-
PDF
- Split View
-
Views
-
Cite
Cite
K. L. DAVIES, M. STPICZYŃSKA, M. P. TURNER, A Rudimentary Labellar Speculum in Cymbidium lowianum (Rchb.f.) Rchb.f. and Cymbidium devonianum Paxton (Orchidaceae), Annals of Botany, Volume 97, Issue 6, June 2006, Pages 975–984, https://doi.org/10.1093/aob/mcl065
Close - Share Icon Share
Abstract
• Background and Aims The labellar ‘hairs’ of some Cymbidium spp. are said to be thin-walled and to contain ‘plasma’, oil and sugars and it has long been speculated that they may function as food-hairs. However, the present authors' preliminary studies showed that certain atypical papillae may have a different role and, by reflecting light, function as a speculum. The purpose of the paper is to test this hypothesis.
• Methods Light microscopy, scanning electron microscopy, transmission electron microscopy, histochemistry and ultraviolet photography were used to investigate the structure, food content and light-reflecting properties of these papillae.
• Key Results and Conclusions The labellum of Cymbidium lowianum (Rchb.f.) Rchb.f. is densely clothed with obconical to conical papillae with wide bases and pointed tips. However, on either side of the median axis of the lip occur silvery patches comprising papillae with truncated tips and it is thought that these reflect light and thereby attract insect pollinators. Similar patches are also found in Cymbidium devonianum Paxton, and in both species, they are set against a reddish background, which, since bees cannot perceive this colour, probably appears dark to the insect thus enhancing the visual impact of the light-reflecting patches. In Cymbidium tigrinum Parish ex Hook. and Cymbidium mastersii Griff. ex Lindl., however, the labellum is mainly white and no light-reflecting patches were observed. Instead, unlike C.lowianum and C. devonianum, these species are highly fragrant and the attraction of insects probably depends to a greater extent on olfactory cues. In C.lowianum both types of papillae contain protein, starch and lipid bodies but only protein is seemingly present at elevated concentrations. However, lipoidal material also occurs upon the surface of the labellum and it is possible that this may be gathered by insects as reported for C. iridifolium A. Cunn (syn. C. madidum Lindl.). The labellar papillae of C.lowianum, thus, have the potential to function as food-hairs, although direct evidence for this is lacking.
INTRODUCTION
The genus Cymbidium Sw. comprises some 44 species widely distributed in south-east Asia from north-west India to Japan and south to Australia with the greatest concentration occurring in north-east India, south-west China, Indo-China and Malesia (Du Puy and Cribb, 1988). Most are melittophilous and are pollinated by bumble bees, carpenter bees, honey bees or stingless bees (Meliponini), although some records indicate that certain species may also be pollinated by wasps. For example, C. aloifolium (L.) Sw. is said to be pollinated by Xylocopa bees as well as the wasp Vespa cincta (Ridley, 1894—cited in van der Pijl and Dodson, 1969), whereas C. finlaysonianum Lindl. is pollinated by both the honey bee Apis dorsata (Burkill, 1919—cited in van der Pijl and Dodson, 1969) and bumble bees (Chan et al., 1994). Bumble bees also visit and probably pollinate the flowers of C. atropurpureum (Lindl.) Rolfe (Chan et al., 1994). The pollinator of the unstriped form of C. insigne Rolfe is Bombus eximius (Kjellson et al., 1985; Du Puy and Cribb, 1988) and this bumble bee is also a regular pollinator of Dendrobium infundibulum Lindl. and Rhododendron lyi Leveille in north-eastern Thailand. Since these three plant species coexist and have flowers that resemble each other in shape, size and colour, it has been suggested that floral mimicry operates here especially since the flowering of both orchid species in early spring coincides with that of R. lyi and the only occurrence of B. eximius workers. Both C. insigne and D. infundibulum are largely pollinated by female worker bees, although pollination can, to a lesser extent, also result from the activity of queen bees. On occasion, Bombus rotundiceps was seen carrying the pollinia of D. infundibulum but no pollinia were observed on the various other insect species that visit these orchids, namely the bees Apis dorsata and Anthophora himalayensis, two species of hawkmoth and Cetoninae beetles (Clinteria sp.). Neither of these orchids produces fragrance, nectar nor indeed any type of floral reward. Furthermore, differences in the morphology and length of their columns tend to favour the deposition of pollinia upon different parts of the pollinator. Thus in C. insigne, the column is relatively long and pollinia are placed upon the back of the visiting bee. Conversely, the shorter column in D. infundibulum ensures that the pollinia are placed nearer to the head of the insect with the result that both orchids are able to share the same pollinator. In short, the resemblance of C. insigne and D. infundibulum to R. lyi with its copious nectar and pollen is thought to deceive bees into visiting these rewardless orchids.
Sasaki et al. (1991) and Sasaki and Ono (1993) report that drones of the honey bee Apis ceranajaponica are responsible for the pollination of C. pumilum Rolfe (syn. C. floribundum Lindl.) and these insects are attracted to the nectarless flowers by fragrances as they engage in mating flights during spring. However, the Australian orchid species C. canaliculatum R. Br., C. madidum Lindl. and C. suave R. Br. are all pollinated by stingless bees (Trigona sp.) (Adams et al., 1992; Adams and Lawson, 1993; Bartareau, 1993). Macpherson and Rupp (1935) studied a population of C. iridifolium A. Cunn. (syn. C. madidum Lindl.) near Prosperine, Queensland and observed that small, unidentified, native bees attracted to the flowers by their sweet fragrance, both gnawed at a viscid secretion produced along the median axis of the labellum and scratched the labellar surface. The exudate was transferred from the first to the second pair of legs and thence, gathered in pollen sacs borne upon the rear legs. It is speculated that this material is used as ‘bee glue’ for repairing holes and cracks in the nest. The labellum of this species is ‘irritable’ in that, as the bee moves further into the flower, the lip closes up against the column in a series of ‘jerky movements’, thereby imprisoning the insect and bringing its back into contact with the sticky, stigmatic surface. On reversing out of the flower, pollinia are deposited on the hairs upon the back of the bee. Field studies indicate that C. madidum and C. suave are pollinated by both Trigona hockingsii and T. carbonaria (Smythe, 1970; Bartareau, 1993) whereas Ghose (1955) (cited in van der Pijl and Dodson, 1969), who did not elaborate further, claimed that bees leaving the flowers of C. devonianum Paxton appeared to be intoxicated and this may perhaps facilitate pollination.
It would thus appear that in Cymbidium, floral fragrances play an important role in attracting potential pollinators. Nectar and other floral rewards, however, are generally absent. Gas chromatography of fragrances derived from C. tracyanum L. Castle yielded four main peaks. Of these, the second peak was identified as alpha-pinene, whereas the fourth was identified as 1, 8-cineole. By contrast, only two main peaks were recorded for C. hookerianum Rchb.f. and, of these, one was identified as alpha-pinene (Du Puy, 1986). The general absence of floral rewards in Cymbidium spp. indicates that pollination by deceit largely operates here (van der Cingel, 2001, and references cited therein). However, early reports would suggest that certain Cymbidium spp. may perhaps reward insect visitors either with a viscid material produced upon the labellum (Macpherson and Rupp, 1935) or other food materials. Indeed, Beck (1912) described the labellar hairs of Cymbidium as being thin-walled and containing ‘plasma’, oil and sugar and this observation is supported by other authors such as Porsch (1908) and Gellert (1923) (both cited in von Kirchner, 1925) who reported the presence of oils in the labellar hairs of C. lowianum (Rchb.f.) Rchb.f.
Given the authors' interest in food-hairs, pseudopollen and indeed, floral rewards in general (Davies and Winters, 1998; Davies et al., 2000, 2002, 2003a, b, 2005; Davies and Turner, 2004a–c; Matusiewicz et al., 2004; Stpiczyńska et al., 2004, 2005), the original aim of this present paper was to describe the labellar micromorphology of C. lowianum and to investigate the nutritional value of its labellar hairs. However, observations were to show that some of the labellar papillae may attract potential pollinators in other ways.
MATERIALS AND METHODS
Labella of C. lowianum were carefully examined by means of a hand lens for floral nectar and for any epidermal structures that might play a role in the production of floral rewards. Hand-cut, unstained, transverse sections of the labellum were prepared for light microscopy (LM).
Scanning electron microscopy (SEM)
Pieces of labellar tissue were excised, dehydrated through a graded ethanol series and subjected to critical-point drying using liquid CO2. The specimens were then mounted on stubs by means of double-sided, carbon adhesive tabs, coated with gold and examined using a JSM 5200 LV-SEM at an accelerating voltage of 20 kV (Davies and Turner, 2004a, b, c).
Transmission electron microscopy (TEM)
Excised tissue was fixed, dehydrated, embedded, cut and stained for TEM as described previously (Stpiczyńska et al., 2004) and examined by means of a TESLA BS-340 at an accelerating voltage of 60 kV. Similarly, semi-thin sections approx. 1 μm thick were cut, stained with toluidine blue (Stpiczyńska et al., 2004) and examined using a Nikon Eclipse 600 light microscope.
Histochemistry
Samples of labellar epidermal tissue and underlying parenchyma were tested for starch and proteins using a dilute iodine/potassium iodide solution and the xanthoproteic test, respectively, as described previously (Davies et al., 2000, 2003a, b). On this occasion, however, a saturated ethanolic solution of Sudan Black B was substituted for Sudan III when testing for lipids so as to distinguish more clearly between the extent of staining and the red anthocyanin pigmentation that occurs within the labellar tissue of this species. The labella of C. devonianum, C. tigrinum Parish ex Hook. and C. mastersii Griff. ex Lindl. were also examined by means of LM and, in the case of C. devonianum, SEM.
Ultraviolet (UV) photography
Flowers of C. lowianum were subjected to UV photography as described by Kay (1975).
RESULTS
Microscopy
Flowers of C. lowianum are faintly scented and lack nectar. From afar, the red, V-shaped mark found upon the mid-lobe of the labellum appears uniform in colour. However, closer examination revealed the presence of two small and indistinct silvery patches of tissue within this red area and these are arranged symmetrically on either side of the mid-lobe (Fig. 1A and B). Similar patches were also found to occur in C. devonianum, one in each of the dark purple, circular markings that occur upon the labellum (Fig. 1C). To the naked eye, these patches resemble the dried mucilage trails of snails but they are absent from some species such as C. tigrinum and C. mastersii which have light-coloured labella (Fig. 1D).
(A) Flower of Cymbidium lowianum (Rchb.f.) Rchb.f. showing position of labellar patch (arrow). Scale bar = 20 mm. (B) Adaxial surface of labellum of C. lowianum showing reflective patches. Scale bar = 20 mm. (C) Flower of Cymbidium devonianum Paxton showing position of labellar patch within darkly pigmented area (arrow). Scale bar = 20 mm. (D) Flower of Cymbidium tigrinum Parish ex Hook. with light-coloured labellum lacking reflective patches. Scale bar = 20 mm.
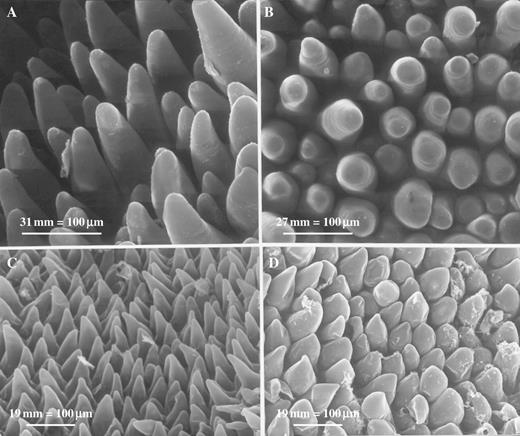
SEM studies of both C. lowianum and C. devonianum revealed that whereas the mid-lobe of the labellum largely bears obconical to conical papillae with pointed tips, those that comprise the silvery patches tend to be more densely packed with abruptly truncated or somewhat angular or faceted tips (Fig. 2A–D).
(A) SEM of labellum of C. lowianum showing typical conical papillae with pointed tips. Scale bar = 100 μm. (B) SEM showing conical papillae with light-reflecting, truncated tips from the labellar patches of C. lowianum. Scale bar = 100 μm. (C) SEM showing typical, conical, labellar papillae of C. devonianum. Scale bar = 100 μm. (D) SEM showing conical papillae with angular, light-reflecting tips from the labellar patches of C. devonianum. Scale bar = 100 μm.
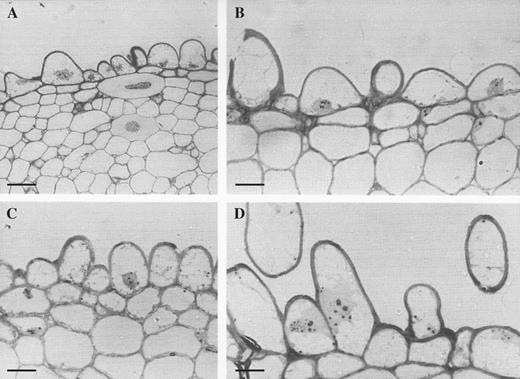
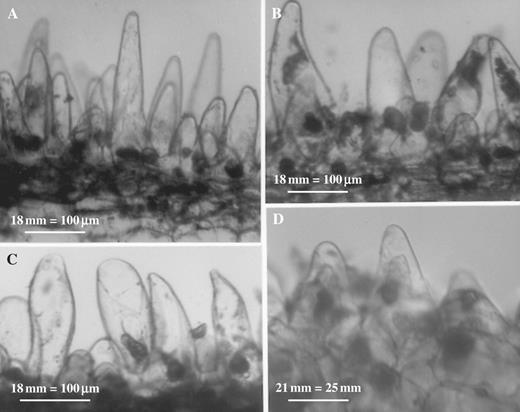
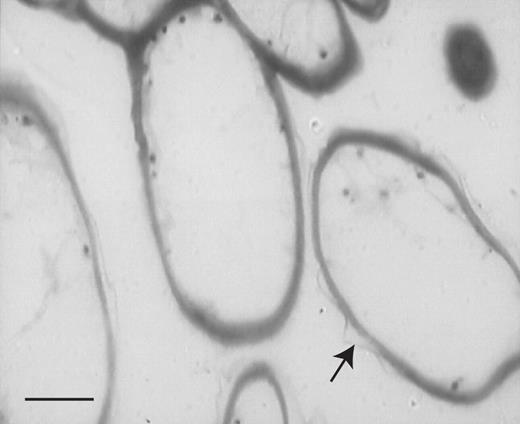
LM revealed that the labellar mid-lobe of C. lowianum and C. devonianum consists mainly of tightly packed parenchyma with small, intercellular spaces and idioblastic cells that contain raphides (Fig. 3A). It also confirmed that the labellar epidermis of both species is largely papillose but that in C. lowianum, bicellular trichomes occur towards the median axis of the lip. Although the papillae of both species are mainly obconical to conical with wide, polygonal bases, those at the margin tend to be clavate. It is difficult to distinguish patch papillae from typical papillae in semi-thin or hand-cut sections since, even within patches, papillae vary greatly in shape (Figs 3A–D and 4A–D). Both types of papillae contain nuclei and are highly vacuolated (Figs 3D and 4A–D) and LM showed that anthocyanin is confined to the vacuolar sap. Secreted material is associated with the outer walls of papillae (Fig. 5).
(A) Semi-thin, toluidine blue-stained section through labellar patch of C. lowianum showing obconical to conical epidermal papillae with sub-epidermal parenchyma and idioblasts containing raphides. Scale bar= 50 μm. (B) A similar section through typical labellar papillae of C. lowianum. Scale bar = 20 μm. (C) A similar section through the labellar patch of C. lowianum. Scale bar = 20 μm. (D) Semi-thin section through typical labellar papillae showing cytoplasmic organelles. Scale bar = 20 μm.
Hand-cut, unstained sections through labellar patches of C. lowianum (A–C) and C. devonianum (D), respectively, showing variation in shape of papillae. Scale bars = 100 μm (A–C) and 25 μm (D).
Semi-thin, toluidine blue-stained section through labellar papillae of C. lowianum. Note the profiles of extra-cellular secretion (arrow) associated with the cell wall. Scale bar = 20 μm.
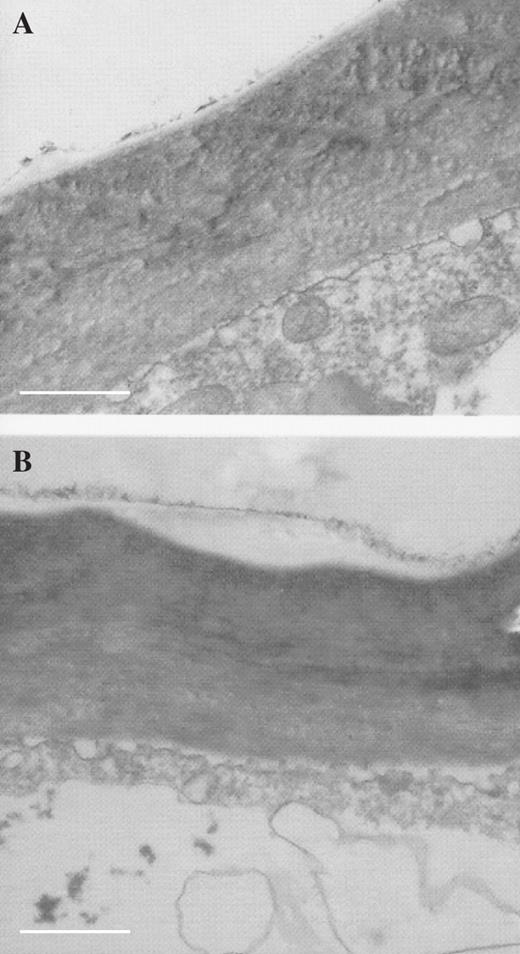
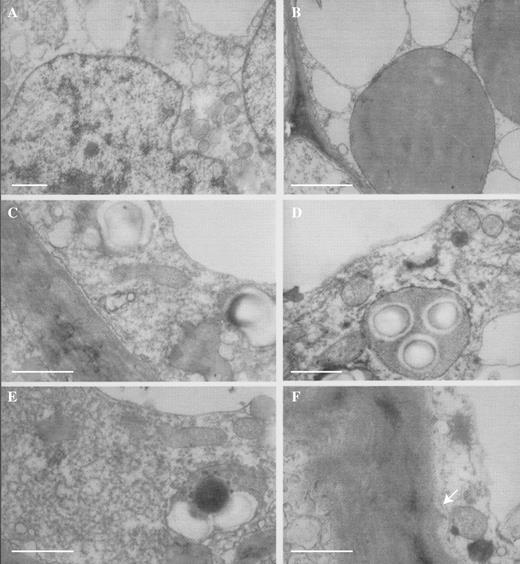
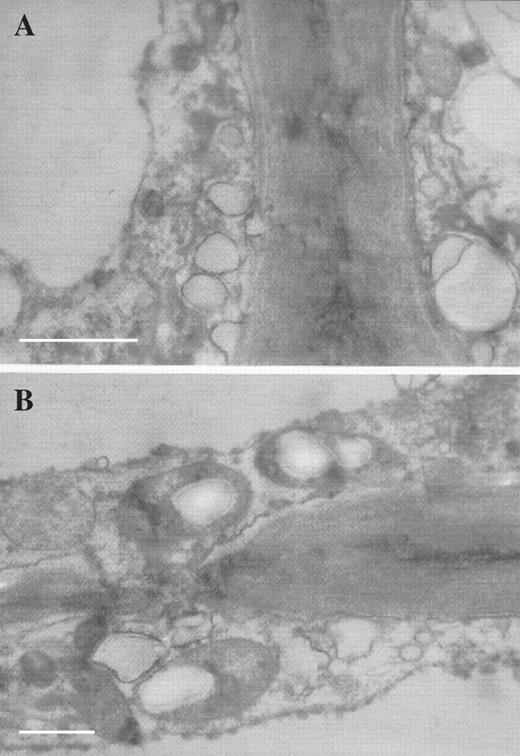
TEM studies also revealed that a layer of diffuse, osmiophilic material occurs upon the outer wall of both patch and typical papillae (Fig. 6A, B) and that both types of papillae contain a similar complement of cytoplasmic organelles comprising nuclei (Fig. 7A), proplastids and vacuolar profiles (Fig. 7B), mitochondria and amyloplasts (Figs 6A, Figs 7C–E and 8B), rough endoplasmic reticulum (Fig. 7E), lipid bodies (Fig. 7D–F), dictyosomes (Fig. 7F) and small vesicles that aggregate at the plasmalemma (Figs 6B and 8A).Towards the base of the papillae, the cell walls often have small ingrowths and, here, the plasmalemma becomes invaginated (Fig. 7F). Pits with plasmodesmata connect contiguous cells (Fig. 8B). Thus, it would appear that, with the exception of their shape and relative density, both types of papillae are otherwise identical.
TEM sections through outer wall of patch papilla (A) and typical labellar papilla (B), respectively, showing diffuse, osmiophilic material associated with outer cell wall. Scale bars = 1 μm.
(A) TEM section of patch papilla showing nucleus, vacuolar profiles, proplastids and mitochondria. Scale bar = = 1 μm. (B) A similar section of a patch papilla showing proplastids with few internal lamellae and numerous vacuolar profiles. Scale bar = 1 μm. (C) TEM section of patch papilla showing plasmalemma, proplastids, amyloplasts, mitochondria and tonoplast. Scale bar = 1 μm. (D) TEM section of patch papilla showing amyloplast with starch grains, mitochondria and vacuolar profiles. Note also the osmiophilic, lipid bodies. Scale bar = 1 μm. (E) A similar section showing dense, rough endoplasmic reticulum, mitochondria, amyloplast and osmiophilic, lipid bodies. Scale bar = 1 μm. (F) Detail of cell wall of patch papilla with small ingrowths (arrow). Note also the dictyosome and lipid bodies in the parietal cytoplasm. Scale bar = 1 μm.
(A) TEM section of typical labellar papilla showing aggregation of small vesicles at plasmalemma. Scale bar = 0.5 μm. (B) TEM section of typical labellar papilla showing pit with plasmodesmata connecting contiguous cells. Scale bar = 1 μm.
Histochemistry
In C. lowianum, starch and protein are present in both types of papillae as well as in the underlying parenchyma. However, it is possible, based upon the relative intensity of staining using the xanthoproteic test, that the papillae contain greater concentrations of aromatic amino acids than the parenchyma. Given their relatively small size (approx. 0·5 μm or less), staining with Sudan Black B did not reveal the presence of many lipid droplets in the parenchyma nor in the papillae, although TEM studies showed conclusively the presence of spherical, osmiophilic oil bodies in the latter. However, extra-cellular material associated with the outer cell walls of papillae derived from both regions of the lip stained intensely with Sudan Black B and demonstrated a strong affinity for this stain.
UV photography did not demonstrate that the labellar patches reflect UV light.
DISCUSSION
Staining with Sudan Black B (LM) and osmium tetroxide (TEM) revealed that lipid material is associated with the outer walls of papillae from both regions of the labellum of C. lowianum. Similarly, staining with Sudan III has revealed the presence of lipid droplets upon the tips of the unicellular, clavate, labellar trichomes of Cymbidium dayanum Rchb.f. (K. L. Davies, 2006, unpubl. res.). These observations support those made by Macpherson and Rupp (1935)—that bees visiting the flowers of C. madidum gnaw at a viscid substance produced along the median axis of the lip. Lipid droplets both within the papillae and underlying parenchyma of C. lowianum, as revealed by TEM, also confirm the observations of Porsch (1908) and Gellert (1923) (both cited in von Kirchner, 1925).
Although both Porsch (1908) and Gellert (1923) (cited in von Kirchner, 1925) recorded the presence of sugars in C. lowianum, the present study failed to demonstrate the presence of floral nectar in this species. Indeed, TEM revealed the presence of starch-laden amyloplasts both in the papillae and in the underlying parenchyma, and it is well established that starch disappears from amyloplasts during nectar formation as it becomes converted to sugar (Stpiczyńska et al., 2004, and references therein). Furthermore, it is most improbable that C. lowianum produces floral nectar, since extra-floral nectaries occur at the junction of the pedicel and rachis and these secrete copious amounts of a sugary solution, which, when tested with a hand-held refractometer (Bellingham & Stanley Eclipse 45–82), gave a reading of 78·9 % (w/w) sugar. With such an ample supply of sugar so freely available, it seems unlikely that floral nectar would be an effective reward for pollinators in this instance.
It is well known that proteins are essential for the reproductive cycle of bees—the most likely pollinators of C. lowianum and, although no quantitative data are available, the relative intensity of histochemical staining for aromatic amino acids indicated that the papillae may contain higher concentrations of these compounds than the sub-epidermal parenchyma. This, coupled with the fact that the papillae are able to secrete copious, lipoidal material, which presumably has some nutritive value, would indicate that they have the potential to function as food-hairs. The secretion is most obvious on the cell walls of obpyriform papillae borne upon the callus. However, the histochemical detection of aromatic amino acids within the secretion was inconclusive since, although the latter appeared to stain pale-amber with the xanthoproteic reaction, the test was frustrated by the intense staining of the underlying cytoplasm.
Since both types of papillae have an identical complement of cytoplasmic organelles, they thus, presumably, have a similar physiology and since, they differ from each other only in shape, it is reasonable to assume that differences in function are largely due to the latter.
The labellar papillae of both C. lowianum and C. devonianum are generally obconical to conical with wide bases and relatively pointed tips and, according to Kay et al. (1981), this type of papilla with its rounded apex, slightly concave, outer walls and expanded, convex base is the most common type found amongst angiosperms. Surface striations are absent and the underlying tissue comprises tightly packed parenchyma with small, intercellular spaces.
Kay et al. (1981) compared the optical geometry of papillate and planar epidermises of flowers and concluded that whereas the former traps light over most of its surface, the latter reflects light at a shallow angle. These authors further proposed that the primary function of the papillate epidermis of a petal is to trap incident light. This is achieved by a combination of external reflection, refraction and internal reflection processes. In petals, light is mainly reflected by the cell wall/air interfaces of the mesophyll. Following refraction by the convex, inner, tangential wall of the conical papilla, the light is reflected by the converging, outer walls with the result that it must firstly pass through a more uniform length of pigment solution, contained within the vacuolar sap, than had the epidermis been planar before finally emerging. Furthermore, the convex base of the papilla would tend to reduce the amount of light that would otherwise be lost by reflecting it back into the mesophyll. It is thought that the presence of surface striations also affect the degree of reflection since comparisons of epidermal tissue having or lacking striations have shown that their presence generally reduces the degree to which light is reflected. However, surface striations probably also scatter emergent light with the result that the brightness of the petal remains more or less constant (Kay et al., 1981). These authors have also clearly demonstrated for petals of Viola tricolor L. that the rounded tips of conical papillae are capable of reflecting incident light. The patch papillae of C. lowianum and C. devonianum, however, have truncated, faceted or angular apices and these would, in effect, increase the reflective surface area. Moreover, since the labella of both species consist of tightly packed parenchyma rather than aerenchyma as found in typical petals, then one would expect the reflection of light by the cell wall/air interfaces of this tissue to be relatively low with much of the emergent light being reflected back into the sub-epidermal parenchyma by the convex, inner, tangential walls of the epidermal papillae.
Vision in bees is highly developed and largely geared towards foraging. It is estimated that the compound eye of a honey bee has approx. 5500 facets or ommatidia and the insect is thus able to recognize patterns and geometric correlations. This is particularly important since flowers are composed of intricate pattern complexes and it has been shown that bees prefer highly subdivided structures when foraging but low-contour structures when returning to the nest (van der Cingel, 2001). Colour vision in insects is usually tri-chromatic with different receptors responding to green, blue and UV light. Some bees, including bumble bees and honey bees fail to respond to the colour red. However, they have a preference for blue and yellow and perceive blue-green and UV as distinct (Proctor et al., 1996). This sensitivity to the UV part of the electromagnetic spectrum and lack of sensitivity to red light is common to most insects and the visible spectrum of an insect is generally in the range of 300–650 nm. Bees also have a high flicker-fusion frequency (above which individual images appear continuous) of about 400 images s−1 (van der Cingel, 2001) and are thus well-equipped for perceiving visual cues.
Since bees are red-blind, the visual contrast between the typical light-trapping papillae found within the red, V-shaped mark upon the labellum of C. lowianum and the light-reflecting patch papillae is likely to be great with the patches appearing as bright reflective areas against a dark background. A similar effect is likely to be produced in C. devonianum since the reflective patches in this species occur within circular reddish-purple marks on either side of the lip.
In Ophrys spp., the attraction of pollinators is due in part to visual cues including the reflection of light by a labellar speculum. Since the speculum is bluish in colour, it has been proposed that male bees, on alighting upon the lip, are deceived by its resemblance to the wings of an insect and that this, together with olfactory and tactile stimuli, initiates pseudocopulation. In Ophrys, the speculum comprises two regions, a central zone and a margin some four or five cells wide of non-pigmented cells (Servettaz et al., 1994). The remainder of the lip is hirsute and heavily clothed with unicellular, often flexuous trichomes that contain anthocyanin. The central zone is often glabrous and is formed of flattened, epidermal cells, although in some species such as O. drumana P. Delforge, O. saratoi E.G. Camus, O. promontorii O. Danesch & E. Danesch, O. garganica (E. Nelson) O. Danesch & E. Danesch, O. fusca Link. and O. lutea (Gouan) Cav., these cells carry a short, central hair or papilla (Servettaz et al., 1994; Ascensão et al., 2005). In O. fuciflora (Crantz) Moench and the hybrid O. × chiesesica J. Kleynen, the central region is papillose and the papillae are conical to dome-shaped with cuticular striations (Servettaz et al., 1994). The marginal zone usually contains papillose cells to a greater or lesser degree but in O. × chiesesica, trichomes are present (Servettaz et al., 1994). In all cases, long trichomes predominate on reaching the outer edge of the speculum (Servettaz et al., 1994; Ascensão et al., 2005).
Comparison with Ophrys spp. would indicate that the patch papillae of C. lowianum and C. devonianum may have a dual role in the attraction of insects, functioning both as a speculum and, since the patches are symmetrically arranged on either side of the median axis of the lip, as ‘markers’ or ‘guides’ for the accurate positioning of the insect as it alights. Indeed, the similarity in shape between the patch papillae of these Cymbidium spp. and the speculum papillae of certain Ophrys taxa such as O. fuciflora and O. × chiesesica is quite remarkable. Despite this similarity, the patch papillae of Cymbidium and the speculum papillae of Ophrys differ from each other in two important respects, namely, the papillae of Cymbidium are smooth and lack striations and also, their apices are truncated, faceted or angular and thus have a greater reflective surface area than those of Ophrys. It has been suggested, on the grounds that the expanded bases of the cells that constitute the speculum of Ophrys spp. become narrower on approaching the speculum margin, that these bases may enhance the surface reflection of light much in the same way as a planar epidermis would (Ascensão et al., 2005). However, labellar papillae with similar bases occur in a number of orchid species (e.g. Maxillaria meleagris Lindl., M. densa Lindl. and M. variabilis Bateman ex Lindl.) not known for their light-reflecting qualities (Davies and Winters, 1998; Davies and Turner, 2004a).
It would also appear that the patch papillae of C. lowianum reflect incident light but not UV light. UV photography revealed that, although UV-absorbing nectar-guides corresponding in position to veins run the length of the tepals, there is no evidence whatsoever that UV light is reflected by the patches. However, the latter reflect incident light more strongly than other parts of the labellum and this is most obvious during flash photography or when plants are subjected to strong sunlight. This supports the observation of Biedinger and Barthlott (1993) which showed that flowers of C. lowianum only weakly reflect UV light.
In conclusion, it is possible that the labellar papillae of C. lowianum may function as food-hairs since they appear to contain protein at relatively higher concentrations than the sub-epidermal parenchyma. Amyloplasts are also present, albeit in low numbers. Furthermore, lipoidal material was present upon the outer walls of papillae, supporting the results obtained by Beck (1912) and the field observations of Macpherson and Rupp (1935) which showed that bees visiting some Cymbidium spp. graze the labellum and gather a viscid substance from its surface. However, although the papillae, as revealed by TEM, contained oil bodies as reported by Porsch (1908) and Gellert (1923) (both cited in von Kirchner 1925), their numbers were relatively small. Unfortunately, hitherto, C. lowianum and its pollination biology have not been extensively studied in the field. Until such information is forthcoming, we cannot categorically state that the labellar papillae of this species function as food-hairs although, on the basis of their contents, they have the potential to do so. It is, however, probable that the flattened, faceted or angular tips of the patch papillae of both C. lowianum and C. devonianum reflect incident light and thereby provide visual cues that attract potential pollinators and help to orientate them upon the labellar surface. In each case, these patch papillae are set against a red or reddish-purple background and, owing to the inability of bees to perceive red coupled with the light-trapping properties of typical labellar papillae, the visual contrast between the light-reflecting papillae and the darker background is enhanced. Of course, not all Cymbidium spp. have darkly coloured labella and it is thus interesting to note that in C. tigrinum and C. mastersii, two species in which the labellum is predominantly white, the labella lack obvious reflective patches. Instead, it is likely that insects are attracted to flowers of these species by their strong, honey-like and almond-like fragrances, respectively. It would therefore be interesting to compare the responses of bees to flowers of both typical C. lowianum and to C. lowianum var. concolor Rolfe in which the V-shaped mark is yellow. Similarly, field studies to compare the behaviour of insects visiting flowers of C. lowianum whose labellar patches have been covered with those of control populations may also yield useful information and help determine the extent to which these rudimentary specula really influence pollinator behaviour.
K.L.D. is grateful to the Stanley Smith (UK) Trust for their generous grant. The authors also acknowledge the help of Alan Gregg, Swansea Botanical Complex, Swansea, UK and Ian Tew, Swansea University, Swansea, UK.
LITERATURE CITED
Adams PB, Lawson SD.
Adams PB, Bartareau T, Walker KL.
Ascensão L, Francisco A, Cotrim H, Pais MS.
Bartareau T.
Beck G.
Biedinger N, Barthlott W.
van der Cingel NA.
Davies KL, Turner MP.
Davies KL, Turner MP.
Davies KL, Turner MP.
Davies KL, Winters C.
Davies KL, Winters C, Turner MP.
Davies KL, Roberts DL, Turner MP.
Davies KL, Turner MP, Gregg A.
Davies KL, Turner MP, Gregg A.
Davies KL, Stpiczyńska M, Gregg A
Du Puy DJ.
Kay QON, Daoud HS, Stirton C.
von Kirchner O.
Kjellson G, Rasmussen FN, Du Puy D.
Macpherson K, Rupp HMR.
Matusiewicz J, Stpiczyńska M, Davies KL.
van der Pijl L, Dodson CH.
Proctor M, Yeo P, Lack A.
Sasaki M, Ono M.
Sasaki M, Ono M, Asada S, Yoshida T.
Servettaz O, Bini Maleci L, Grünanger P.
Stpiczyńska M, Davies KL, Gregg A.
Author notes
1School of Earth, Ocean and Planetary Sciences, Cardiff University, Main Building, Park Place, Cardiff CF10 3YE, UK, 2Department of Botany, Agricultural University, Akademicka 15, 20-950 Lublin, Poland and 3School of Biosciences, Cardiff University, Park Place, Cardiff CF10 3TL, UK