-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Mockler, Luisa F Escobar-Hoyos, Ali Akalin, Jamie Romeiser, A Laurie Shroyer, Kenneth R Shroyer, Keratin 17 Is a Prognostic Biomarker in Endocervical Glandular Neoplasia, American Journal of Clinical Pathology, Volume 148, Issue 3, September 2017, Pages 264–273, https://doi.org/10.1093/ajcp/aqx077
Close - Share Icon Share
Abstract
Previous work in our laboratory identified keratin 17 (K17) as a specific and sensitive biomarker for high-grade squamous intraepithelial lesions and cervical squamous cell carcinoma (SCC). K17, however, has not been previously evaluated in endocervical glandular neoplasia. Based on the similar pathogenesis of squamous and glandular lesions of the cervix, we hypothesized that K17 overexpression could also be a diagnostic and/or prognostic biomarker for endocervical neoplasia.
Cases of endocervical adenocarcinoma (n = 90), adenocarcinoma in situ (AIS) (n = 32), benign glandular lesions (n = 36), and normal endocervical mucosa (n = 5) were selected from Stony Brook Medicine and the University of Massachusetts from 2002 to 2013. Immunohistochemical staining for K17 was performed by an indirect immunoperoxidase method and was scored based on the proportion of cells that showed strong (2+) staining.
K17 was highly expressed in 21 (65.6%) of 32 AIS and in 75 (83.0%) of 90 adenocarcinoma cases. In adenocarcinomas, K17 staining was detected in a mean of 33.9% of malignant cells. Staining tended to be strongest at the periphery of pseudoglandular groups and at the invasive front of tumors. K17 was not detected in the epithelial cells of benign glandular lesions, but groups of cuboidal cells, residing beneath the epithelial layer of benign glands, were frequently positive for K17, especially in cases of microglandular hyperplasia. High levels of K17 expression were significantly associated with decreased patient survival.
K17 is highly expressed in most cases of both invasive adenocarcinoma and in AIS and is a powerful, negative prognostic marker for patient survival.
Uterine cervical cancer is a significant health concern, being the second most common cancer and the third leading cause of cancer mortality in women worldwide.1 However, incidence and mortality rates have declined in the United States and other developed countries due largely to the introduction of the Papanicolaou stain in the 1940s and the implementation of screening programs that detect preinvasive lesions.2-4
Cervical cancer has multiple histologic subtypes, with squamous cell carcinoma (SCC) accounting for approximately 75% of cases and invasive adenocarcinoma accounting for 10% to 15% of cases.5 It has been shown that histologic type is an independent prognostic factor in cervical cancer, with adenocarcinoma subtypes being associated with a poorer prognosis compared with SCC.6,7 When adenocarcinomas are present, they tend to be larger in size and have a propensity for early lymphatic and hematogenous metastasis. Although screening programs have been very effective in decreasing the incidence of SCC by as much as 80%,8 studies have shown a contrasting increase in the incidence of cervical adenocarcinoma, particularly in younger women.5,9,10
Although p16INK4a has been well established as sensitive and accurate diagnostic biomarker for endocervical adenocarcinoma in situ and invasive adenocarcinoma in histologic sections and in cervical cytology specimens, clinical and diagnostic features alone have limited power to predict the survival of patients with endocervical adenocarcinoma.11-13 By contrast, we discovered, using laser capture microdissection and mass spectrometry, that keratin 17 (K17) could predict the overall survival of patients with cervical squamous cell carcinoma more accurately than grade, stage, or any other clinicopathologic features.14 Subsequent work in our laboratory demonstrated that K17 serves as a nuclear shuttle of p27KIP1, resulting in sustained cell cycle progression.15 Other studies have shown K17 to be a negative prognostic indicator in gastric adenocarcinoma, bladder carcinoma, epithelial ovarian carcinoma, and triple-negative breast carcinoma.16-20 There remains a critically important, unmet clinical need, however, to identify prognostic (as opposed to diagnostic) biomarkers of endocervical adenocarcinoma. Thus, the goal of this study was to determine if K17 expression is a negative prognostic biomarker associated with decreased survival of patients with endocervical adenocarcinoma.
Materials and Methods
Institutional Review Board Approval
The study was coordinated as part of the Stony Brook University Institutional Review Board Committee on Research in Human Subjects (CORIHS protocol 94651). This study’s biomarker assessments used formalin-fixed, paraffin-embedded (FFPE) surgical tissue specimens that had been linked to deidentified patient records that included information on patient risk characteristics, pathology/histological tumor assessments, radiation and chemotherapy treatment details, and clinical outcomes (ie, survival status, disease recurrence, and cause of death assessments).
Case Selection
This study included a total of 178 FFPE surgical tissue blocks that were retrospectively selected from the archival collections of both the Stony Brook BioBank and the Department of Pathology at the University of Massachusetts, in compliance with institutional review board–approved protocols. For cases from the Stony Brook BioBank, a search for each diagnostic category was performed encompassing the years 1980 to 2010, and all of the endocervical adenocarcinoma cases were selected while the most recent cases were selected for the other diagnostic categories as described below. For cases from the Department of Pathology at the University of Massachusetts, two separate searches encompassing the years 2000 to 2013 were performed for the diagnosis of adenocarcinoma in situ (AIS) and invasive endocervical adenocarcinoma. Thirty-six cases of invasive adenocarcinoma (between 2000 and 2013) and 19 of the most recent cases of AIS (between 2009 and 2013) were selected. These study cases comprised the following diagnostic categories: adenocarcinoma (n = 90), AIS (n = 32), microglandular hyperplasia (n = 16), tubal metaplasia (n = 13), endometriosis (n = 5), tunnel clusters (n = 2), and normal (n = 5). In all cases, a tissue block was selected following histologic review by a pathologist (K.R.S., D.M., and A.A.) of H&E-stained sections from hysterectomy, cervical cone, loop electrosurgical excision procedure, and/or cervical biopsy to confirm that diagnostic tissue as originally reported was adequately represented in the remaining tissue blocks. Cases that had insufficient residual tissue were not included in the study.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks were sectioned at 5 µm, and the whole tissue sections were mounted on charged glass slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA). After incubation at 60°C for 1 hour, tissue sections were deparaffinized in xylene and rehydrated using graded alcohols. Antigen retrieval was performed using a decloaking chamber at 120°C for 10 minutes in citrate buffer (20 mmol, pH 6.0; Vector Laboratories, Burlingame, CA) for K17 and p16, as well as a high pH antigen unmasking solution (Vector Laboratories) for Ki-67. Endogenous peroxidase activity was blocked by applying 3% hydrogen peroxide for 5 minutes. Nonspecific antibody binding was blocked by preincubation with 5% horse serum. Primary antibodies used were mouse monoclonal anti–human K17 antibody (clone E3; Abcam, Cambridge, MA; 4°C overnight), p16INK4a (CINtec, 60 minutes at room temperature; Ventana Medical Systems, Tucson, AZ), and Ki-67 (clone MIB-1, 1:100 dilution, 60 minutes at room temperature; DAKO, Carpentaria, CA). Negative controls were performed on all cases using an equivalent concentration of a subclass-matched mouse immunoglobulin, generated against unrelated antigens (BD, Franklin Lakes, NJ), in place of primary antibody. Following incubation with the primary antibody, slides were processed by an indirect avidin-biotin–based immunoperoxidase method using biotinylated horse secondary antibodies (R.T.U. Vectastain Universal Elite ABC kit; Vector Laboratories), developed in 3,3’-diaminobenzidine (DAKO) and counterstained with hematoxylin. K17 staining was scored using PathSQ,14 a manual semiquantitative scoring system that is based on the proportion of tumor cells with strong (2+) staining. Ki-67 staining was scored as a percentage of tumor cells with positive nuclear staining, and p16INK4a-stained sections were scored positive when strong (2+) nuclear and cytoplasmic staining of more than 90% of lesional cells was present.
Immunofluorescence
Briefly, sections were cut at 4 μm and heat immobilized at 60°C for 60 minutes. After deparaffinization with xylene and rehydration through graded alcohols, antigen retrieval was performed in a High PH buffer (Vector Laboratories) at 120°C for 10 minutes in a Decloaking Chamber (Biocare Medical, Walnut Creek, CA). Sections were rinsed in phosphate-buffered saline (PBS) and blocked for 1 hour at room temperature using 5% nonfat milk. Primary antibodies were added and incubated overnight at 4°C using the following: monoclonal CK17 (E3 clone) prediluted (Abcam) and rabbit polyclonal CK7, diluted 1:1,000 (Abcam). Sections were rinsed in PBS and incubated with Alexa secondary 488 goat anti-mouse and 594 goat anti-rabbit, both diluted to 1:250, incubated for 2 hours at room temperature in a light-proof chamber. The slides were rinsed in PBS and cover slipped using Vectashield mounting media with 4',6-diamidino-2-phenylindole (Vector Laboratories). Negative controls were performed on all cases.
Statistical Analysis
Diagnostic Value of K17
To determine an optimal diagnostic K17 staining cutoff value to differentiate between normal vs abnormal tissue, biomarker findings were dichotomized into two groups: normal tissue vs abnormal (AIS + cancer) tissue. As a visual inspection of the K17 scoring distribution identified potential natural data-driven cut-points, a negative likelihood ratio (NLR) analysis was used to identify the lowest K17 value (as the new “threshold”) that would differentiate a normal vs an abnormal diagnosis; the NLR represents the probability of a negative test result given the presence of the disease (ie, false-negative probability), divided by the probability of a negative test result given the absence of disease (ie, true-negative probability). A low NLR would indicate a low chance of misclassifying tissue as “normal” when actually the tissue is abnormal. NLRs are known for ruling conditions out and thus are an appropriate measure to use when defining normal tissue using K17 thresholds.
Prognostic Value of 17
Cox proportional hazards models for survival time from date of index surgical biopsy were generated for K17 as both a continuous variable and through an exploratory categorical cutoff process using increments of 10, from 10% or more to 90% or more. Once the cutoffs were determined, a second reviewer (D.M.) rescored the K17 score for all cancer tissue slides. This second reviewer was blinded to the first reviewer’s (K.R.S.) scores. Interrater reliability was examined using a weighted κ score. All disagreements were then reviewed by both raters, and a consensus value was achieved. Kaplan-Meier survival curves were then generated for the categorical K17 variable. Finally, the relationship between K17, p16, stage, and survival was explored as univariate predictors of survival, testing for interactions with K17, and then as subgroup analyses. Finally, a multivariable Cox regression was performed using any predictors with a univariate significance of P < .1.
As this was an exploratory, pilot study analysis of K17 biomarker findings, all calculations were performed at a 95% confidence level using SAS 9.4 software (SAS Institute, Cary, NC).
Results
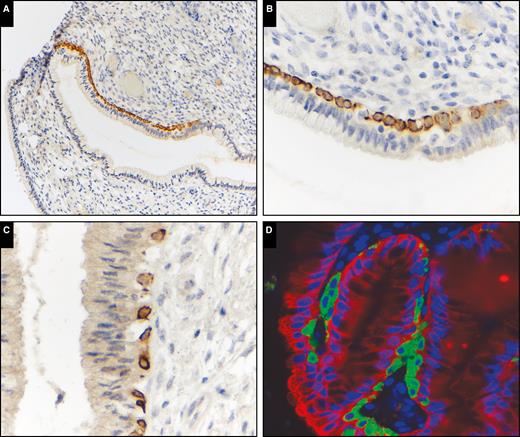
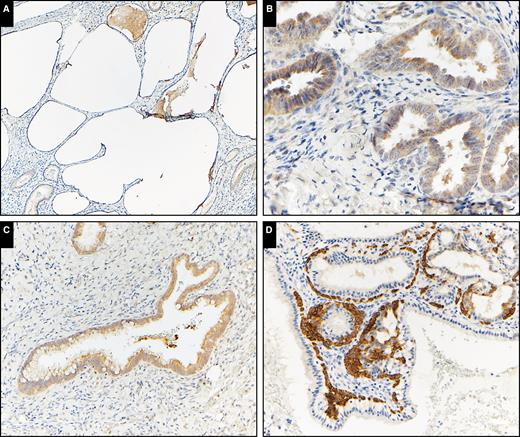
Immunohistochemical analysis for K17 in normal endocervical tissue showed sporadic staining of subcolumnar reserve cells, predominately located near the squamocolumnar junction. Dual immunofluorescence staining for K7 and K17 showed these reserve cells to be K17+/K7– and were a discrete population of cells present subjacent to K7-positive endocervical columnar epithelium Image 1. K17 staining was not detected in endocervical columnar epithelium with the exception of occasional staining of immature squamous metaplasia in five (12%) of 41 benign cases. K17 was not detected in columnar epithelium in the other benign categories with the exception of intense and diffuse staining of reserve cells only in 16 (100%) of 16 microglandular hyperplasia cases Image 2.
Keratin 17 (K17) expression in normal endocervical mucosa. Keratin immunohistochemistry: (A, ×200), (B, ×600), (C, ×600), and (D) dual immunofluorescence staining, K17 (green), K7 (red) (×600). K17 immunohistochemistry highlights a population of subcolumnar cells that are discrete from the surface K7-positive columnar cells. These cells are predominately located near the squamocolumnar junction.
Keratin 17 (K17) immunohistochemical expression in benign endocervical patterns. A, Tunnel clusters (×100). B, Endometriosis (×400). C, Tubal metaplasia (×200). D, Microglandular hyperplasia (×200). Intense K17 staining in subcolumnar reserve cells in microglandular hyperplasia but only faint staining in other benign endocervical patterns.
For the 90 adenocarcinoma tissue specimens analyzed, the mean patient age was 49 years. The mean time a patient was followed was 105 months (or 8.75 years). Around 16% of study patients had a high-stage (stage III or IV) tumor; 3.3% had positive lymph nodes and 10% had distant metastasis. p16 was present in 79% of cases. The mean K17 score was 34% Table 1 and Table 2.
Categorical Descriptive Characteristics
| Characteristic . | All, No. (%) . | Survived, No. (%) . | Did Not Survive, No. (%) . |
|---|---|---|---|
| Total | 90 (100) | 62 (69) | 28 (31) |
| Keratin 17 | |||
| 0%-39% | 58 (64.4) | 43 (74.1) | 15 (25.9) |
| 40%-89% | 20 (22.2) | 13 (65.0) | 7 (35.0) |
| 90%-100% | 12 (13.3) | 6 (50.0) | 6 (50.0) |
| Stage | |||
| Low | 75 (83.3) | 58 (78.5) | 17 (21.5) |
| High | 14 (15.6) | 3 (15.4) | 11 (84.6) |
| Missing | 1 (1.1) | 1 (75.0) | 0 (0.0) |
| Lymph nodes | |||
| Absent | 84 (93.3) | 61 (72.6) | 23 (27.4) |
| Present | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Missing | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Distant metastasis | |||
| No | 80 (88.9) | 61 (76.3) | 19 (23.8) |
| Yes | 10 (11.1) | 1 (10.0) | 9 (90.0) |
| p16 | |||
| Absent | 18 (20.0) | 7 (38.9) | 11 (61.1) |
| Present | 71 (78.9) | 54 (76.1) | 17 (23.9) |
| Missing | 1 (1.1) | 1 (100.0) | 0 (0.0) |
| Characteristic . | All, No. (%) . | Survived, No. (%) . | Did Not Survive, No. (%) . |
|---|---|---|---|
| Total | 90 (100) | 62 (69) | 28 (31) |
| Keratin 17 | |||
| 0%-39% | 58 (64.4) | 43 (74.1) | 15 (25.9) |
| 40%-89% | 20 (22.2) | 13 (65.0) | 7 (35.0) |
| 90%-100% | 12 (13.3) | 6 (50.0) | 6 (50.0) |
| Stage | |||
| Low | 75 (83.3) | 58 (78.5) | 17 (21.5) |
| High | 14 (15.6) | 3 (15.4) | 11 (84.6) |
| Missing | 1 (1.1) | 1 (75.0) | 0 (0.0) |
| Lymph nodes | |||
| Absent | 84 (93.3) | 61 (72.6) | 23 (27.4) |
| Present | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Missing | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Distant metastasis | |||
| No | 80 (88.9) | 61 (76.3) | 19 (23.8) |
| Yes | 10 (11.1) | 1 (10.0) | 9 (90.0) |
| p16 | |||
| Absent | 18 (20.0) | 7 (38.9) | 11 (61.1) |
| Present | 71 (78.9) | 54 (76.1) | 17 (23.9) |
| Missing | 1 (1.1) | 1 (100.0) | 0 (0.0) |
Categorical Descriptive Characteristics
| Characteristic . | All, No. (%) . | Survived, No. (%) . | Did Not Survive, No. (%) . |
|---|---|---|---|
| Total | 90 (100) | 62 (69) | 28 (31) |
| Keratin 17 | |||
| 0%-39% | 58 (64.4) | 43 (74.1) | 15 (25.9) |
| 40%-89% | 20 (22.2) | 13 (65.0) | 7 (35.0) |
| 90%-100% | 12 (13.3) | 6 (50.0) | 6 (50.0) |
| Stage | |||
| Low | 75 (83.3) | 58 (78.5) | 17 (21.5) |
| High | 14 (15.6) | 3 (15.4) | 11 (84.6) |
| Missing | 1 (1.1) | 1 (75.0) | 0 (0.0) |
| Lymph nodes | |||
| Absent | 84 (93.3) | 61 (72.6) | 23 (27.4) |
| Present | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Missing | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Distant metastasis | |||
| No | 80 (88.9) | 61 (76.3) | 19 (23.8) |
| Yes | 10 (11.1) | 1 (10.0) | 9 (90.0) |
| p16 | |||
| Absent | 18 (20.0) | 7 (38.9) | 11 (61.1) |
| Present | 71 (78.9) | 54 (76.1) | 17 (23.9) |
| Missing | 1 (1.1) | 1 (100.0) | 0 (0.0) |
| Characteristic . | All, No. (%) . | Survived, No. (%) . | Did Not Survive, No. (%) . |
|---|---|---|---|
| Total | 90 (100) | 62 (69) | 28 (31) |
| Keratin 17 | |||
| 0%-39% | 58 (64.4) | 43 (74.1) | 15 (25.9) |
| 40%-89% | 20 (22.2) | 13 (65.0) | 7 (35.0) |
| 90%-100% | 12 (13.3) | 6 (50.0) | 6 (50.0) |
| Stage | |||
| Low | 75 (83.3) | 58 (78.5) | 17 (21.5) |
| High | 14 (15.6) | 3 (15.4) | 11 (84.6) |
| Missing | 1 (1.1) | 1 (75.0) | 0 (0.0) |
| Lymph nodes | |||
| Absent | 84 (93.3) | 61 (72.6) | 23 (27.4) |
| Present | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Missing | 3 (3.3) | 1 (33.3) | 2 (66.7) |
| Distant metastasis | |||
| No | 80 (88.9) | 61 (76.3) | 19 (23.8) |
| Yes | 10 (11.1) | 1 (10.0) | 9 (90.0) |
| p16 | |||
| Absent | 18 (20.0) | 7 (38.9) | 11 (61.1) |
| Present | 71 (78.9) | 54 (76.1) | 17 (23.9) |
| Missing | 1 (1.1) | 1 (100.0) | 0 (0.0) |
Categorical Descriptive Characteristics
| Variable . | No. . | Mean (95% CI) . | Median . | Q1-Q3 . | Minimum-Maximum . |
|---|---|---|---|---|---|
| Total | |||||
| Age | 90 | 48.69 (45.74-51.64) | 45 | 39-56 | 24-87 |
| Months followed | 90 | 105.94 (86.68-125.21) | 76.5 | 39-134 | 1-341 |
| K17 score | 90 | 33.94 (26.68-41.20) | 20 | 5-70 | 0-100 |
| Survived | |||||
| Age | 62 | 45.60 (42.34-48.85) | 43 | 38-53 | 24-86 |
| Months followed | 62 | 131.40 (107.43-155.38) | 101 | 61-230 | 10-341 |
| K17 score | 62 | 31.47 (23.21-39.73) | 20 | 5-60 | 0-100 |
| Did not survive | |||||
| Age | 28 | 55.54 (49.87-61.20) | 54 | 45-65 | 32-87 |
| Months followed | 28 | 49.57 (28.45-70.69) | 33 | 13.5-60 | 1-239 |
| K17 score | 28 | 39.43 (24.29-54.57) | 35 | 2-75 | 0-100 |
| Variable . | No. . | Mean (95% CI) . | Median . | Q1-Q3 . | Minimum-Maximum . |
|---|---|---|---|---|---|
| Total | |||||
| Age | 90 | 48.69 (45.74-51.64) | 45 | 39-56 | 24-87 |
| Months followed | 90 | 105.94 (86.68-125.21) | 76.5 | 39-134 | 1-341 |
| K17 score | 90 | 33.94 (26.68-41.20) | 20 | 5-70 | 0-100 |
| Survived | |||||
| Age | 62 | 45.60 (42.34-48.85) | 43 | 38-53 | 24-86 |
| Months followed | 62 | 131.40 (107.43-155.38) | 101 | 61-230 | 10-341 |
| K17 score | 62 | 31.47 (23.21-39.73) | 20 | 5-60 | 0-100 |
| Did not survive | |||||
| Age | 28 | 55.54 (49.87-61.20) | 54 | 45-65 | 32-87 |
| Months followed | 28 | 49.57 (28.45-70.69) | 33 | 13.5-60 | 1-239 |
| K17 score | 28 | 39.43 (24.29-54.57) | 35 | 2-75 | 0-100 |
CI, confidence interval; K17, keratin 17; Q1, first quartile; Q3, third quartile.
Categorical Descriptive Characteristics
| Variable . | No. . | Mean (95% CI) . | Median . | Q1-Q3 . | Minimum-Maximum . |
|---|---|---|---|---|---|
| Total | |||||
| Age | 90 | 48.69 (45.74-51.64) | 45 | 39-56 | 24-87 |
| Months followed | 90 | 105.94 (86.68-125.21) | 76.5 | 39-134 | 1-341 |
| K17 score | 90 | 33.94 (26.68-41.20) | 20 | 5-70 | 0-100 |
| Survived | |||||
| Age | 62 | 45.60 (42.34-48.85) | 43 | 38-53 | 24-86 |
| Months followed | 62 | 131.40 (107.43-155.38) | 101 | 61-230 | 10-341 |
| K17 score | 62 | 31.47 (23.21-39.73) | 20 | 5-60 | 0-100 |
| Did not survive | |||||
| Age | 28 | 55.54 (49.87-61.20) | 54 | 45-65 | 32-87 |
| Months followed | 28 | 49.57 (28.45-70.69) | 33 | 13.5-60 | 1-239 |
| K17 score | 28 | 39.43 (24.29-54.57) | 35 | 2-75 | 0-100 |
| Variable . | No. . | Mean (95% CI) . | Median . | Q1-Q3 . | Minimum-Maximum . |
|---|---|---|---|---|---|
| Total | |||||
| Age | 90 | 48.69 (45.74-51.64) | 45 | 39-56 | 24-87 |
| Months followed | 90 | 105.94 (86.68-125.21) | 76.5 | 39-134 | 1-341 |
| K17 score | 90 | 33.94 (26.68-41.20) | 20 | 5-70 | 0-100 |
| Survived | |||||
| Age | 62 | 45.60 (42.34-48.85) | 43 | 38-53 | 24-86 |
| Months followed | 62 | 131.40 (107.43-155.38) | 101 | 61-230 | 10-341 |
| K17 score | 62 | 31.47 (23.21-39.73) | 20 | 5-60 | 0-100 |
| Did not survive | |||||
| Age | 28 | 55.54 (49.87-61.20) | 54 | 45-65 | 32-87 |
| Months followed | 28 | 49.57 (28.45-70.69) | 33 | 13.5-60 | 1-239 |
| K17 score | 28 | 39.43 (24.29-54.57) | 35 | 2-75 | 0-100 |
CI, confidence interval; K17, keratin 17; Q1, first quartile; Q3, third quartile.
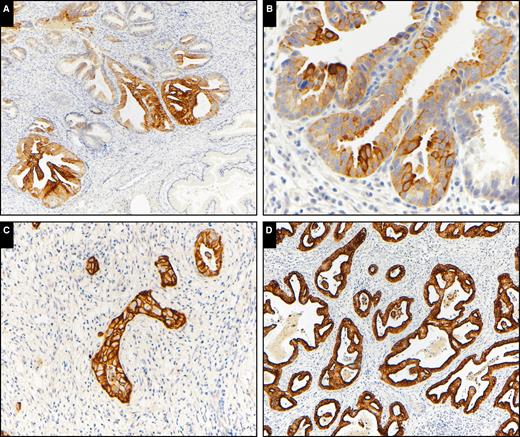
K17 was detected in most AIS and adenocarcinoma cases (88% and 83%, respectively), but there was no K17 expression seen in the columnar epithelium of any normal/benign tissue samples Image 3. To distinguish AIS/adenocarcinoma from normal mucosa/benign endocervical lesions, a potential diagnostic cutoff value of K17 was determined using a two-step process: a histogram, first generated for all 178 tissue samples, revealed a natural cutoff point for K17 at or below 10%. Then, NLR tests confirmed the K17 cutoff value of 0% or more to have the lowest NLR (NLR = .16), indicating a moderate refinement in the diagnostic process. Thus, this threshold may likely be useful for clinical decision-making purposes when combined with other relevant and/or expert clinician judgment. At this K17 diagnostic level of 0% or more, sensitivity was maximized (84.4%), as was specificity (100%).
Keratin 17 immunohistochemical expression in adenocarcinoma in situ (AIS) and invasive adenocarcinoma. A, AIS (×100). B, AIS (×400). C, Invasive adenocarcinoma (×200). D, Invasive adenocarcinoma (×100).
Within the cancer-specific tissues, there was no difference in continuous K17 score between low and high stage, presence or absence of p16, or mortality. There was a significant difference in rank score between tissues that were originally from Massachusetts compared with Stony Brook (table not shown).
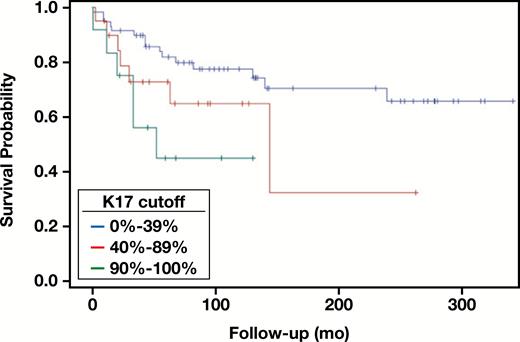
Distribution of K17 was examined and two values were identified as natural break points in the distribution. A polychotomous K17 variable was created to indicate those with K17 values from 0% to 39%, 40% to 89%, and 90% to 100% Image 4. Using these categories, a second reviewer (blinded to the first reviewer’s scores) reviewed and scored each slide. Interrater reliability was very strong (raw agreement = 96.7%; weighted κ = 0.95; 95% confidence interval, 0.90-1.0). After the consensus process, Kaplan-Meier survival curves were plotted Figure 1, and the Cox proportional hazards model found K17 to be a significant predictor of survival (Wald P = .03) Table 3 |and Table 4. While the 40% to 89% category did not significantly differ from the less than 40% category (hazard ratio [HR], 2.12; P = .9), those with 90% to 100% K17 scores had 3.47 times higher risk of death compared with the risk of death for those with a less than 40% K17 score (P = .01). Univariate analyses also revealed that the absence of p16 and high stage were both significantly associated with an increase in survival HR (p16 HR, 3.2 [P = .003]; stage HR, 5.62 [P < .01]) (Tables 3 and 4). While the relationship seemed to slightly attenuate after controlling for both stage and p16 in the multivariable analysis, the 90%+ K17 group remained a significant predictor of hazard of death (HR, 2.76; P = .0479). There were no significant interactions between K17 and p16 or K17 and stage; thus, no interaction variables were included in the final multivariable analysis. Finally, a subgroup analysis revealed that for those with p16 present, survival HRs increased for both K17 medium and high groups (40%-89% vs <40%: HR, 3.71 [P = .03]; 90%-100% vs <40%: HR, 5.19 [P = .01]). Within the p16 absent subgroup, the relationship between K17 and survival became insignificant. However, this may be likely due to the limited sample size (n = 18). Additional subgroup analyses revealed that within the low-stage subgroup, the 90%+ K17 group showed a marginally significant higher hazard of death compared with the less than 40% K17 group (HR, 3.09; P = .097).
Survival Analyses, K17 Univariate and Multivariable Hazard Ratios
| Description . | Point Estimate (95% CI) . | P Value . |
|---|---|---|
| Univariate hazard ratios | ||
| K17 40-89 vs <40 | 2.188 (0.826-5.267) | .092 |
| K17 90+ vs <40 | 3.469 (1.205-8.895) | .013 |
| p16 (no vs yes) | 3.197 (1.449-6.767) | .003 |
| Stage (high vs low) | 5.62 (2.612-12.106) | <.0001 |
| Multivariable hazard ratios | ||
| K17 40-89 vs <40 | 1.896 (0.718-4.539) | .167 |
| K17 90+ vs <40 | 2.758 (1.01-7.533) | .0479 |
| Stage (high vs low) | 4.174 (1.82-9.178) | .0005 |
| p16 (negative vs positive) | 2.472 (1.093-5.365) | .0243 |
| Description . | Point Estimate (95% CI) . | P Value . |
|---|---|---|
| Univariate hazard ratios | ||
| K17 40-89 vs <40 | 2.188 (0.826-5.267) | .092 |
| K17 90+ vs <40 | 3.469 (1.205-8.895) | .013 |
| p16 (no vs yes) | 3.197 (1.449-6.767) | .003 |
| Stage (high vs low) | 5.62 (2.612-12.106) | <.0001 |
| Multivariable hazard ratios | ||
| K17 40-89 vs <40 | 1.896 (0.718-4.539) | .167 |
| K17 90+ vs <40 | 2.758 (1.01-7.533) | .0479 |
| Stage (high vs low) | 4.174 (1.82-9.178) | .0005 |
| p16 (negative vs positive) | 2.472 (1.093-5.365) | .0243 |
CI, confidence interval; K17, keratin 17.
Survival Analyses, K17 Univariate and Multivariable Hazard Ratios
| Description . | Point Estimate (95% CI) . | P Value . |
|---|---|---|
| Univariate hazard ratios | ||
| K17 40-89 vs <40 | 2.188 (0.826-5.267) | .092 |
| K17 90+ vs <40 | 3.469 (1.205-8.895) | .013 |
| p16 (no vs yes) | 3.197 (1.449-6.767) | .003 |
| Stage (high vs low) | 5.62 (2.612-12.106) | <.0001 |
| Multivariable hazard ratios | ||
| K17 40-89 vs <40 | 1.896 (0.718-4.539) | .167 |
| K17 90+ vs <40 | 2.758 (1.01-7.533) | .0479 |
| Stage (high vs low) | 4.174 (1.82-9.178) | .0005 |
| p16 (negative vs positive) | 2.472 (1.093-5.365) | .0243 |
| Description . | Point Estimate (95% CI) . | P Value . |
|---|---|---|
| Univariate hazard ratios | ||
| K17 40-89 vs <40 | 2.188 (0.826-5.267) | .092 |
| K17 90+ vs <40 | 3.469 (1.205-8.895) | .013 |
| p16 (no vs yes) | 3.197 (1.449-6.767) | .003 |
| Stage (high vs low) | 5.62 (2.612-12.106) | <.0001 |
| Multivariable hazard ratios | ||
| K17 40-89 vs <40 | 1.896 (0.718-4.539) | .167 |
| K17 90+ vs <40 | 2.758 (1.01-7.533) | .0479 |
| Stage (high vs low) | 4.174 (1.82-9.178) | .0005 |
| p16 (negative vs positive) | 2.472 (1.093-5.365) | .0243 |
CI, confidence interval; K17, keratin 17.
Survival Analyses, K17 Compared to Stage and p16 Status
| Subgroup/K17 Group . | HR (95% CI) . | P Value . |
|---|---|---|
| Stage = low | ||
| K17 40-89 vs <40 | 1.879 (0.505-5.815) | .298 |
| K17 90+ vs <40 | 3.086 (0.674-10.644) | .097 |
| Stage = high | ||
| K17 40-89 vs <40 | 1.487 (0.297-6.222) | .594 |
| K17 90+ vs <40 | 1.689 (0.325-7.842) | .501 |
| p16 = absent | ||
| K17 40-89 vs <40 | 1.382 (0.202-5.887) | .691 |
| K17 90+ vs <40 | 2.593 (0.368-12.102) | .258 |
| p16 = present | ||
| K17cat 40-89 vs <40 | 3.714 (1.062-12.238) | .031 |
| K17cat 90+ vs <40 | 5.191 (1.292-18.824) | .013 |
| Subgroup/K17 Group . | HR (95% CI) . | P Value . |
|---|---|---|
| Stage = low | ||
| K17 40-89 vs <40 | 1.879 (0.505-5.815) | .298 |
| K17 90+ vs <40 | 3.086 (0.674-10.644) | .097 |
| Stage = high | ||
| K17 40-89 vs <40 | 1.487 (0.297-6.222) | .594 |
| K17 90+ vs <40 | 1.689 (0.325-7.842) | .501 |
| p16 = absent | ||
| K17 40-89 vs <40 | 1.382 (0.202-5.887) | .691 |
| K17 90+ vs <40 | 2.593 (0.368-12.102) | .258 |
| p16 = present | ||
| K17cat 40-89 vs <40 | 3.714 (1.062-12.238) | .031 |
| K17cat 90+ vs <40 | 5.191 (1.292-18.824) | .013 |
CI, confidence interval; HR, hazard ratio; K17, keratin 17.
Survival Analyses, K17 Compared to Stage and p16 Status
| Subgroup/K17 Group . | HR (95% CI) . | P Value . |
|---|---|---|
| Stage = low | ||
| K17 40-89 vs <40 | 1.879 (0.505-5.815) | .298 |
| K17 90+ vs <40 | 3.086 (0.674-10.644) | .097 |
| Stage = high | ||
| K17 40-89 vs <40 | 1.487 (0.297-6.222) | .594 |
| K17 90+ vs <40 | 1.689 (0.325-7.842) | .501 |
| p16 = absent | ||
| K17 40-89 vs <40 | 1.382 (0.202-5.887) | .691 |
| K17 90+ vs <40 | 2.593 (0.368-12.102) | .258 |
| p16 = present | ||
| K17cat 40-89 vs <40 | 3.714 (1.062-12.238) | .031 |
| K17cat 90+ vs <40 | 5.191 (1.292-18.824) | .013 |
| Subgroup/K17 Group . | HR (95% CI) . | P Value . |
|---|---|---|
| Stage = low | ||
| K17 40-89 vs <40 | 1.879 (0.505-5.815) | .298 |
| K17 90+ vs <40 | 3.086 (0.674-10.644) | .097 |
| Stage = high | ||
| K17 40-89 vs <40 | 1.487 (0.297-6.222) | .594 |
| K17 90+ vs <40 | 1.689 (0.325-7.842) | .501 |
| p16 = absent | ||
| K17 40-89 vs <40 | 1.382 (0.202-5.887) | .691 |
| K17 90+ vs <40 | 2.593 (0.368-12.102) | .258 |
| p16 = present | ||
| K17cat 40-89 vs <40 | 3.714 (1.062-12.238) | .031 |
| K17cat 90+ vs <40 | 5.191 (1.292-18.824) | .013 |
CI, confidence interval; HR, hazard ratio; K17, keratin 17.
Kaplan-Meier curves for keratin 17 as a polychotomous variable.
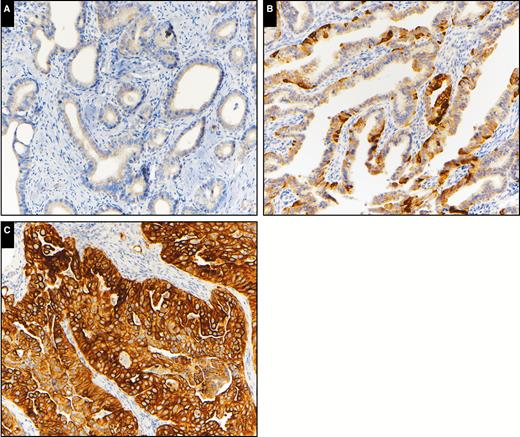
Keratin 17 immunohistochemical expression in invasive adenocarcinoma. A, Low K17 expression (×200). B, Intermediate K17 expression (×200). C, High K17 expression (×200).
Discussion
K17 was identified in normal cervical tissue and cervical adenocarcinoma (n = 1) in the mid-1980s while profiling intermediate filaments for diagnostic purposes.21 Early studies of K17 in tissue were greatly limited by lack of access to antibodies that were reactive in FFPE.22 In 1989, the monoclonal antibody (E3) directed against K17 was identified,23 and subsequent studies in the 1990s looking at a limited number of cases of glandular neoplasia found weak- to moderate-intensity staining in most cells of adenocarcinoma cases as well as identifying a reserve cell (RC)/stem cell phenotype (CK 8, 14, 16, 17, 18, and 19 and bcl-2) of the normal endocervix.24,25 It was hypothesized that cervical lesions that maintain an RC phenotype may develop into malignant lesions while loss of an RC phenotype may predict lesions that are regressive in nature and that cervical adenocarcinomas do not arise from the columnar cells of the endocervix but rather the RC compartment, where K17 expression was always found.24 This bipotential nature/keratin expression profile of RC could suggest that a cervical intraepithelial neoplasia lesion could give rise to adenocarcinoma with persistence of all of the cytokeratins except K5, K6, and K16.22 RCs also have been studied in microglandular hyperplasia, a common endocervical glandular proliferation that can either produce a stable population of RCs or terminate in mature squamous metaplasia. It has been postulated that the plastic nature of RC cells and the molecular mechanisms governing these phenotype switches in microglandular hyperplasia might explain the multiplicity of neoplastic phenotypes (squamous and glandular) observed in cervical neoplasia.26
Since the first microscopic investigations of the endocervical canal, the presence of RCs has been noted. On light microscopy, these cells are small cuboidal cells with relatively large nuclei that are located subjacent to the columnar epithelium and between the basement membrane of endocervical glands. Anatomically, these cells are present in the proximal endocervix and have a discontinuous distribution pattern, with the greatest concentration being present at the squamo-columnar junction.27 It is believed that these RCs regenerate the columnar epithelium as well as respond to trauma and hormonal cues by enabling columnar to squamous metaplasia.26 RCs have been shown to have a complex yet distinct keratin expression pattern, which includes K5, K8, K14, K17, K18, and K19,28 with K17 being the most specific marker for RCs and RC hyperplasia.29 These cells are noncycling (Ki-67 negative) and unrelated to human papillomavirus (HPV) status (p16 negative), and it is believed that these RCs function as the stem cell population of the cervix, which is supported by studies showing ubiquitous expression of Bcl-2 and p63 in these cells.25,29,30
A columnar epithelial hypothesis of cervical carcinogenesis has been proposed. In this hypothesis, the HPV target cell is not the basal layer of the squamous epithelium but the subcolumnar RCs of the endocervical mucosa.31 This HPV target cell can be identified by the combined expression of K17 and p63, and once this target cell is infected by HPV, it has two potential fates: the cells can remain in their original form or become benign squamous epithelium by normal metaplasia; however, if the cells undergo HPV-mediated transformation, they can produce AIS or further undergo atypical metaplasia to produce a squamous intraepithelial lesion, which is a logical explanation for why endocervical glandular and squamous lesions often occur together.29 This theory also correlates with the anatomic location of RCs, which are located near the squamocolumnar junction and could explain why recurrence rates are lower following excisional or ablative treatments of the proximal endocervical canal.32
K17 has been studied previously in small numbers of adenocarcinoma cases secondarily in the context of cytokeratin profiling, and to our knowledge, no studies have looked specifically at K17 expression in human FFPE tissue as a clinical prognostic indicator in cervical adenocarcinoma and adenocarcinoma in situ.22,24,25 Prior studies have shown that K17 expression can be correlated with poor prognosis since CD44+/K17+ cells derived from primary cervical carcinoma have been shown to have stem-like properties, including the capacity for cell renewal, chemoresistance, and in vivo tumorigenicity.33 In bladder cancer, K17 has been shown to mark a basal-like population of highly tumorigenic cancer cells that are located at the invasive tumor front.17 K17 has also been shown to be a poor prognostic indicator in other epithelial malignancies, including gastric adenocarcinoma, bladder carcinoma, ovarian carcinoma, and triple-negative breast carcinoma.16-19 Since K17 is overexpressed in many different types of cancer, it is unlikely to be a specific marker for primary cervical cancer vs carcinomas that have spread to the cervix from other anatomic sites. Mechanistic insight into its role as a prognostic biomarker, however, is based on recent work from our laboratory that has shown that K17 functions as an oncoprotein by mediating cancer cell-cycle progression by promoting p27KIP1 nuclear export and degradation in cervical SCC, and similar mechanisms are likely to be important in endocervical adenocarcinoma.15
It should be noted that we did not detect p16 staining in 18 (20%) of 90 cases. Although most cervical adenocarcinomas are associated with high-risk HPV, it should be noted that in recent years, it has been reported that the more uncommon morphologic subtypes of endocervical adenocarcinoma, such as clear cell and adenoma malignum (which were included in our study), are often unassociated with HPV infection.34
In summary, we have shown that K17 expression in normal endocervix is limited to subcolumnar RCs near the squamocolumnar junction, and expression in the columnar endocervical epithelium was highly specific for glandular neoplasia of the cervix. Most important, we further determined that high K17 expression is a powerful, negative prognostic biomarker that can be used to identify patients who have the shortest survival probability following the diagnosis of endocervical adenocarcinoma. Thus, K17 immunohistochemical test results could provide important additional data to help inform clinical decisions related to optimal therapeutic intervention.
This work was supported by academic enrichment funds of the Department of Pathology at Stony Brook Medicine.
Drs Escobar-Hoyos and Shroyer served as members of the advisory board for OncoGenesis and were compensated for stock options.
Acknowledgments
We thank Stephanie Burke and Mallory Korman for their expert histologic assistance, as well as Grace Swennson and the staff at the Cancer Registry Database of Stony Brook Medicine for generously providing their time and help with this study.
References
Author notes
Corresponding author: Kenneth R. Shroyer, MD, PhD, Dept of Pathology, Basic Science Tower, Level 9, Stony Brook Medicine, Stony Brook, New York, NY 11794-8691; kenneth.shroyer@stonybrookmedicine.edu.