-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher J. Bowman, Katie J. Turner, Madhabananda Sar, Norman J. Barlow, Kevin W. Gaido, Paul M. D. Foster, Altered Gene Expression During Rat Wolffian Duct Development following Di(n-Butyl) Phthalate Exposure, Toxicological Sciences, Volume 86, Issue 1, July 2005, Pages 161–174, https://doi.org/10.1093/toxsci/kfi172
Close - Share Icon Share
Abstract
Di(n-butyl) phthalate (DBP) is a common plasticizer and solvent that disrupts androgen-dependent male reproductive development in rats. In utero exposure to 500 mg/kg/day DBP on gestation days (GD) 12 to 21 decreases androgen biosynthetic enzymes, resulting in decreased fetal testicular testosterone levels. One consequence of prenatal DBP exposure is malformed epididymides in adult rats. Reduced fetal testosterone levels may be responsible for the malformation, since testosterone is required for Wolffian duct stabilization and their development into epididymides. Currently, little is understood about the molecular mechanisms of Wolffian duct differentiation. The objective of this study was to identify changes in gene expression associated with altered morphology of the proximal Wolffian duct following in utero exposure to DBP. Pregnant Crl:CD® (SD) rats were gavaged with corn oil vehicle or 500 mg/kg/day DBP from GD 12 to GD 19 or 21. There were only small morphological differences between control and DBP-exposed Wolffian ducts on GD 19. On GD 21, 89% of male fetuses in the DBP dose group showed marked underdevelopment of Wolffian ducts, characterized by decreased coiling. RNA was isolated from Wolffian ducts on GD 19 and 21. Together with empirical information, cDNA microarrays were used to help identify candidate genes that could be associated with the morphological changes observed on GD 21. These candidate genes were analyzed by real-time RT-PCR. Changes in mRNA expression were observed in genes within the insulin-like growth factor (IGF) pathway, the matrix metalloproteinase (MMP) family, the extracellular matrix, and in other developmentally conserved signaling pathways. On GD 19, immunolocalization of IGF-1 receptor protein demonstrated an increase in cytoplasmic expression in the mesenchymal and epithelial cells. There was also a variable decrease in androgen receptor protein in ductal epithelial cells on GD 19. This study provides insight into the effects of antiandrogens on the molecular mechanisms involved in Wolffian duct development. The altered morphology and changes in gene expression following DBP exposure are suggestive of altered paracrine interactions between ductal epithelial cells and the surrounding mesenchyme during Wolffian duct differentiation due to lowered testosterone production.
Fetal testicular testosterone production during gestation is critical for stabilization and differentiation of the Wolffian ducts (WD) into epididymides, vasa deferentia, and seminal vesicles in male rats (George and Wilson, 1994). Fetal testosterone synthesis begins in the testes of male rats around gestation day (GD) 15.5 and peaks around GD 19.5 (Warren et al., 1973), during which time the upper WD are stabilized, with subsequent coiling of the duct observed by GD 21. The ducts within undifferentiated urogenital ridge cultures in mice can be induced to coil by androgens and blocked by antiandrogens (Tsuji et al., 1991a), thus demonstrating the necessity of testosterone in WD development. Paracrine factors (such as growth factors) are thought to regulate the mesenchymal-epithelial signaling necessary for testosterone-induced ductal coiling and proliferation of epithelial cells within the male reproductive tract (Cunha, 1996; Cunha et al., 1992; Thomson, 2001). The importance of paracrine control was demonstrated in recombination experiments where seminal vesicle mesenchyme, combined with upper WD epithelium, induced the differentiation of a seminal vesicle rather than an epididymis (Higgins et al., 1989). A model has been proposed whereby testosterone and growth factors regulate mesenchymal-epithelial interactions with involvement of the extracellular matrix components during androgen-dependent reproductive tract development (Luke and Coffey, 1994). However, the available information on WD development regarding this model is limited.
Di(n-butyl) phthalate (DBP), one of many phthalate esters, is primarily used as a plasticizer and solvent and is widely distributed as an environmental contaminant (IPCS, 1997). Adverse effects on male reproductive development have been observed in animals exposed pre- and postnatally to DBP, in the absence of maternal toxicity (Mylchreest et al., 1998; NTP, 1991; Wine et al., 1997). Male rats, exposed to DBP only during late gestation, exhibited similar adverse effects to those observed in previous studies of longer duration, including malformed epididymides, vasa deferentia, seminal vesicles, and prostates in addition to hypospadias, cryptorchidism, decreased anogenital distance, and increased nipple retention (Barlow and Foster, 2003; Gray et al., 1999; Mylchreest et al., 1999, 2000). Malformations of the epididymis are one of the most sensitive and consistent lesions in adult male rats associated with prenatal DBP exposure, with an overall pup incidence of 40 to 50% (82 to 89% litter incidence) at 500 mg/kg/day when administered daily from GD 12 to 21 (Mylchreest et al., 1999, 2000). DBP-exposed epididymides are characterized by decreased coiling of the epididymis and an increased amount of intervening fibrous connective tissue stroma. Neither DBP nor its monoester metabolite bind to the androgen receptor (AR) (Foster et al., 2001); however, DBP exposure during gestation reduces fetal testicular testosterone levels (Mylchreest et al., 2002; Shultz et al., 2001). This decrease in fetal testicular testosterone following prenatal DBP exposure (500 mg/kg/day) is a result of decreased fetal testicular mRNA and protein expression of several proteins required for cholesterol transport and steroidogenesis (Thompson et al., 2004). Lehmann et al. (2004) has demonstrated through dose-response studies that fetal testosterone and testicular expression of several proteins required for cholesterol transport and steroidogenesis are decreased significantly following in utero DBP exposure as low as 50 mg/kg/day. This decrease in fetal testicular testosterone following DBP exposure is the likely primary mode of action responsible for altered WD differentiation that results in epididymal malformations observed in adult rats (Mylchreest et al., 2002). GD 19 and 21 are sensitive time points for evaluation of WD development because ductal coiling has not yet started on GD 19, but by GD 21 the WD is highly coiled and differentiated in controls, but following exposure to 500 mg/kg/day of DBP, lack of coiling is observed by GD 21 (Barlow and Foster, 2003). In utero exposure to the androgen receptor antagonist linuron induces a similar abnormal phenotype of the WD, but fetal testicular testosterone levels do not appear to be altered (McIntyre et al., 2002). The decreased coiling of the WD induced by linuron has been examined at the molecular level, and some critical developmental pathways have been identified (Turner et al., 2003) that may begin to explain the malformations of the epididymis in adult rats following prenatal exposure to this androgen receptor antagonist (McIntyre et al., 2002).
The epididymal malformations induced by prenatal DBP exposure represent an experimental model to improve understanding of the mechanisms responsible for androgen-dependent development of the male reproductive tract. Knowledge of the mechanisms involved in the induction of epididymal malformations could ultimately be useful in determining the relative risk to human health from prenatal exposure to DBP. The objective of the present study was to identify gene expression changes associated with the abnormal differentiation of the upper WD in DBP-exposed rat fetuses. Pregnant rats were treated with DBP (500 mg/kg/day) from GD 12 to GD 19 or 21, since this has been shown in previous studies to maximize the incidence of epididymal abnormalities (Barlow and Foster, 2003). Microarrays and real-time reverse transcription polymerase chain reaction (RT-PCR) were utilized to identify candidate genes altered by DBP exposure during WD stabilization and differentiation. In addition, insulin-like growth factor 1 receptor (IGF1R) and AR protein were evaluated by immunohistochemistry to identify corresponding changes in the localization and/or intensity of protein expression on GD 19 in an effort to further characterize the effects of DBP on paracrine and androgen signaling during development in a cell-specific manner.
MATERIAL AND METHODS
Animals.
This study was conducted in accordance with federal guidelines for the care and use of laboratory animals (National Research Council, 1996) and was approved by the Institutional Animal Care and Use Committee at the CIIT Centers for Health Research (CIIT). Time-mated, 8- to 10-week-old, nulliparous Crl:CD® (SD) rats were obtained from Charles River Laboratories Inc. (Raleigh, NC) on gestation day (GD) 0. GD 0 was defined as the day sperm was found in the vagina of the mated female. Animal allocation to treatment groups was done by body weight randomization to ensure unbiased weight distribution among groups. Animals were housed in the CIIT animal care facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International. Animals were kept in a HEPA-filtered, mass air-displacement room with a 12-h, light-dark cycle at 18–26°C and relative humidity of 30–70%. Animals had access to deionized water and rodent chow ad libitum (NIH-07, Zeigler Brothers, Gardner, PA). Individual dams were housed in polycarbonate cages on ALPHA-dri bedding (Shepherd Specialty Papers, Kalamazoo, MI) until necropsy on GD 19 or 21 (prior to natural delivery).
Treatment.
Sperm-positive rats from each treatment block were gavaged daily (0800–0900) from GD 12 to 19 or 21 with either corn oil vehicle (Sigma Chemical Company, St. Louis, MO) or di(n-butyl) phthalate (99.8%, Aldrich Chemical Company, Milwaukee, WI) in corn oil at 500 mg/kg/day at a volume of 1 ml/kg/day. Dams were examined daily for clinical signs of toxicity. Dam body weights were recorded daily during dosing.
Study design.
The study was performed using three separate blocks of animals. Dams from all blocks were euthanized by carbon dioxide asphyxiation and exsanguination followed by removal of the fetuses by cesarean section. The developing WD and fetal testes were removed from all male fetuses and examined using a dissecting microscope with transillumination, and the gross morphology of the tissues was recorded. Two blocks of animals were euthanized on GD 19. One block consisted of six dams dosed with corn oil and seven dams with 500 mg/kg/day DBP, and the second block consisted of four corn oil-exposed and four DBP-exposed dams. The tissues collected from the first block of animals were snap-frozen in liquid nitrogen immediately after dissection and used for molecular analyses. Tissues collected from the second block of animals were fixed in neutral-buffered formalin for 12 h and subsequently processed for immunohistochemistry. A third block of dams was euthanized on GD 21 and consisted of six dams dosed with corn oil and seven dams with 500 mg/kg/day DBP. The tissues collected from the third block of animals were immediately frozen in liquid nitrogen for subsequent molecular analyses.
cDNA microarrays.
Wolffian ducts were pooled from three to four fetuses within the same litter to obtain sufficient RNA for the molecular analyses (each pool was considered a sample). For GD 21 fetuses, only WD with similar ductal morphology was pooled. Total RNA was isolated from WD on GD 19 and 21 using STAT-60™ (Tel-Test, Inc., Friendswood, TX). Total RNA was DNase-treated with RNase-free DNase (Roche, Indianapolis, IN) at 37°C for 30 min in the presence of RNasin (Applied Biosystems, Foster City, CA). Reverse-transcription (RT) reactions were performed using 3.5 μg of total RNA, 32P-dATP, and Superscript-II MMLV reverse transcriptase (Gibco-BRL, Gaithersburg, MD) for 60 min at 50°C. Following purification with ProbeQuant™ G-50 MicroColumns (Amersham Biosciences, Piscataway, NJ), probes were added to each Clontech (Palo Alto, CA) Atlas Rat Toxicology 1.2 cDNA expression array (1,185 genes). Hybridization and washing were performed according to manufacturer's instructions. Arrays were placed on a phosphor screen for 1–2 days, and images collected using a PhosphorImager™ SI (Molecular Dynamics, Amersham Biosciences Corp., Piscataway, NJ). These images were imported into AtlasImage software (Clontech) for data collection. Arrays were analyzed by AtlasImage 2.01 and GeneSpring 4.2 (Silicon Genetics, Redwood, CA). Array analysis was performed on four to seven pooled samples (representing at least three individual litters) per treatment group conducted with three or four arrays per treatment group per hybridization.
Real-time RT-PCR.
Real-time RT-PCR using the GeneAmp® 5700 Sequence Detection System (Applied Biosystems) was performed to analyze changes in gene expression. DNase-treated total RNA (1 μg) from each tissue pool (described previously) was aliquoted in quadruplicate for RT, with one aliquot receiving no enzyme. RT was performed using the TaqMan® RT reagents (Applied Biosystems) and manufacturer's instructions. The quality of the RT reactions was confirmed by PCR of each triplicate RT compared to the no-enzyme control for each RNA sample using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and manufacturer's protocols (Applied Biosystems) for PCR. For GAPDH RT samples, the PCRs were performed using SYBR® Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions using a reaction volume of 25 μl. Sequences of the rat-specific primer sets used are listed in Table 1. The primer sets with asterisks have been described previously (Turner et al., 2003). New primer sets were designed by Primer Express 2.0 software (Applied Biosystems). For each primer set, the production of a single PCR product was confirmed using gel electrophoresis, and primer efficiency was determined according to the manufacturer's protocols (Applied Biosystems). Real-time RT-PCR analyses were performed in triplicate on four to ten pooled samples (representing three to seven individual litters) per treatment group. PCR reactions for specific mRNAs of interest were performed in parallel with reactions using GAPDH specific primers to allow for standardization across samples for cDNA input. Relative quantitation of gene expression for samples from DBP-exposed fetuses compared to controls was performed according to User Bulletin #2 (Applied Biosystems).
Sequences of Primer Sets Used for Real-Time RT-PCR Analysis of Gene Expression on GeneAmp® 5700 Sequence Detection System
Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| AR | TGATTGCACCATTGATAAATTTCG | GCTTACGAGCTCCCAGAGTCA |
| BMP4* | ATGTGACACGGTGGGAAACTTTC | ACCTCAATGGCCAGCCCATA |
| Collagen | TCACTCAAGTCTGTTAACGGACAAA | GGTGGCAGAATTTCAGGTCTCT |
| DLK* | CGTGCTCACCAGCCTGGTT | GTGGTTGTAGCGCAGGTTGGA |
| EGFR | AACCAACAAAACTGGGCTTAGG | CGCACAGCACCGATCAGA |
| FGF10* | GCTGCTGTTGCTGCTTCTTGTTG | GCTGCTGTTGCTGCTTCTTGTTG |
| FGFR2* | TTCCCCAGATTACCTGGAGATAGC | TCGTGGTCTTCATTCGGCAA |
| Fibronectin* | ACGTGCAGGCTGACAGAGATGA | GGGTGTGGATTGACCTTGGTAGA |
| GAPDH* | AATGTATCCGTTGTGGATCTGA | GCCTGCTTCACCACCTTCT |
| IGF1* | CACAGGCTATGGCTCCAGCAT | TCTCCAGCCTCCTCAGATCACA |
| IGF2 | TCTCGAGGCACCCTAAATTACC | CAGAGGATGGTCAGCAAATGC |
| IGF1R* | CGCTGTGTGGACCGGGATTT | GCATGCACTCGCCATCGTG |
| IGFBP5* | GTGACCGCAAAGGATTCTACAAGA | GGCAGCTTCATCCCATACTTGTC |
| IntegrinA5 | CACCGCAGGCTGACTTCATC | TGCCGGGTTTGGTTTTCTGT |
| IntegrinB1 | TTGGTCAGCAGCGCATATCTG | GCCAATCAGCGACCCACAA |
| MGP | GCCGCCTACAACCGCTACTT | CAGACTCCGTAACAAAGCGACTGT |
| MMP2 | GTGCTGAAGGACACCCTCAAGAA | TCCGCATGGTCTCGATGGT |
| MMP14 | CCAAGGCGACTGCGCTCTAG | GCTCTCTTGGGCGCCTTTG |
| MMP16* | AGGAGCCAAACCCAACATCTGT | CCAAAACCACTGGTCCTTGAAAA |
| Notch2* | AGGCCCCTTGCCCTCTATGT | TGATAGGTTGGGACAATGGTCTGA |
| TIMP1 | GGGCTACCAGAGCGATCACTTT | GGTATTGCCAGGTGCACAAATC |
| TIMP2 | CGCTTAGCATCACCCAGAAGA | TGCACTCGCAGCCCATCT |
| TIMP3* | TACCCTGGCTATCAGTCCAAACA | GCTGCAGTAGCCACCCTTCT |
Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| AR | TGATTGCACCATTGATAAATTTCG | GCTTACGAGCTCCCAGAGTCA |
| BMP4* | ATGTGACACGGTGGGAAACTTTC | ACCTCAATGGCCAGCCCATA |
| Collagen | TCACTCAAGTCTGTTAACGGACAAA | GGTGGCAGAATTTCAGGTCTCT |
| DLK* | CGTGCTCACCAGCCTGGTT | GTGGTTGTAGCGCAGGTTGGA |
| EGFR | AACCAACAAAACTGGGCTTAGG | CGCACAGCACCGATCAGA |
| FGF10* | GCTGCTGTTGCTGCTTCTTGTTG | GCTGCTGTTGCTGCTTCTTGTTG |
| FGFR2* | TTCCCCAGATTACCTGGAGATAGC | TCGTGGTCTTCATTCGGCAA |
| Fibronectin* | ACGTGCAGGCTGACAGAGATGA | GGGTGTGGATTGACCTTGGTAGA |
| GAPDH* | AATGTATCCGTTGTGGATCTGA | GCCTGCTTCACCACCTTCT |
| IGF1* | CACAGGCTATGGCTCCAGCAT | TCTCCAGCCTCCTCAGATCACA |
| IGF2 | TCTCGAGGCACCCTAAATTACC | CAGAGGATGGTCAGCAAATGC |
| IGF1R* | CGCTGTGTGGACCGGGATTT | GCATGCACTCGCCATCGTG |
| IGFBP5* | GTGACCGCAAAGGATTCTACAAGA | GGCAGCTTCATCCCATACTTGTC |
| IntegrinA5 | CACCGCAGGCTGACTTCATC | TGCCGGGTTTGGTTTTCTGT |
| IntegrinB1 | TTGGTCAGCAGCGCATATCTG | GCCAATCAGCGACCCACAA |
| MGP | GCCGCCTACAACCGCTACTT | CAGACTCCGTAACAAAGCGACTGT |
| MMP2 | GTGCTGAAGGACACCCTCAAGAA | TCCGCATGGTCTCGATGGT |
| MMP14 | CCAAGGCGACTGCGCTCTAG | GCTCTCTTGGGCGCCTTTG |
| MMP16* | AGGAGCCAAACCCAACATCTGT | CCAAAACCACTGGTCCTTGAAAA |
| Notch2* | AGGCCCCTTGCCCTCTATGT | TGATAGGTTGGGACAATGGTCTGA |
| TIMP1 | GGGCTACCAGAGCGATCACTTT | GGTATTGCCAGGTGCACAAATC |
| TIMP2 | CGCTTAGCATCACCCAGAAGA | TGCACTCGCAGCCCATCT |
| TIMP3* | TACCCTGGCTATCAGTCCAAACA | GCTGCAGTAGCCACCCTTCT |
Primer sets previously described by Turner et al. (2003).
Sequences of Primer Sets Used for Real-Time RT-PCR Analysis of Gene Expression on GeneAmp® 5700 Sequence Detection System
Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| AR | TGATTGCACCATTGATAAATTTCG | GCTTACGAGCTCCCAGAGTCA |
| BMP4* | ATGTGACACGGTGGGAAACTTTC | ACCTCAATGGCCAGCCCATA |
| Collagen | TCACTCAAGTCTGTTAACGGACAAA | GGTGGCAGAATTTCAGGTCTCT |
| DLK* | CGTGCTCACCAGCCTGGTT | GTGGTTGTAGCGCAGGTTGGA |
| EGFR | AACCAACAAAACTGGGCTTAGG | CGCACAGCACCGATCAGA |
| FGF10* | GCTGCTGTTGCTGCTTCTTGTTG | GCTGCTGTTGCTGCTTCTTGTTG |
| FGFR2* | TTCCCCAGATTACCTGGAGATAGC | TCGTGGTCTTCATTCGGCAA |
| Fibronectin* | ACGTGCAGGCTGACAGAGATGA | GGGTGTGGATTGACCTTGGTAGA |
| GAPDH* | AATGTATCCGTTGTGGATCTGA | GCCTGCTTCACCACCTTCT |
| IGF1* | CACAGGCTATGGCTCCAGCAT | TCTCCAGCCTCCTCAGATCACA |
| IGF2 | TCTCGAGGCACCCTAAATTACC | CAGAGGATGGTCAGCAAATGC |
| IGF1R* | CGCTGTGTGGACCGGGATTT | GCATGCACTCGCCATCGTG |
| IGFBP5* | GTGACCGCAAAGGATTCTACAAGA | GGCAGCTTCATCCCATACTTGTC |
| IntegrinA5 | CACCGCAGGCTGACTTCATC | TGCCGGGTTTGGTTTTCTGT |
| IntegrinB1 | TTGGTCAGCAGCGCATATCTG | GCCAATCAGCGACCCACAA |
| MGP | GCCGCCTACAACCGCTACTT | CAGACTCCGTAACAAAGCGACTGT |
| MMP2 | GTGCTGAAGGACACCCTCAAGAA | TCCGCATGGTCTCGATGGT |
| MMP14 | CCAAGGCGACTGCGCTCTAG | GCTCTCTTGGGCGCCTTTG |
| MMP16* | AGGAGCCAAACCCAACATCTGT | CCAAAACCACTGGTCCTTGAAAA |
| Notch2* | AGGCCCCTTGCCCTCTATGT | TGATAGGTTGGGACAATGGTCTGA |
| TIMP1 | GGGCTACCAGAGCGATCACTTT | GGTATTGCCAGGTGCACAAATC |
| TIMP2 | CGCTTAGCATCACCCAGAAGA | TGCACTCGCAGCCCATCT |
| TIMP3* | TACCCTGGCTATCAGTCCAAACA | GCTGCAGTAGCCACCCTTCT |
Gene . | Forward primer . | Reverse primer . |
|---|---|---|
| AR | TGATTGCACCATTGATAAATTTCG | GCTTACGAGCTCCCAGAGTCA |
| BMP4* | ATGTGACACGGTGGGAAACTTTC | ACCTCAATGGCCAGCCCATA |
| Collagen | TCACTCAAGTCTGTTAACGGACAAA | GGTGGCAGAATTTCAGGTCTCT |
| DLK* | CGTGCTCACCAGCCTGGTT | GTGGTTGTAGCGCAGGTTGGA |
| EGFR | AACCAACAAAACTGGGCTTAGG | CGCACAGCACCGATCAGA |
| FGF10* | GCTGCTGTTGCTGCTTCTTGTTG | GCTGCTGTTGCTGCTTCTTGTTG |
| FGFR2* | TTCCCCAGATTACCTGGAGATAGC | TCGTGGTCTTCATTCGGCAA |
| Fibronectin* | ACGTGCAGGCTGACAGAGATGA | GGGTGTGGATTGACCTTGGTAGA |
| GAPDH* | AATGTATCCGTTGTGGATCTGA | GCCTGCTTCACCACCTTCT |
| IGF1* | CACAGGCTATGGCTCCAGCAT | TCTCCAGCCTCCTCAGATCACA |
| IGF2 | TCTCGAGGCACCCTAAATTACC | CAGAGGATGGTCAGCAAATGC |
| IGF1R* | CGCTGTGTGGACCGGGATTT | GCATGCACTCGCCATCGTG |
| IGFBP5* | GTGACCGCAAAGGATTCTACAAGA | GGCAGCTTCATCCCATACTTGTC |
| IntegrinA5 | CACCGCAGGCTGACTTCATC | TGCCGGGTTTGGTTTTCTGT |
| IntegrinB1 | TTGGTCAGCAGCGCATATCTG | GCCAATCAGCGACCCACAA |
| MGP | GCCGCCTACAACCGCTACTT | CAGACTCCGTAACAAAGCGACTGT |
| MMP2 | GTGCTGAAGGACACCCTCAAGAA | TCCGCATGGTCTCGATGGT |
| MMP14 | CCAAGGCGACTGCGCTCTAG | GCTCTCTTGGGCGCCTTTG |
| MMP16* | AGGAGCCAAACCCAACATCTGT | CCAAAACCACTGGTCCTTGAAAA |
| Notch2* | AGGCCCCTTGCCCTCTATGT | TGATAGGTTGGGACAATGGTCTGA |
| TIMP1 | GGGCTACCAGAGCGATCACTTT | GGTATTGCCAGGTGCACAAATC |
| TIMP2 | CGCTTAGCATCACCCAGAAGA | TGCACTCGCAGCCCATCT |
| TIMP3* | TACCCTGGCTATCAGTCCAAACA | GCTGCAGTAGCCACCCTTCT |
Primer sets previously described by Turner et al. (2003).
Immunohistochemistry.
Immunohistochemistry was performed to evaluate changes in localization and/or intensity of IGF-1Rβ and AR protein expression. Immunohistochemistry was performed on at least 15 fetuses from a minimum of three individual litters per exposure group. GD 19 WDs were carefully removed from the fetus, fixed in neutral-buffered formalin, and embedded in paraffin; 5-μm sections were placed on charged slides and stored at room temperature. The sections were deparaffinized with xylene and treated with 3% H2O2 in methanol for 10 min to minimize interference from endogenous peroxidase activity. Antigen retrieval was performed by heating the sections for 3 min and 9 min in citrate buffer (1:10 dilution in distilled water, pH 5.5–5.7) (BioGenex, San Ramon, CA) for IGF-1Rβ and AR, respectively. Immunostaining was performed using the avidin-biotin peroxidase method described previously (Sar and Welsch, 1999). The sections were treated with 10% powdered milk in PBS for 10 min followed by 2% normal rabbit serum in PBS for 20 min to reduce nonspecific staining. The tissue sections were incubated for 15 min each with avidin and biotin to suppress endogenous biotin activity. The sections were incubated with the antibodies overnight at 4°C. IGF-1Rβ was localized using a polyclonal anti-IGF-1Rβ antibody (1.0 μg/ml) from Santa Cruz Biotechnology (Santa Cruz, CA), and AR was localized using a polyclonal anti-AR antibody (2.0 μg/ml) from Affinity Bioreagents, Inc. (Golden, CO) (You and Sar, 1998; You et al., 2002). Following incubation with the primary antibody, the slides were washed in PBS for 5 min, followed by incubation with a biotinylated secondary antibody anti-rabbit IgG (1:200) and then with avidin-biotin peroxidase (1:200) (Vector Labs, Burlingame, CA) for 30 min at room temperature. Following a 5-min wash in PBS, the sections were exposed to liquid diaminobenzidine (BioGenex). The slides were then counterstained with hematoxylin, and mounted with Permount.
Statistical analysis.
Statistical analyses were conducted using JMP (version 4.0.4, SAS Institute, Cary, NC), and p < 0.05 was considered statistically significant. For analyses of dam body weight, the number of fetuses was used as a covariant in the analysis of covariance (ANCOVA) used to test for significance of treatment effects. The Fisher's exact test was used to determine whether the incidence of decreased coiling of the WDs was significantly different between groups. Statistical analysis of the real-time RT-PCR data was performed on the relative gene expression data as described in User Bulletin #2 (Applied Biosystems). The data represented in the figures reflects relative gene expression in comparison to controls and does not represent mRNA abundance. Significant differences between control and treated were determined by performing a t-test.
RESULTS
Maternal and Reproductive Parameters Following DBP Exposure
At 500 mg/kg/day, prenatal DBP exposure had no significant effect on dam body weight or body weight gain in any of the study blocks over the course of the study (data not shown). This is consistent with previous reports that exposure to DBP during gestation (up to 500 mg/kg/day) does not alter dam body weight, body weight gain, food consumption, litter size, pup survival, sex ratio, or body weights of male pups compared to controls (Barlow and Foster, 2003; Mylchreest et al., 2000).
Effects of DBP on Wolffian Duct Morphology During Late Gestation
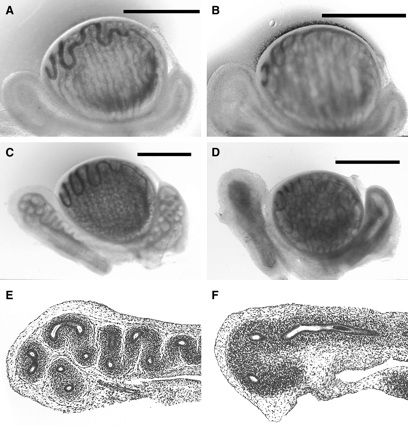
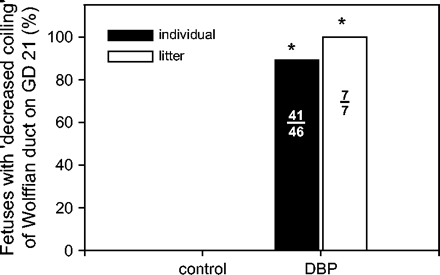
In general, WDs dissected from GD 19 DBP-exposed fetuses compared to control fetuses were slightly smaller in size (underdeveloped), the ducts themselves were more fragile, and the adipose tissue surrounding the duct had a gelatinous appearance. As shown in Figures 1A and 1B, the changes in WD morphology from DBP-exposed GD 19 fetuses were very subtle; as such no quantitation of these changes was performed. By GD 21, WDs from control fetuses were markedly coiled, whereas WD from fetuses exposed to DBP exhibited less coiling (Figs. 1C and 1D). The WD from DBP-exposed fetuses on GD 21 exhibited variably decreased coiling, from complete lack of coiling (Fig. 1D) to decreased coiling of only the caput or cauda regions (not shown). Since there was no detectable pattern as to which fetuses exhibited no coiling or regions of decreased coiling, all of these variations were classified as ‘decreased coiling’ (Fig. 2). On GD 21, 89% of fetuses (100% of litters) showed decreased coiling following exposure to DBP (Fig. 2), whereas on GD 19 no coiling of the WD was observed in any fetus. The macroscopic pathology of the differentiating WD following DBP exposure in the present study is consistent with that described previously by Barlow and Foster (2003). Consistent with the lack of coiling in the WDs from DBP-exposed fetuses, the GD 21 WD sections showed an overall decrease in the number of ductal cross-sections (Fig. 1F) in comparison with the controls (Fig. 1E). Since macroscopic changes in WD morphology were observed on GD 21, it is possible that the proportion of ductal epithelial cells to the surrounding mesenchyme may be altered relative to controls. Thus, the increases in mRNA expression on GD 21 described below may reflect alterations in cell numbers. Unfortunately, the differentiating rat WD would be extremely difficult to evaluate using standard morphometry techniques due to its extremely small size and frailty. As such, quantification of cellular ratios was not attempted in the present study.
Effect of DBP exposure (500 mg/kg/day) on gestation day (GD) 19 and 21 Wolffian ducts. Shown are representative images from control fetuses on GD 19 (A), and GD 21 (C, E); and from DBP-exposed fetuses on GD 19 (B), and GD 21 (D, F). Images (A) through (D) were captured using a dissecting microscope with transillumination. Note the lack of coiling in the Wolffian duct from a DBP-exposed fetus (D) compared to control (C). Representative images of hematoxylin and eosin-stained sections of the caudal region of Wolffian duct from control (E) and DBP-exposed (F) fetuses on GD 21. Note the reduction in ductal coiling as represented by the decreased numbers of ductal cross-sections within the Wolffian duct in the DBP-exposed tissue (F) compared to control (E). Scale bars are 1.0 mm (A, B, C, D), and images for E and F were taken at 20× magnification.
Incidence of decreased Wolffian duct coiling in DBP-exposed fetuses on gestation day 21. None of the control fetuses exhibited decreased coiling. The black bar represents the percent of individual fetuses in the DBP group with decreased coiling of the Wolffian duct. The white bar represents the percent of litters in the DBP group with decreased coiling of the Wolffian duct. The numbers of individuals and litters with altered Wolffian duct morphology compared to the total are printed in the respective bars.
Microarray Results
Microarrays were utilized as a guide to evaluate the gene expression of many mRNAs to identify key developmental pathways in the WD affected by prenatal DBP exposure. Many different methods of normalization and interpretation were used to explore the data and to find candidate genes that could be involved in the DBP-induced phenotype. In general, following normalization and removal of genes with expression changes within 10–15% of background, Welch's t-test was used to compare control and DBP groups. Evaluation of seven control and seven DBP arrays on GD 19 and six control and seven DBP arrays on GD 21 revealed that DBP exposure tended to increase overall gene expression. There was considerable variability in gene expression levels within the treatment group at each age, and this made it difficult to identify specific mRNAs that were consistently altered in expression on the limited number of arrays that were evaluated. However, it was possible to identify mRNAs from similar pathways that were suggestive of specific pathways being altered by DBP exposure (growth factors, matrix metalloproteinases and components of the extracellular matrix). No microarray results are presented, due to the high variability across arrays and since candidate genes were more critically analyzed by real-time RT-PCR. This information, together with empirically determined genes from the literature, was used to select individual mRNAs for evaluation by real-time RT-PCR and is described in the following sections.
Effect of DBP Exposure on mRNA Expression of Members of the Insulin-Like Growth Factor Gene Family and Epidermal Growth Factor Receptor
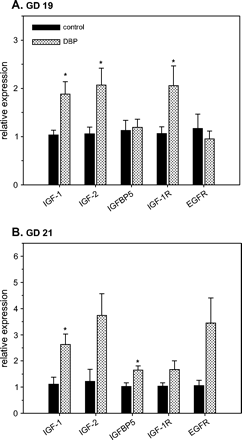
Daily exposure to DBP from GD 12 to 19 or 21 increased mRNA expression of different members of the insulin-like growth factor (IGF) family in the developing WD (Fig. 3). Real-time RT-PCR analysis indicated that mRNAs for IGF1, IGF2, IGF1 receptor (IGF1R), and IGF binding protein 5 (IGFBP5) were up-regulated following DBP exposure. Specifically, IGF1 mRNA expression was increased 1.82- and 2.37-fold (p < 0.05) with DBP on GD 19 and 21, respectively (Fig. 3). Both IGF2 and IGF1R mRNAs were significantly increased on GD 19 (1.95- and 1.93-fold, respectively) with DBP. There was a 1.6-fold increase (p < 0.05) in IGFBP5 mRNA on GD 21, but no change on GD 19. Epidermal growth factor receptor (EGFR) mRNA was unchanged on GD 19 and GD 21.
Effects of prenatal DBP exposure mRNA expression of insulin-like growth factor-1 (IGF1), IGF2, IGF binding protein 5 (IGFBP5), IGF1 receptor (IGF1R), and epidermal growth factor receptor (EGFR) in Wolffian ducts from control and DBP-exposed fetuses on gestation day (GD) 19 (A) and 21 (B). Total RNA from developing Wolffian ducts was subjected to real-time RT-PCR analyses. The data presented show the relative expression (means ± SE) compared to the control group, where the mean expression is assumed to be 1. Data on GD 19 (A) have n = 8 control samples (six litters) and n = 7 DBP-exposed samples (seven litters). Data on GD 21 (B) have n = 4 control samples (three litters) and n = 10 DBP-exposed samples (six litters). *p < 0.05, significantly different from the corresponding control group.
Effect of DBP Exposure on mRNA Expression of Androgen Receptor, Bone Morphogenetic Protein 4, Fibroblast Growth Factor Receptor 2 and Fibroblast Growth Factor 10
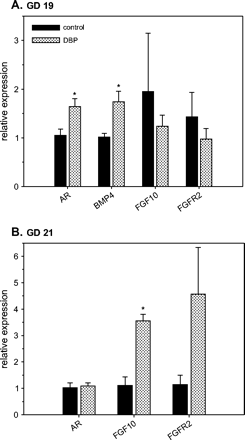
Prenatal exposure to DBP resulted in altered mRNA expression of the androgen receptor (AR), bone morphogenetic protein 4 (BMP4), and fibroblast growth factor 10 (FGF10) (Fig. 4). The array analysis was suggestive of an increase in FGF10 mRNA on GD 21 (data not shown). AR, BMP4, and the FGF Receptor 2 (FGFR2) mRNAs were analyzed, because they are considered critical growth regulators in epithelial cell differentiation, and were shown to be altered in the developing epididymis following prenatal exposure to the weak AR antagonist, linuron (Turner et al., 2003). Following DBP exposure, WD AR mRNA expression was increased slightly (1.56-fold) on GD 19, but not on GD 21. Similarly, BMP4 mRNA expression was increased 1.71-fold (p < 0.05) on GD 19 following DBP exposure (BMP4 mRNA was not quantitated on GD 21). FGF10 mRNA was increased 3.2-fold on GD 21 compared to controls, but not on GD 19. FGFR2 was unchanged on GD 19, but appeared to be increased on GD 21, though this value was not statistically significant.
Effects of prenatal DBP exposure on mRNA expression of androgen receptor (AR), bone morphogenetic protein 4 (BMP4), fibroblast growth factor 10 (FGF10), and fibroblast growth factor receptor 2 (FGFR2) in Wolffian ducts from control and DBP-exposed fetuses on gestation day (GD) 19 (A) and 21 (B). Total RNA from developing Wolffian ducts were subjected to real-time RT-PCR analyses. The data presented show the relative expression (means ± SE) compared to the control group where the mean expression is assumed to be 1. Data on GD 19 (A) have n = 8 control samples (six litters) and n = 7 DBP-exposed samples (seven litters). Data on GD 21 (B) have n = 4 control samples (three litters) and n = 10 DBP-exposed samples (six litters). *p < 0.05, significantly different from the corresponding control group.
Effect of DBP Exposure on Notch2 and Delta Like Receptor mRNA Expression
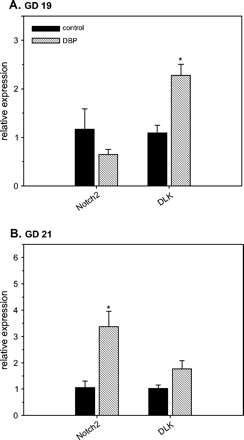
Analysis of GD 19 and 21 WD microarray data indicated that the mRNA expression of the genes for Notch2 receptor (Notch2) and delta like (Dlk) were altered by DBP exposure. Array analysis suggested that Notch2 mRNA expression was decreased on GD 19, but increased on GD 21, while Dlk mRNA expression was increased with DBP exposure at both ages (data not shown). Confirmation of these results by RT-PCR demonstrated significant increases (p < 0.05) in Notch2 mRNA expression on GD 21 (3.18-fold) and the increased Dlk mRNA expression on GD 19 (2.08-fold) (Fig. 5).
Altered mRNA expression of Notch 2 receptor (Notch2) and delta-like (Dlk) in Wolffian ducts from control and DBP-exposed fetuses on gestation day (GD) 19 (A) and 21 (B). Total RNA from developing Wolffian ducts were subjected to real-time RT-PCR analyses. The data presented show the relative expression (means ± SE) compared to the control group where the mean expression is assumed to be 1. Data on GD 19 (A) have n = 8 control samples (six litters) and n = 7 DBP-exposed samples (seven litters). Data on GD 21 (B) have n = 4 control samples (three litters) and n = 10 DBP-exposed samples (six litters). *p < 0.05, significantly different from the corresponding control group.
Effect of DBP Exposure on mRNA Expression of Members of the Matrix Metalloproteinase Gene Family
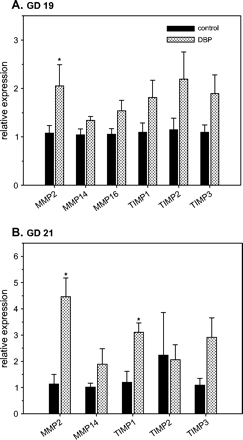
Following prenatal exposure to DBP, array analysis indicated that mRNAs for various members of the matrix metalloproteinase (MMP) family were increased in the developing WD (data not shown). Real-time RT-PCR analysis demonstrated an increase in MMP2, MMP16, and tissue-inhibitors of MMPs (TIMPs) 1, 2, and 3 on GD 19 following DBP exposure (Fig. 6). On GD 19, only the increase in MMP2 mRNA expression (1.91-fold) was significant (p < 0.05). On GD 21, significant increases in MMP2 and TIMP1 mRNA expression were observed in WD from DBP-exposed fetuses (3.93- and 2.59-fold respectively) (Fig. 6). Analysis on MMP16 mRNA was not successful in GD 21 WD.
Altered mRNA expression of matrix metalloproteinases (MMPs) 2, 14, 16 and tissue-inhibitors of MMPs (TIMPs) 1, 2, and 3 in Wolffian ducts from control and DBP-exposed fetuses on gestation day (GD) 19 (A) and 21 (B). Total RNA from developing Wolffian ducts was subjected to real-time RT-PCR analyses. The data presented show the relative expression (means ± SE) compared to the control group, where the mean expression is assumed to be 1. Data on GD 19 (A) have n = 8 control samples (six litters) and n = 7 DBP-exposed samples (seven litters). Data on GD 21 (B) have n = 4 control samples (three litters) and n = 10 DBP-exposed samples (six litters). *p < 0.05, significantly different from the corresponding control group.
Effects of DBP Exposure on mRNA Expression of Components of the Extracellular Matrix
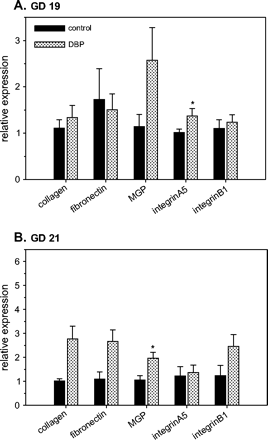
Microarray analysis of the WD from DBP-exposed fetuses compared to controls indicated alterations in the expression of several components of the extracellular matrix (ECM), specifically collagen, fibronectin, and the matrix Gla protein (MGP) (data not shown). The mRNAs for these genes were analyzed by real-time RT-PCR as well as the integrin subunits A5 and B1, which form a heterodimer that functions as a fibronectin surface receptor (Fig. 7). On GD 19, only mRNA expression of A5 integrin subunit showed a significant increase in expression in comparison with controls (1.35-fold, p < 0.05), while on GD 21, mRNAs for collagen, fibronectin, and MGP appeared to be increased by 2.74-, 2.42-, and 1.86-fold, respectively, in the WD from DBP-exposed fetuses, but only the increase in MGP expression was statistically significant (Fig. 7B).
Altered mRNA expression of collagen, fibronectin, matrix Gla protein (MGP), integrin A5, and integrin B1 in Wolffian ducts from control and DBP-exposed fetuses on gestation day (GD) 19 (A) and 21 (B). Total RNA from developing Wolffian ducts was subjected to real-time RT-PCR analyses. The data presented show the relative expression (means ± SE) compared to the control group, where the mean expression is assumed to be 1. Data on GD 19 (A) have n = 8 control samples (six litters) and n = 7 DBP-exposed samples (seven litters). Data on GD 21 (B) have n = 4 control samples (three litters) and n = 10 DBP-exposed samples (six litters). *p < 0.05, significantly different from the corresponding control group.
Immunolocalization of IGF1 Receptor β and Androgen Receptor
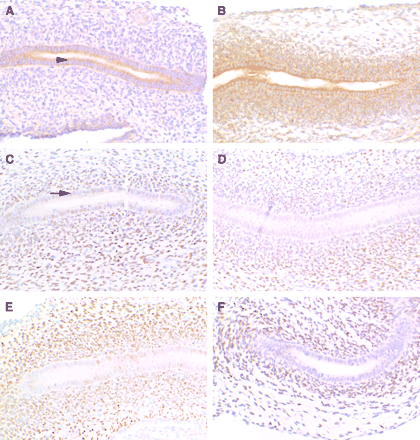
IGF1Rβ protein was localized in WD of the fetuses from control and DBP-exposed dams on GD 19. In control WD, low levels of IGF1Rβ protein immunostaining were detected in the cytoplasm of the ductal epithelial cells (arrowhead, Fig. 8A) and very little immunostaining in the mesenchymal cells surrounding the epithelial cell layer. In WDs from DBP-exposed fetuses, the intensity of IGF1Rβ immunostaining was high in the cytoplasm of the ductal epithelial cells, and markedly increased in the cytoplasm of mesenchymal cells (Fig. 8B) when compared with the controls. Specificity of the immunostaining (for IGF1Rβ and AR) was confirmed by the absence of staining in sections incubated with antisera preadsorbed with the peptide used as the immunogen (not shown).
Immunolocalization of insulin-like growth factor 1 receptor (IGF1R) and androgen receptor (AR) in control (A, C) and DBP-exposed (B, D, E, F) fetuses on gestation day 19. Representative images shown are from the same region of the Wolffian duct (future corpus region) except for F (future cauda region). Epithelial cells lining the duct are surrounded by the condensing mesenchymal cells. All images were collected using 20× magnification. In control animals, IGF1R exhibited moderate cytoplasmic staining in the ductal epithelia (arrowhead, A), and with DBP-exposure there was more intense staining of the epithelial cells in addition to intense cytoplasmic staining of the mesenchyme (B). AR exhibited moderate nuclear staining of the ductal epithelial cells in control animals (arrow, C), and with DBP-exposure there was a decrease in ductal epithelial cell staining in the nucleus in some animals (D, F), but not as clearly in others (E). Nuclear AR staining of the mesenchyme was variable and did not change in Wolffian duct from DBP-exposed fetuses.
In developing WD from fetuses exposed to DBP, the immunostaining of AR protein compared to controls on GD 19 was variably decreased in the epithelial cells. In GD 19 control WD, AR immunostaining was localized in nuclei of both epithelial and mesenchymal cells (arrow, Fig. 8C) with variability of staining intensity. In general, WD from DBP-exposed fetuses exhibited a reduction of AR immunostaining in the nuclei of ductal epithelial cells (Figs. 8D and 8F). This decrease in epithelial immunostaining was not as pronounced in some DBP-exposed WD (Fig. 8E). There was no discernable trend in AR mesenchymal staining between WD from control or DBP-exposed fetuses. Specificity of the immunoreaction was confirmed by the absence of immunostaining in sections incubated with antisera preadsorbed with peptide (not shown).
DISCUSSION
In utero exposure to di(n-butyl) phthalate (DBP) results in decreased fetal testicular testosterone (Mylchreest et al., 2002; Shultz et al., 2001) and lack of differentiation of the upper WD into the epididymis. Malformed epididymides in DBP-exposed rats causes obstruction of testicular fluid outflow, resulting in secondary pressure atrophy of the seminiferous epithelium in the testes of adult rats (Barlow and Foster, 2003; Mylchreest et al., 1999, 2000, 2002). Consistent with a previous study (Barlow and Foster, 2003), the WDs in DBP-exposed fetuses appeared slightly smaller, and the adipose tissue surrounding the developing duct was more gelatinous on GD 19, while on GD 21 a significant lack of coiling was observed (Fig. 1). In normal development, androgens induce epithelial cell proliferation by stimulating the transition of mesenchymal cells to epithelial cells (Cunha et al., 1992; Thomson, 2001; Tsuji et al., 1991b). Proliferation of the epithelium coincides with coiling of the WD (Tsuji et al., 1991b). One possible explanation for the altered WD morphology in DBP-exposed fetuses is that decreased fetal testosterone levels result in a decrease in ductal epithelial cell proliferation, and this may alter the proportion of epithelial to mesenchymal cells and composition of the extracellular matrix. In female fetuses where the WD involutes, a lack of testosterone has been suggested to promote an epithelial to mesenchyme transition (Jirsova and Vernerova, 1993). The mechanisms by which testosterone induces alterations in cellular proliferation within the reproductive tract have still to be elucidated; however, there is considerable interest in the effects of androgens on paracrine interactions between the epithelial and mesenchymal cells. Thus, the objective of the present study was to identify gene expression changes within paracrine signaling pathways associated with the abnormal differentiation of the upper WD in DBP-exposed fetuses.
Proposed Model of Altered Wolffian Duct Differentiation
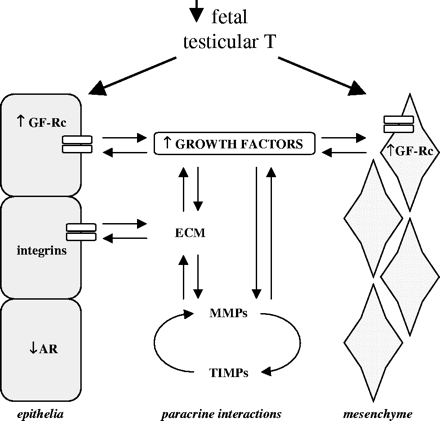
Based on the results of these studies, we propose the following model for increased paracrine signaling in the developing WD (Fig. 9). This model suggests a coherent molecular response between the mesenchyme, extracellular matrix, and epithelia that is secondary to the decrease in fetal testicular testosterone and ultimately results in the altered phenotype following in utero DBP exposure. Specifically, prenatal DBP exposure (and the subsequent decrease in fetal testicular testosterone) during WD development appears to increase paracrine signaling of growth factors (e.g., IGFs) and other developmentally conserved pathways (e.g., BMP4) followed by increased proteinase activity (MMPs), which may induce altered morphogenesis of the extracellular matrix (e.g., collagen and fibronectin) ultimately resulting in the abnormal WD phenotype observed on GD 21. In addition to the above changes, AR protein appears to be decreased in the ductal epithelia on GD 19 (present study) and 21 (Mylchreest et al., 2002), coincident with decreased levels of fetal testicular testosterone. Both mRNA and protein data from the current study suggest that the IGF pathway is up-regulated in the WD with DBP exposure. We speculate this may be due to one of two reasons. The increased IGF signaling may be a compensatory response to decreased testosterone, or the IGF system may be a functional repressor normally leading to WD regression in females, but in normal males is repressed by testosterone; therefore decreased testosterone may result in an increase in IGF signaling in DBP-exposed fetuses in comparison to the controls. There is evidence suggesting that another growth factor (transforming growth factor beta—TGFβ) is a functional repressor (Roy et al., 1999) that is normally down-regulated by androgens (Kyprianou and Isaacs, 1989) but can inhibit androgen-induced growth and ductal morphogenesis (Cunha et al., 1992). This growth factor was not evaluated in the current study.
Proposed model of altered paracrine signaling in the developing Wolffian duct following di(n-butyl) phthalate exposure. The mRNA data from the present study support a model where the paracrine control of the mesenchymal-epithelial signaling is altered, resulting in abnormal morphogenesis and changes in the extracellular matrix. Immunohistochemistry data from the present study and that from Mylchreest et al. (2002) demonstrate that decreased fetal testicular testosterone, increased insulin-like growth factor signaling, and decreased epithelial androgen receptor are associated with disruption of Wolffian duct differentiation following prenatal DBP exposure. T = testosterone, GF-Rc = growth factor receptor, ECM = extracellular matrix, AR = androgen receptor, MMP = matrix metalloproteinase, and TIMP = tissue inhibitors of MMPs.
Altered Signaling Pathways
In the current study, increased mRNA expression of IGF1, IGF2, IGF1R, and IGFBP5 and immunoexpression of IGF1R protein in WD from DBP-exposed fetuses suggest that the IGF pathway is upregulated. This pattern is consistent with the increase in IGFBP5 mRNA expression in luminal epithelial cells in the mammary gland and prostate following androgen ablation (Guenette and Tenniswood, 1994). In addition, IGF involvement in reproductive development is demonstrated in several other studies, one of which found that IGF1 null mice are infertile and exhibit small epididymides with decreased ductal coiling (Baker et al., 1996). Interestingly, testicular testosterone production was decreased by 80% in these mice. These data support a critical role for the IGF pathway in male reproductive development. In vitro, testosterone or insulin increased protein content and mesenchymal DNA content of the neonatal mouse seminal vesicle, but only testosterone induced epithelial branching morphogenesis and increased epithelial cell content (Tsuji et al., 1991b). These in vitro data show that insulin by itself has no effect on epithelial proliferation and branching morphogenesis, but instead amplifies the morphogenetic and proliferative effects of androgen (Tsuji et al., 1991b). Support for a combined role of EGF and testosterone in WD differentiation comes from studies using undifferentiated WD explants where neither EGF nor testosterone alone were completely effective as masculinizing agents (Gupta et al., 1991; Gupta and Singh, 1996). In addition, EGF has been shown to induce partial WD differentiation in female fetuses (in the absence of testosterone) in vivo, as well as inducing WD differentiation in vitro (without testosterone), suggesting that growth factors may be capable of independent differentiation of the WD, at least in females (Gupta et al., 1991). In the present study, we did not analyze EGF mRNA expression, and the increase in EGFR mRNA expression in WDs from DBP-exposed fetuses was variable and thus not statistically significant. The real-time RT-PCR data did not detect a change in the EGF signaling pathway in WD from DBP-exposed fetuses in vivo.
During normal fetal development in the male rat, AR is initially expressed in the mesenchymal cells surrounding the WD, with subsequent induction of expression within the epithelial cells by GD 18 (Bentvelsen et al., 1995; Cooke et al., 1991; Majdic et al., 1995; You and Sar, 1998). DBP induces its antiandrogenic effects upstream of the AR, by decreasing testosterone biosynthesis (Mylchreest et al., 2002; Shultz et al., 2001; Thompson et al., 2004). However, it was important to evaluate the expression and localization of the AR in WD from DBP-exposed fetuses, since circulating testosterone levels are lower than controls. There is some evidence that AR transcriptional activity may be enhanced by low levels of androgens (Orio et al., 2002). In the present study, AR mRNA expression appeared to be slightly increased on GD 19, but was unchanged on GD 21 in the WD from DBP-exposed fetuses. However, interpretation of the GD 21 mRNA data is complicated by potential changes in the proportions of epithelial to mesenchymal cells. Consequently, overall GD 21 AR mRNA expression in the WD may not change if the number of mesenchymal cells increased in parallel with a decrease in the number of ductal epithelial cells. AR expression was further evaluated on GD 19 using immunohistochemistry. Epithelial cell AR protein expression was variably decreased on GD 19 (Fig. 8), but there was no apparent change in mesenchymal AR protein expression. The apparent disconcordance between increased AR mRNA and variably decreased epithelial AR protein on GD 19 may be explained by small sample size, variability, a posttranscriptional event precluding increased protein synthesis, or by increased protein turnover. The decrease in ductal epithelial AR protein expression is consistent with previous data demonstrating a decrease in ductal epithelial AR protein in WD from DBP-exposed fetuses on GD 21 (Mylchreest et al., 2002). A decrease in AR protein in the epithelia of the developing WD has also been shown with the AR-antagonists linuron (Turner et al., 2003) and flutamide (Bentvelsen et al., 1995; Mylchreest et al., 2002).
The bone morphogenetic protein (BMP) and fibroblast growth factor (FGF) families, together with their receptors, are highly conserved signaling pathways of great importance in development (Gerhart, 1999; Hogan, 1999, Jung et al., 1998). In the present study, BMP4 mRNA expression was increased in the WD from GD 19 DBP-exposed fetuses and is consistent with reports that BMP4 is considered a negative growth factor, inhibiting epithelial cell proliferation (Hogan, 1999; Lamm et al., 2001). In contrast, decreases in BMPs and BMP-receptors were observed in developing WDs on GD 21 and epididymides on PND 7 from linuron-exposed fetuses (Turner et al., 2003). In DBP-exposed fetuses, FGF10 mRNA expression was unaltered in WDs on GD 19, but increased on GD 21. BMP4 and FGF10 are expressed in the mesenchyme of developing organs and together regulate branching morphogenesis (Thomson and Cunha, 1999; Weaver et al., 2000). BMP4 is though to act as a negative growth factor, by inhibiting epithelial cell proliferation and bud formation (Hogan, 1999; Lamm et al., 2001), whereas FGF10 promotes budding (Weaver et al., 2000). In addition BMP4 is expressed in the mesenchyme surrounding the developing WD and appears to play a role in the development of the mouse external genitalia (Miyazaki et al., 2000). It is difficult to know if the alterations in BMP4 and FGF10 expression represent changes in their respective signaling pathways, because it was not possible to quantify all the relevant receptors. However, the fact that BMP4 and FGF10 are growth factors that are known to act in a paracrine fashion between the mesenchyme and epithelia in many developing organs suggests that they could be involved in regulating epithelial cell proliferation in WDs and thus ductal coiling.
Both Notch2 and Dlk are members of highly conserved developmental signaling pathways that regulate cell-to-cell communication during tissue morphogenesis (Artavanis-Tsakonas et al., 1999; Gerhart, 1999). Therefore it is not surprising that these signaling pathways may be disrupted in developing WD from DBP-exposed fetuses. Down regulation of Dlk has been associated with cellular differentiation (Laborda, 2000). Thus, the increased Dlk mRNA expression observed in the current study following DBP exposure might suggest a lack of cell maturation (mesenchyme-epithelia transition or epithelial proliferation). This is consistent with an increase in Dlk mRNA expression observed in GD 21 WDs from linuron-exposed fetuses that exhibited decreased ductal coiling (Turner et al., 2003).
MMPs are a family of extracellular proteinases that regulate many developmental processes, including branching morphogenesis, extracellular matrix degradation, growth factor release, and cleavage of cell adhesion molecules (Seiki, 2002; Vu and Werb, 2000). The family of MMPs also has their own family of inhibitors, the TIMPs. The overall balance of active MMPs and TIMPs (thought to be primarily regulated at the level of transcription) dictates specific proteinase activity and function (Basbaum and Werb, 1996; Talhouk et al., 1992; Werb, 1997). MMP2 specifically is considered one of the first target genes downstream of paracrine signaling during normal Mullerian ductal epithelial regression in the male rat (Roberts et al., 2002). In the developing WD, we propose that paracrine signals may be altered to induce MMP activity, thus preventing WD differentiation as a consequence of prenatal DBP exposure (Fig. 9).
In general, the ECM environment consists of soluble factors (growth factors and MMPs) interacting with matrix components (collagen and fibronectin) and the cell surface receptors (integrins, growth factor receptors, and MMPs) to regulate tissue morphogenesis and differentiation during development (Adams and Watt, 1993; Basbaum and Werb, 1996; Jones et al., 1993; Luke and Coffey, 1994; Sakurai, 2003; Slater, 1996; Sternlicht and Werb, 2001; Streuli, 1999; Werb, 1997; Werb et al., 1996). For example, MMP proteolysis of IGFBPs results in increased IGF activity, and IGFBPs bound to the ECM serve as a reservoir of IGF1 and 2 (Fowlkes et al., 1999). IGF1 and IGF1R can regulate MMP2 and MMP14 synthesis (Zhang and Brodt, 2003). Interestingly, fibronectin increases MMP activity and epithelial cell loss in hormone withdrawal-induced mammary gland involution (Schedin et al., 2000). Signals from both integrins and growth factor receptors are thought to be necessary for the G1 to S-phase transition and cell proliferation (Danen and Yamada, 2001). Synergy between these receptors results from binding of ECM ligands and growth factors that act on different components of the same signaling pathways involved with normal morphogenesis (Miyamoto et al., 1998; Werb, 1997). In addition, recent data demonstrates that MGP (a calcification inhibitor in bone) may also regulate BMPs (Zebboudj et al., 2002).
In summary, prenatal DBP exposure (and subsequent decrease in fetal testicular testosterone) appears to alter the mesenchyme-epithelial signaling of growth factors (e.g., IGFs) and other developmentally conserved pathways (e.g., BMP4), followed by changes in the macroscopic and microscopic morphology on GD 21 (Fig. 9). Although the mRNA and immunohistochemical data support this hypothesis, further study is necessary to validate this proposed model. Based on the current DBP study and the linuron-induced changes in the WD (Turner et al., 2003) there are distinct differences in the molecular and hormonal mechanisms responsible for the similar epididymal malformation observed in adult animals following in utero exposure to antiandrogens. We believe this effect on WD differentiation by DBP is likely a consequence of decreased fetal testicular testosterone, but direct effects of DBP on the developing WD independent of testosterone are possible. This study identifies candidate genes involved with paracrine signaling during rat WD differentiation not previously reported. In addition, a model has been proposed for the DBP-induced changes observed in the developing WD. The present study builds on previous work within our laboratory to understand the various modes of action underlying the sensitivity of male reproductive tract development to environmental antiandrogens. Future studies should further evaluate the IGF pathway and its relationship to testosterone-regulated development of the reproductive tract.
Present address: Merck Research Laboratories, West Point, PA 19486.
Present address: Sanofi-Aventis, Bridgewater, NJ 08807.
Present address: National Institute of Environmental Health Sciences, Research Triangle Park, NC 27709.
The authors would like to thank Dr. David C. Dorman, Dr. Kamin J. Johnson, Dr. Elena V. Kleymenova, and Dr. Jeanelle Martinez for critical review of this manuscript. The authors would like to thank Ms. Kim P. Lehmann and Mr. Duncan G. Wallace for technical assistance. The authors are very grateful to Mr. Paul Ross and the animal care staff and to Ms. Elizabeth Gross-Bermudez and the necropsy and histology staff. This study was made possible by funds from the American Chemistry Council.
References
Adams, J. C., and Watt, F. M. (
Artavanis-Tsakonas, S., Rand, M. D., and Lake, R. J. (
Baker, J., Hardy, M. P., Zhou, J., Bondy, C., Lupu, F., Bellve, A. R., and Efstratiadis, A. (
Barlow, N. J., and Foster, P. M. D. (
Basbaum, C. B., and Werb, Z. (
Bentvelsen, F. M., Brinkmann, A. O., van der Schoot, P., van der Linden, J. E., van der Kwast, T. H., Boersma, W. J., Schroder, F. H., and Nijman, J. M. (
Cooke, P. S., Young, P., and Cunha, G. R. (
Cunha, G. R. (
Cunha, G. R., Alarid, E. T., Turner, T., Donjacour, A. A., Boutin, E. L., and Foster, B. A. (
Danen, E. H., and Yamada, K. M. (
Foster, P. M. D., Mylchreest, E., Gaido, K. W., and Sar, M. (
Fowlkes, J. L., Serra, D. M., Nagase, H., and Thrailkill, K. M. (
George, F. W., and Wilson, J. D. (
Gerhart, J. (
Gray, L. E., Jr., Wolf, C., Lambright, C., Mann, P., Price, M., Cooper, R. L., and Ostby, J. (
Guenette, R. S., and Tenniswood, M. (
Gupta, C., Siegel, S., and Ellis, D. (
Gupta, C., and Singh, M. (
Higgins, S. J., Young, P., and Cunha, G. R. (
International Programme on Chemical Safety (IPCS) (
Jirsova, Z., and Vernerova, Z. (
Jones, P. L., Schmidhauser, C., and Bissell, M. J. (
Kyprianou, N., and Isaacs, J. T. (
Jung, H. S., Francis-West, P. H., Widelitz R. B., Jiang, T. X., Ting-Berreth, S., Tickle, C., Wolpert, L., and Chuoung, C. M. (
Laborda, J. (
Lamm, M. L., Podlasek, C. A., Barnett, D. H., Lee, J., Clemens, J. Q., Hebner, C. M., and Bushman, W. (
Lehmann, K. P., Phillips, S., Sar, M., Foster, P. M. D., and Gaido, K. W. (
Luke, M. C., and Coffey, D. S. (
Majdic, G., Millar, M. R., and Saunders, P. T. (
McIntyre, B. S., Barlow, N. J., Sar, M., Wallace, D. G., and Foster, P. M. D. (
Miyamoto, S., Katz, B. Z., Lafrenie, R. M., and Yamada, K. M. (
Miyazaki, Y., Oshima, K., Fogo, A., Hogan, B. L., and Ichikawa, I. (
Mylchreest, E., Cattley, R. C., and Foster, P. M. D. (
Mylchreest, E., Sar, M., Cattley, R. C., and Foster, P. M. D. (
Mylchreest, E., Sar, M., Wallace, D. G., and Foster, P. M. D. (
Mylchreest, E., Wallace, D. G., Cattley, R. C., and Foster, P. M. D. (
National Research Council (
National Toxicology Program (NTP) (
Orio, F., Terouanne, B., Georget, V., Lumbroso, S., Avances, C., Siatka, C., and Sultan, C. (
Roberts, L. M., Visser, J. A., and Ingraham, H. A. (
Roy, A. K., Lavrovsky, Y., Song, C. S., Chen, S., Jung, M. H., Velu, N. K., Bi, B. Y., and Chatterjee, B. (
Sakurai, H. (
Sar, M., and Welsch, F. (
Schedin, P., Strange, R., Mitrenga, T., Wolfe, P., and Kaeck, M. (
Seiki, M. (
Shultz, V. D., Phillips, S., Sar, M., Foster, P. M. D., and Gaido, K. W. (
Slater, M. (
Sternlicht, M. D., and Werb, Z. (
Streuli, C. (
Talhouk, R. S., Bissell, M. J., and Werb, Z. (
Thompson, C. J., Ross, S. M., and Gaido, K. W. (
Thomson, A. A. (
Thomson, A. A., and Cunha, G. R. (
Tsuji, M., Shima, H., and Cunha, G. R. (
Tsuji, M., Shima, H., and Cunha, G. R. (
Turner, K. J., McIntyre, B. S., Phillips, S. L., Barlow, N. J., Bowman, C. J., and Foster, P. M. D. (
Vu, T. H., and Werb, Z. (
Warren, D. W., Haltmeyer, G. C., and Eik-Nes, K. B. (
Weaver, M., Dunn, N. R., and Hogan, B. L. (
Werb, Z., Sympson, C. J., Alexander, C. M., Thomasset, N., Lund, L. R., MacAuley, A., Ashkenas, J., and Bissell, M. J. (
Wine, R. N., Li, L. H., Barnes, L. H., Gulati, D. K., and Chapin, R. E. (
You, L., and Sar, M. (
You, L., Sar, M., Bartolucci, E. J., McIntyre, B. S., and Sriperumbudur, R. (
Zebboudj, A. F., Imura, M., and Bostrom, K. (
- androgens
- pregnancy
- testosterone
- gene expression
- extracellular matrix
- dna, complementary
- fetus
- genes
- insulin-like growth factor i
- matrix metalloproteinases
- insulin-like-growth factor i receptor
- androgen receptor
- reproductive physiological process
- reverse transcriptase polymerase chain reaction
- rna, messenger
- mesonephric duct
- epididymis
- prenatal care
- rats
- testosterone measurement
- rna
- epithelial cells
- signal pathway
- paracrine
- mesenchyme
- signal transduction pathways
- candidate disease gene
- phthalates




Comments