-
PDF
- Split View
-
Views
-
Cite
Cite
Cedric F Garland, Frank C Garland, Do sunlight and vitamin D reduce the likelihood of colon cancer?, International Journal of Epidemiology, Volume 35, Issue 2, April 2006, Pages 217–220, https://doi.org/10.1093/ije/dyi229
Close - Share Icon Share
It is proposed that vitamin D is a protective factor against colon cancer. This hypothesis arose from the inspection of the geographic distribution of colon cancer deaths in the US, which revealed that colon cancer mortality rates were highest in places where populations were exposed to the least amounts of natural light—major cities, and rural areas at high latitudes. The hypothesis is supported by a comparison of colon cancer mortality rates in areas that vary in mean daily solar radiation penetrating the atmosphere. A mechanism involving cholecalciferol (vitamin D3) is suggested. The possibility that an ecological fallacy or another indirect association explains the findings is explored.
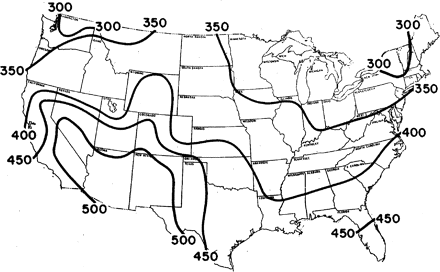
The amount of sunlight reaching the earth's surface varies greatly from area to area in the US. Solar radiation (including ultraviolet and visible light) reaching the ground is measured by the US Weather Bureau at a number of weather stations which are distributed throughout the country.1 The mean daily solar radiation values for the contiguous United States, which vary between 300 and 500 gm-cal/cm2, are shown in Figure 1.
An estimate of the amount of sunlight reaching the earth in any particular state can be obtained by selecting the solar radiation contour that passes through that state or selecting the closest contours and taking their mean. This was done, and solar radiation values were calculated for each state.
New Mexico and Arizona had the highest statewide mean solar radiation values (500 gm-cal/cm2). These states experienced colon cancer rates for white males of 6.7 and 10.1 per 100 000 population, respectively, over the period 1959–61.2 New York, New Hampshire, and Vermont had the lowest statewide mean solar radiation values (300 gm-cal/cm2) and experienced colon cancer rates for white males of 17.3, 15.3, and 11.3 per 100 000 populations, respectively, during the same period.2
Since solar radiation contours provide an estimate of the amount of sunlight due to gross geographic and meteorological factors over large areas, they do not necessarily provide a completely accurate indication of the amount of solar radiation penetrating the atmosphere in cities. Even in areas where sunlight is intense, persons who live and work in cities may not receive much exposure to it. Behar has noted that rickets, a disease, which is believed to be related to inadequate solar exposure, and, consequently, vitamin D3 deficiency, occurs in large cities even in tropical and subtropical areas.3 He attributes this to shading provided by the buildings which are present in large cities.
Since shading by structures, and a predominance of indoor occupations in metropolitan areas, would be likely to reduce the dosages of sunlight received in metropolitan areas, it was felt that it would be appropriate to analyse the inverse association between sunlight and colon cancer in states with large metropolitan populations separately from the inverse association between sunlight and colon cancer in non-metropolitan states. Metropolitan states were defined as states in which at least 80% of the population lived in Standard Metropolitan Statistical Areas (SMSAs) in 1970.4
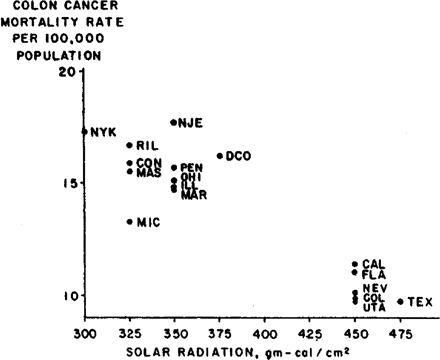
A scatter plot of the inverse association between solar radiation and age-adjusted colon cancer mortality rates in metropolitan states for white males is shown in Figure 2. The colon cancer mortality rates are from Cancer in the United States, by Lilienfeld and associates.2 The solar radiation values are computed from annual mean daily solar radiation maps compiled by the US Weather Bureau.1 The Pearson product-moment correlation is −0.9 (the Spearman rank-order correlation coefficient is −0.7). This strong inverse association demonstrates that colon cancer mortality rates are lower in metropolitan states located in sunny climates than in metropolitan states located in relatively darker climates.
Annual mean daily solar radiation (gm-cal/cm2) and annual age-adjusted colon cancer death rates per 100 000 population, white males, 17 non-metropolitan states, United States, 1959–61
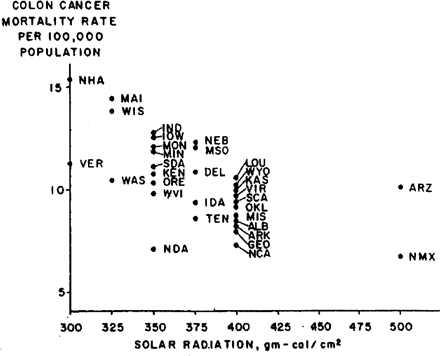
A scatter plot of the inverse association between solar radiation and age-adjusted colon cancer mortality rates in non-metropolitan states for white males is shown in Figure 3. The Pearson correlation is −0.6 (the Spearman rank-order correlation coefficient is also −0.6). The inverse association is not as strong as it is in metropolitan states, but the general effect is similar.
Annual mean daily solar radiation (gm-cal/cm2) and annual age-adjusted colon cancer death rates per 100 000 population, white males, 32 non-metropolitan states, United States, 1959–61
Pearson product-moment correlations were also calculated using annual mean daily solar radiation and age-adjusted mortality rates for cancer of the colon in white males for the period 1950–69, as reported by Mason and McKay in Cancer Mortality in the United States, 1950–69.5 These rates may be slightly less accurate than those reported by Lilienfeld et al., since they are partly based on estimates of population during the periods between censuses. Nevertheless, there were Spearman correlations between annual mean daily solar radiation and colon cancer in white males of −0.7 for metropolitan states and −0.6 for non-metropolitan states, which are the same as the values obtained using the mortality data reported by Lilienfeld et al.2
Mechanism. Rickets is apparently due to a deficiency in vitamin D, and can be prevented by exposure to sunlight or by dietary supplements of Vitamin D. Vitamin D is uncommon in the diet in the US, except in milk, which is fortified with 400 IU of vitamin D per quart. The only other principal natural source of vitamin D is fish; butter and eggs contain considerably less.6
The strong inverse association of sunlight and colon cancer raises the possibility that vitamin D, which prevents rickets, may also act in the prevention of colon cancer.
The association between vitamin D and rickets was slow to be recognised.7 Until the 1900s, rickets was thought to be due to noxious air, urban crowding, damp surroundings, and other aspects of life in large cities. This was because it occurred far more commonly in heavily industrialized areas than in rural surroundings.8 Colon cancer is also much more common in large cities (and darker latitudes), and a mechanism can be hypothesized that would account for the parallels between rickets, a deficiency disease of childhood, and colon cancer, a progressive disease of the later decades of life.
Vitamin D (which is converted in the body to 1,25-dihydroxycholecalciferol) promotes gastro-intestinal absorption of calcium via induction of the synthesis of a calcium-binding protein (CaBP), possibly as a result of unmasking a specific DNA in the epithelial cells of the small intestine. This protein appears to play a role in the transport of calcium from the brush border facing the lumen of the gut across the cell, and into the circulation.9
Calcium, once it has entered the circulation, is carried to a variety of tissues, including the skin. It has been shown to decrease the reactivity of the skin to inflammatory stimuli.10,11 It is proposed here that calcium exerts a similar effect on the epithelial cells of the large intestine, reducing the inflammatory response to bacterial flora and other agents present in the lumen of the colon. Inflammatory bowel disease would be expected to occur more commonly in persons with inadequate intracellular calcium levels due to insufficient concentration of vitamin D in plasma. Similarly, diminished integrity of cell structures in the colon could conceivably result from inadequate calcium, and this might predispose to preneoplastic and neoplastic changes in the colonic epithelium. Abnormal morphology of intestinal cells has been observed in chicks fed a ricketogenic diet.12 Biochemical abnormalities in the intestinal cells of chicks fed on a ricketogenic diet have also been reported.13
Geographic and occupational variation in serum 25-hydroxycholecalciferol has been documented. It has been observed that even the ‘northern Briton’ can increase his serum 25-hydroxycholecalciferol level some 2–3 times and achieve concentrations as high as 60 ng/ml by 2 weeks exposure to sunshine in southern Europe.14 Nuclear submarine crewmen who were excluded from ultraviolet light for 3 months showed a fall in an already low circulating 25-hydroxycholecalciferol level from 13.7 ± 1.1 to 7.9 ± 1.2 ng/ml.15
Much has been hypothesized about the association between the consumption of meat, especially beef, and the occurrence of colon cancer, and it has been reported from reliable sources that consumption of meat is the single best correlate of colon cancer.16 As shown in Table 1, however, the variation in consumption of meat by region in the United States is not what one would expect from a hypothesis implicating consumption of meat in colon cancer.17
Quantity in pounds of beef, pork, other meats, poultry, and fish consumed per person per week, by region, United States, 1965–6617
| . | Red meat . | . | . | . | Poultry and fish . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region* . | Beef . | Pork . | Other . | Total . | Poultry . | Fish . | Total . | Colon cancer death rate, white males2 . | |||||
| North-east | 1.6 | 1.0 | 0.7 | 3.3 | 0.9 | 0.4 | 1.3 | 16.4 | |||||
| North Central | 1.8 | 1.2 | 0.6 | 3.6 | 0.8 | 0.3 | 1.1 | 13.4 | |||||
| South | 1.4 | 1.2 | 0.5 | 3.2 | 0.9 | 0.5 | 1.4 | 9.8 | |||||
| West | 1.9 | 1.0 | 0.6 | 3.5 | 0.8 | 0.3 | 1.1 | 10.7 | |||||
| . | Red meat . | . | . | . | Poultry and fish . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region* . | Beef . | Pork . | Other . | Total . | Poultry . | Fish . | Total . | Colon cancer death rate, white males2 . | |||||
| North-east | 1.6 | 1.0 | 0.7 | 3.3 | 0.9 | 0.4 | 1.3 | 16.4 | |||||
| North Central | 1.8 | 1.2 | 0.6 | 3.6 | 0.8 | 0.3 | 1.1 | 13.4 | |||||
| South | 1.4 | 1.2 | 0.5 | 3.2 | 0.9 | 0.5 | 1.4 | 9.8 | |||||
| West | 1.9 | 1.0 | 0.6 | 3.5 | 0.8 | 0.3 | 1.1 | 10.7 | |||||
Survey regions—North-east: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont; North Central: Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, Wisconsin; South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, West Virginia; West: Arizona, California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, Wyoming. Alaska and Hawaii were not included in this survey.
Quantity in pounds of beef, pork, other meats, poultry, and fish consumed per person per week, by region, United States, 1965–6617
| . | Red meat . | . | . | . | Poultry and fish . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region* . | Beef . | Pork . | Other . | Total . | Poultry . | Fish . | Total . | Colon cancer death rate, white males2 . | |||||
| North-east | 1.6 | 1.0 | 0.7 | 3.3 | 0.9 | 0.4 | 1.3 | 16.4 | |||||
| North Central | 1.8 | 1.2 | 0.6 | 3.6 | 0.8 | 0.3 | 1.1 | 13.4 | |||||
| South | 1.4 | 1.2 | 0.5 | 3.2 | 0.9 | 0.5 | 1.4 | 9.8 | |||||
| West | 1.9 | 1.0 | 0.6 | 3.5 | 0.8 | 0.3 | 1.1 | 10.7 | |||||
| . | Red meat . | . | . | . | Poultry and fish . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region* . | Beef . | Pork . | Other . | Total . | Poultry . | Fish . | Total . | Colon cancer death rate, white males2 . | |||||
| North-east | 1.6 | 1.0 | 0.7 | 3.3 | 0.9 | 0.4 | 1.3 | 16.4 | |||||
| North Central | 1.8 | 1.2 | 0.6 | 3.6 | 0.8 | 0.3 | 1.1 | 13.4 | |||||
| South | 1.4 | 1.2 | 0.5 | 3.2 | 0.9 | 0.5 | 1.4 | 9.8 | |||||
| West | 1.9 | 1.0 | 0.6 | 3.5 | 0.8 | 0.3 | 1.1 | 10.7 | |||||
Survey regions—North-east: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont; North Central: Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, Wisconsin; South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, West Virginia; West: Arizona, California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, Wyoming. Alaska and Hawaii were not included in this survey.
Burkitt, Trowell, and others, who originally worked in Africa, have suggested that dietary fibre is an important protective factor for colon cancer.18,19 However, observed patterns of consumption of fruit and vegetables, which are important sources of dietary fibre, are hardly what one would predict from this hypothesis. These patterns are shown in Table 2.17
Quantity in pounds of fruits and vegetables consumed per person per week, by region, United States, 1965–6617
| Region* . | Fruits . | Vegetables . | Colon cancer death rate, white males2 . |
|---|---|---|---|
| North-east | 4.2 | 5.4 | 16.4 |
| North Central | 3.8 | 5.4 | 13.4 |
| South | 3.2 | 5.3 | 9.8 |
| West | 4.0 | 5.3 | 10.7 |
| Region* . | Fruits . | Vegetables . | Colon cancer death rate, white males2 . |
|---|---|---|---|
| North-east | 4.2 | 5.4 | 16.4 |
| North Central | 3.8 | 5.4 | 13.4 |
| South | 3.2 | 5.3 | 9.8 |
| West | 4.0 | 5.3 | 10.7 |
Composition of regions is specified in Table 1.
Quantity in pounds of fruits and vegetables consumed per person per week, by region, United States, 1965–6617
| Region* . | Fruits . | Vegetables . | Colon cancer death rate, white males2 . |
|---|---|---|---|
| North-east | 4.2 | 5.4 | 16.4 |
| North Central | 3.8 | 5.4 | 13.4 |
| South | 3.2 | 5.3 | 9.8 |
| West | 4.0 | 5.3 | 10.7 |
| Region* . | Fruits . | Vegetables . | Colon cancer death rate, white males2 . |
|---|---|---|---|
| North-east | 4.2 | 5.4 | 16.4 |
| North Central | 3.8 | 5.4 | 13.4 |
| South | 3.2 | 5.3 | 9.8 |
| West | 4.0 | 5.3 | 10.7 |
Composition of regions is specified in Table 1.
Occupational groups with different solar exposures, e.g. fishermen and farmers vs accountants and lawyers, ought to differ in levels of circulating vitamin D metabolites, and, presumably, in rates of colon cancer. A study of individuals in different occupations ought to be carried out to determine if this is the case. This is because correlations of aggregate data, as in these analyses, are subject to an ecological fallacy.20,21
The possibility that an indirect association exists must also be considered. If living in higher latitudes were associated with aetiologically important differences in behaviour, dietary constituents, or genetic characteristics, an indirect association might exist that was not directly attributable to differences in exposure to sunlight.
Dietary sources of vitamin D appear to be relatively minor in the US diet when compared with vitamin D, which is produced endogenously in response to light. Milk, which is fortified with 400 IU of vitamin D per quart, is the most important single source. It is, therefore, of some interest to note that the relative risk for milk consumption in a retrospective study of colon cancer cases was 0.3, which, although not statistically significant, suggests a protective effect of vitamin D-fortified milk.22
The authors express their appreciation for the advice from Prof. Abraham M Lilienfeld, in the preparation of the paper, and to Dr Albert I Mendeloff who also read the manuscript. This work was supported by Grant No. CA11489 from the National Cancer Institute and a grant from Dr James Gamble, a former student at The Johns Hopkins School of Hygiene and Public Health.
References
United States Department of Commerce. Maps of Annual Mean Daily Solar Radiation for the United States. Washington, DC: US Government Printing Office,
Lilienfeld AM, Levin ML, Kessler II. Cancer in the United States. Cambridge: Harvard University Press,
Behar M. Miscellaneous deficiencies. In: Jelliffe DB (ed.). Diseases of Children in the Subtropics and Tropics. London: Edward Arnold Ltd,
United States Department of Commerce. US Census of the Population, 1970, vol. I, part A. Washington, DC: US Government Printing Office,
Mason TJ, McKay FW. US Cancer Mortality by County: 1950–1969. Washington, DC: US Government Printing Office,
Davidson S, Passmore R, Brock JF. Human Nutrition and Dietetics. Baltimore, MD: Williams and Wilkins Company,
Ihde AJ. Studies on the history of rickets. II. The roles of cod liver oil and light.
World Health Organization. Nutrition and Preventive Medicine. Geneva: World Health Organization,
DeLuca HF. Metabolism and function of vitamin D. In: Deluca HF, Suttie JW (eds). The Fat-Soluble Vitamins. Madison: University of Wisconsin Press,
Luithlen F. Das gegenseitige kationenverhaltnis bei verschiedener ernahrung und bei saurevergiftung.
Spielvogel AM, Farley RD, Norman AW. Studies on the mechanism of action of calciferol.
Goodman DBP, Haussler MR, Rasmussen H. Vitamin D3 induced alteration of microvillar membrane lipid composition.
Stanbury SW, Mawer EB, Hill LF, Taylor CM, DeSilva P, Lumb GA. Vitamin D metabolism in adult man in health and in disease. In: Talmage RV, Owen M, Parsons JA (eds). Proceedings of the Fifth Parathyroid Conference on Calcium Regulating Hormones. Amsterdam: Excerpta Medica,
Preece MA, Tomlinson S, Pietrek J et al. Serum 25-hydroxy-vitamin D concentration in man. In: Talmage RV, Owen M, Parsons JA (eds). Proceedings of the Fifth Parathyroid Conference on Calcium Regulating Hormones. Amsterdam: Excerpta Medica,
Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices.
United States Department of Agriculture. Dietary Levels of Households in the Northeast, North Central, South, and West. Washington, DC: US Government Printing Office,
Burkitt DP, Walker ARP, Painter NS. Effect of dietary fibre on stools and transit-times, and its role in the causation of disease.
Robinson WS. Ecological correlations and the behaviour of individuals.