information associated with enemies (Stauffer and Semlitsch 1993; Kiesecker et al. 1996; Mathis and Vincent 2000; Hickman et al. 2004; Saidapur et al. 2009). Dragonfly predators utilize the tadpoles as one of various prey items, including snails, small fish, and other invertebrates, including conspecifics (Crumrine and Crowley 2003; Ferris and Rudolf 2007; Turner and Chislock 2007). Using visual cues to find a variety of prey species may be more efficient for dragonfly predators than would be recognizing species-specific chemical cues.
This study estimated the experimental responses of prey against predator cues in an artificial short channel. Our apparatus had no directional flow, and thus the diffusion of predator chemical cues must have been relatively slow. Responses to chemical cues took the tadpoles 20 min even at 5 cm from the predatory nymphs. In the field, an effect of water movement may influence the detection of chemical cues, if water movement disperses the chemical cues more quickly than the chemical cues diffuse (e.g., Smee and Weissburg 2006). Further study is needed, linking laboratory and field research, to better understand the extent to which diffusion affects the chemical cues causing inducible defenses in nature.
In the present study, we found that tadpoles exhibited defensive behavior in response to distance gradients away from predator-originated chemical cues. The differences in the ranges and the cue types utilized by the tadpoles and dragonfly predators to detect one another may be related to predator avoidance for tadpoles and to effective foraging strategies for dragonfly predators. The differences in the cues utilized by prey and predator would be an evolutionally consequence related to their costs and benefits. The detection of chemical cues by prey may shape predator–prey relationships and drive population dynamics in aquatic habitats.
FUNDING
This study was supported by grants from the Research Fellowship grant from the Japan Society for the Promotion of Science for young scientists (No. 07071) to T.T. All experiments were performed in agreement with the laws of Japan.
We are grateful to D. C. Blackburn (University of Kansas) for useful comments on the manuscript and language revision. We would like to thank Dr T. Nisimura (Nihon University) for useful comments on experimental design and the early draft, and the members of the Laboratory of Animal Ecology, Kyoto University for further discussion and comments on the study. The Venture Laboratory of the Kyoto Institute of Technology allowed us the use of their facility for the experiments.