-
PDF
- Split View
-
Views
-
Cite
Cite
Isabelle C. Van Gelder, D. George Wyse, Mary L. Chandler, Howard A. Cooper, Brian Olshansky, Vincent E. Hagens, Harry J.G.M. Crijns, the RACE, Does intensity of rate-control influence outcome in atrial fibrillation? An analysis of pooled data from the RACE and AFFIRM studies, EP Europace, Volume 8, Issue 11, November 2006, Pages 935–942, https://doi.org/10.1093/europace/eul106
Close - Share Icon Share
Abstract
Aims The AFFIRM and RACE studies showed that rate control is an acceptable treatment strategy for atrial fibrillation (AF). We examined whether strict rate control offers benefit over more lenient rate control.
Methods and Results We compared the outcome of patients enrolled in the rate-control arms of AFFIRM and RACE, using data from patients who met a composite of overlapping inclusion and exclusion criteria. We evaluated 1091 patients, 874 from AFFIRM and 217 from RACE. In AFFIRM, the rate-control strategy aimed for a resting heart rate ≤80 bpm and heart rate during daily activity of ≤110 bpm. In RACE, a more lenient approach was taken: resting heart rate <100 bpm. Primary endpoint was a composite of mortality, cardiovascular hospitalization, and myocardial infarction. Mean heart rate across all follow-up visits for patients in AF was lower in AFFIRM (76.1 vs. 83.4 bpm). Event-free survival for the occurrence of the primary endpoint did not differ (64% in AFFIRM vs. 66% in RACE). Patients with mean heart rates during AF within the AFFIRM (≤80) or RACE (<100) criteria had a better outcome than patients with heart rates ≥100 (hazard ratios 0.69 and 0.58, respectively, for ≤80 and <100 compared with ≥100 bpm).
Conclusion Stringency of the approach to rate control, based on the comparison of the AFFIRM and RACE studies, was not associated with an important difference in clinical events.
Introduction
The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study and the RAte Control vs. Electrical cardioversion (RACE) study both showed that rate control is an acceptable treatment strategy for atrial fibrillation (AF).1–3 The optimal target heart rate during AF is still unknown. Intuitively, strict rate control should be associated with fewer symptoms, better quality of life, a lower incidence of heart failure, and better survival. Strict rate control with higher drug doses, in contrast, could lead to drug-related adverse effects, causing symptomatic bradycardia, leading to falls, syncope, trauma, and preventable pacemaker implantation. Furthermore, strict rate control does not necessarily lead to fewer symptoms because symptoms may be due to the underlying cardiovascular disease rather than heart rate.
The AFFIRM and RACE studies used different rate-control guidelines. In AFFIRM, a stricter rate-control strategy was chosen compared with RACE. These differences offered the opportunity to determine if the outcome was related to heart rate. Our first hypothesis was that the dissimilarities in rate control in the two trials would not be associated with differences in important clinical endpoints. Secondly, we hypothesized that in the group of patients with AF at each visit, high heart rates would be associated with poorer outcome.
Methods
Study design
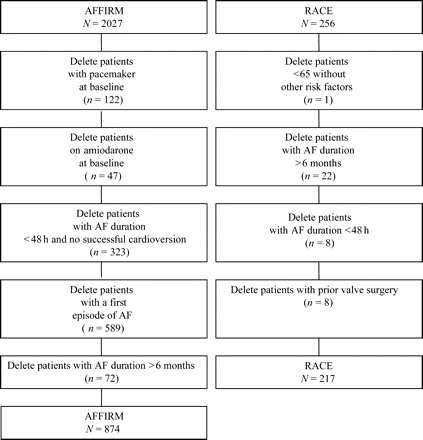
This analysis of the rate-control arms of the AFFIRM and RACE studies evaluated morbidity and mortality, comparing the outcome for patients enrolled in the two studies. We used a composite of overlapping inclusion and exclusion criteria of the two trials to construct the study cohorts. Detailed descriptions of the AFFIRM and RACE designs have been published.1–3 Specifically, we excluded from the AFFIRM study group all patients who had pacemakers or were on amiodarone at baseline, who qualified with their first episode of AF, who had AF <48 h or whose duration of AF was known to be greater than 6 months. From the RACE study group, we excluded patients who were less than 65 years of age with no risk factors for stroke or death, whose duration of AF was known to be greater than 6 months or <48 h, or who had a history of valve surgery (Figure 1). Since not all patients had AF at each visit, the analyses looking at heart rate in AF in relation to survival were performed only in those patients who had AF at all of their visits (n=314 in AFFIRM and n=177 in RACE, respectively).
Outline of patients removed from the rate-control arms of the two trials to form the composite cohort of the present study.
In AFFIRM, a stricter ventricular rate-control strategy was adopted, attempting to keep the resting heart rate ≤80 bpm. Resting heart rates were obtained by apical auscultation for 1 min, following a 5-min period of sitting quietly. In addition, a measure of heart rate control during activity was performed, using either a 6-min walk test or a 24-h Holter monitor. Adequate heart rate control was defined as either (i) heart rate ≤110 bpm during a 6-min walk test, or (ii) a mean heart rate on a 24-h Holter recording <100 bpm and maximum heart rate ≤110% of predicted maximum heart rate during the recording period. In AFFIRM, rate control was evaluated at the 2-month visit and at each 4-monthly clinic visit thereafter. Treatment was adjusted to achieve rate-control goals both at rest and with exercise. Rate-control tests were repeated after drug or dose changes, and whenever major clinical events intervened. Rate control was achieved with beta-blockers, diltiazem, verapamil or digoxin, alone or in combination. Repeated failure of drug therapy because of high rates or drug intolerance could be overcome by radiofrequency ablation of the atrioventricular node and permanent pacemaker implantation. Chronotropic incompetence was managed by pacing. Alternatively, some patients were crossed over to rhythm control.
In RACE, a more lenient approach was taken towards rate control. The target was a resting rate <100 bpm, determined from a 12-lead resting electrocardiogram. Rate control was achieved with the administration of digitalis, a non-dihydropyridine calcium channel blocker or a beta-blocker, alone or in combination. If patients had intolerable symptoms due to AF, unacceptable adverse effects of the atrioventricular node-blocking drugs, or progressive left ventricular dysfunction despite treatment (tachycardiomyopathy), crossover to rhythm control or atrioventricular node ablation and implantation of a pacemaker was performed.
Continuous oral anticoagulation (warfarin in AFFIRM, acenocoumarol or fenprocoumon in RACE) was recommended for patients in the rate-control arms of both studies. The target international normalized ratio (INR) was 2.0–3.0 in the AFFIRM study, and 2.5–3.5 in the RACE study.
The studies comply with the Declaration of Helsinki and were approved by the Institutional Review Boards at each participating hospital. All patients gave written informed consent to participate in the respective trials.
Primary and secondary endpoints
From the endpoints available and collected prospectively in both trials, those the investigators felt might reasonably be affected by a difference in heart rate were a priori considered for the present analysis. The primary endpoint of this study was a composite of all-cause death, cardiovascular hospitalization, and myocardial infarction. In both RACE and AFFIRM, a committee of experts adjudicated major clinical endpoints and their causes unaware of the treatment assignments. Secondary endpoints included individual components of the composite primary endpoint, and pacemaker implantation. As the follow-up time in AFFIRM was longer than in RACE, events experienced by AFFIRM patients at follow-up times later than the maximum follow-up time in RACE (3 years) were not included in the analyses.
Statistical analysis
Means and standard deviations of continuous variables were calculated, and differences between the two study groups were compared using t-tests. For categorical variables, the number and percentage of subjects in each category were calculated, and study groups were compared using χ2 tests for homogeneity or Fisher's exact tests. Kaplan–Meier estimates of cumulative event rates were calculated, and event rates for the two study groups were compared using the log rank statistic. Adjusted hazard ratios were calculated using Cox proportional hazards regression models. For each multivariate Cox analysis comparing the two study groups, the full model included baseline covariates, AF at each visit analysed as a time-dependent covariate, and a study group indicator variable. Next, a stepwise analysis was run. A final, reduced model included study group as well as the variables that remained after the stepwise analysis. For each of the two multivariate models, the accuracy of the assumption of proportional hazards was confirmed by testing a time-by-study group interaction and by examining a plot of negative log-log survivor function estimates vs. the logarithm of time.
Resting heart rates were analysed as time-dependent covariates in models based on patients with AF at all of their visits. Variables at each follow-up visit indicated whether the resting heart rate conformed to the RACE criterion (<100 bpm) and whether it also satisfied the AFFIRM criterion (≤80 bpm). Analyses including these variables can be considered to be ‘per-protocol’ analyses vs. the previously described intention-to-treat analyses that compared AFFIRM with RACE. In order to investigate whether heart rates, as classified in the AFFIRM or RACE criteria, were associated with outcome, Cox models were run, including these two indicator variables based on resting heart rate during AF, analysed as time-dependent covariates. Mean heart rates for all patients, restricted to those in AF at the time of each recorded heart rate measurement, were compared using a repeated measures regression model and assuming an autoregressive covariance structure.
Results
Characteristics of the patients
A total of 874 of 2027 rate-control patients from the AFFIRM study and 217 of 256 rate-control patients from the RACE study were included in the present analysis (Figure 1). AFFIRM patients were more often elderly (P=0.003) or had a history of coronary artery disease (P=0.008), hypertension (P<0.0001), or diabetes mellitus (P=0.009, Table 1). AFFIRM patients were less likely to have had a history of heart failure (P<0.0001) or non-ischaemic cardiomyopathy (P=0.04), and had lower systolic and diastolic blood pressures at study entry (P=0.001, P<0.0001, respectively). Left ventricular function, measured by shortening fraction on echocardiography, was lower in RACE (P=0.0003).
Baseline characteristics
| . | N (total) . | AFFIRM (n=874) . | RACE (n=217) . | P-value . |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age (mean, std. dev.) | 1091 | 69.3 (9.1) | 68.1 (9.2) | 0.07 |
| Age ≥65 years (N, %) | 1091 | 670 (77) | 145 (67) | 0.003 |
| Male (N, %) | 1091 | 517 (59) | 140 (65) | 0.15 |
| History of (N, %) | ||||
| Coronary artery disease | 1091 | 326 (37) | 60 (28) | 0.008 |
| NYHA class II or III | 1091 | 71 (8) | 112 (52) | <0.0001 |
| Myocardial infarction | 1090 | 135 (15) | 35 (16) | 0.78 |
| Hypertension | 1091 | 609 (70) | 93 (43) | <0.0001 |
| Valvular disease | 1091 | 133 (15) | 39 (18) | 0.32 |
| Cardiomyopathy | 1091 | 70 (8) | 27 (12) | 0.04 |
| Diabetes | 1091 | 162 (19) | 24 (11) | 0.009 |
| Stroke | 1091 | 105 (12) | 31 (14) | 0.36 |
| Smoking | 1073 | 101 (12) | 35 (18) | 0.02 |
| Shortening fraction (mean, std. dev.) | 650 | 33.3 (13.7) | 29.8 (9.7) | 0.0003 |
| Blood pressure (mean, std. dev.) | ||||
| Systolic, per mmHg | 1076 | 136.2 (19.7) | 141.5 (21.7) | 0.001 |
| Diastolic, per mmHg | 1076 | 77.2 (11.7) | 84.8 (11.4) | <0.0001 |
| . | N (total) . | AFFIRM (n=874) . | RACE (n=217) . | P-value . |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age (mean, std. dev.) | 1091 | 69.3 (9.1) | 68.1 (9.2) | 0.07 |
| Age ≥65 years (N, %) | 1091 | 670 (77) | 145 (67) | 0.003 |
| Male (N, %) | 1091 | 517 (59) | 140 (65) | 0.15 |
| History of (N, %) | ||||
| Coronary artery disease | 1091 | 326 (37) | 60 (28) | 0.008 |
| NYHA class II or III | 1091 | 71 (8) | 112 (52) | <0.0001 |
| Myocardial infarction | 1090 | 135 (15) | 35 (16) | 0.78 |
| Hypertension | 1091 | 609 (70) | 93 (43) | <0.0001 |
| Valvular disease | 1091 | 133 (15) | 39 (18) | 0.32 |
| Cardiomyopathy | 1091 | 70 (8) | 27 (12) | 0.04 |
| Diabetes | 1091 | 162 (19) | 24 (11) | 0.009 |
| Stroke | 1091 | 105 (12) | 31 (14) | 0.36 |
| Smoking | 1073 | 101 (12) | 35 (18) | 0.02 |
| Shortening fraction (mean, std. dev.) | 650 | 33.3 (13.7) | 29.8 (9.7) | 0.0003 |
| Blood pressure (mean, std. dev.) | ||||
| Systolic, per mmHg | 1076 | 136.2 (19.7) | 141.5 (21.7) | 0.001 |
| Diastolic, per mmHg | 1076 | 77.2 (11.7) | 84.8 (11.4) | <0.0001 |
NYHA, New York Heart Association.
Baseline characteristics
| . | N (total) . | AFFIRM (n=874) . | RACE (n=217) . | P-value . |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age (mean, std. dev.) | 1091 | 69.3 (9.1) | 68.1 (9.2) | 0.07 |
| Age ≥65 years (N, %) | 1091 | 670 (77) | 145 (67) | 0.003 |
| Male (N, %) | 1091 | 517 (59) | 140 (65) | 0.15 |
| History of (N, %) | ||||
| Coronary artery disease | 1091 | 326 (37) | 60 (28) | 0.008 |
| NYHA class II or III | 1091 | 71 (8) | 112 (52) | <0.0001 |
| Myocardial infarction | 1090 | 135 (15) | 35 (16) | 0.78 |
| Hypertension | 1091 | 609 (70) | 93 (43) | <0.0001 |
| Valvular disease | 1091 | 133 (15) | 39 (18) | 0.32 |
| Cardiomyopathy | 1091 | 70 (8) | 27 (12) | 0.04 |
| Diabetes | 1091 | 162 (19) | 24 (11) | 0.009 |
| Stroke | 1091 | 105 (12) | 31 (14) | 0.36 |
| Smoking | 1073 | 101 (12) | 35 (18) | 0.02 |
| Shortening fraction (mean, std. dev.) | 650 | 33.3 (13.7) | 29.8 (9.7) | 0.0003 |
| Blood pressure (mean, std. dev.) | ||||
| Systolic, per mmHg | 1076 | 136.2 (19.7) | 141.5 (21.7) | 0.001 |
| Diastolic, per mmHg | 1076 | 77.2 (11.7) | 84.8 (11.4) | <0.0001 |
| . | N (total) . | AFFIRM (n=874) . | RACE (n=217) . | P-value . |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Age (mean, std. dev.) | 1091 | 69.3 (9.1) | 68.1 (9.2) | 0.07 |
| Age ≥65 years (N, %) | 1091 | 670 (77) | 145 (67) | 0.003 |
| Male (N, %) | 1091 | 517 (59) | 140 (65) | 0.15 |
| History of (N, %) | ||||
| Coronary artery disease | 1091 | 326 (37) | 60 (28) | 0.008 |
| NYHA class II or III | 1091 | 71 (8) | 112 (52) | <0.0001 |
| Myocardial infarction | 1090 | 135 (15) | 35 (16) | 0.78 |
| Hypertension | 1091 | 609 (70) | 93 (43) | <0.0001 |
| Valvular disease | 1091 | 133 (15) | 39 (18) | 0.32 |
| Cardiomyopathy | 1091 | 70 (8) | 27 (12) | 0.04 |
| Diabetes | 1091 | 162 (19) | 24 (11) | 0.009 |
| Stroke | 1091 | 105 (12) | 31 (14) | 0.36 |
| Smoking | 1073 | 101 (12) | 35 (18) | 0.02 |
| Shortening fraction (mean, std. dev.) | 650 | 33.3 (13.7) | 29.8 (9.7) | 0.0003 |
| Blood pressure (mean, std. dev.) | ||||
| Systolic, per mmHg | 1076 | 136.2 (19.7) | 141.5 (21.7) | 0.001 |
| Diastolic, per mmHg | 1076 | 77.2 (11.7) | 84.8 (11.4) | <0.0001 |
NYHA, New York Heart Association.
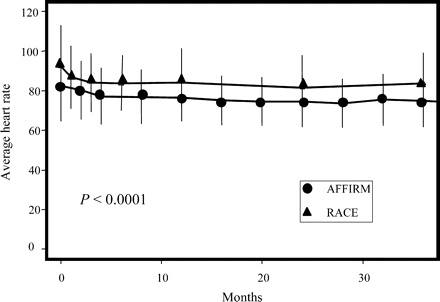
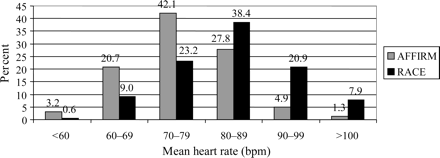
During follow-up, mean heart rate among patients in AF at all their follow-up visits was significantly lower in AFFIRM than in RACE (Figure 2; 76.1 vs. 83.4 bpm, P<0.0001). Figure 3 shows the proportion of patients in each study with mean heart rates (across all follow-up visits) in the indicated category. Sixty-six percent of AFFIRM patients had average resting heart rates <80 bpm, whereas over 67% of the RACE patients had average resting heart rates ≥80 bpm. Only a minority of patients had an average heart rate >90 bpm.
Heart rate over time in all AFFIRM and RACE patients in AF at all assessments.
Proportion of patients in the various categories of heart rates in the AFFIRM and RACE studies. Numbers at the top of each bar are percentages.
Composite primary endpoint
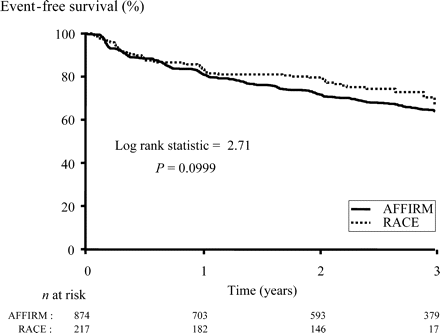
The incidence of the composite endpoint and its components are shown in Table 2. Event-free survival for the primary endpoint did not differ between studies (Figure 4). Multivariate analysis showed that a history of coronary artery disease (P=0.004), New York Heart Association functional class II or III heart failure (P=0.02), and the presence of valve disease (P=0.003) independently increased the risk of the composite endpoint but the study group (RACE vs. AFFIRM, P=0.48) did not (Table 3). Time-dependent AF, i.e. presence of AF during follow up, was associated with a lower incidence of the composite endpoint (P=0.0006).
Incidence of composite endpoint and its components
| . | AFFIRM (n=874) . | RACE (n=217) . |
|---|---|---|
| N (%) | N (%) | |
| Composite endpoint | ||
| Death, cardiovascular hospitalization or myocardial infarction | 299 (34) | 55 (25) |
| Individual endpoints | ||
| Death | 37 (4) | 5 (2) |
| Cardiovascular hospitalization | 247 (28) | 47 (22) |
| Myocardial infarction | 15 (2) | 3 (1) |
| . | AFFIRM (n=874) . | RACE (n=217) . |
|---|---|---|
| N (%) | N (%) | |
| Composite endpoint | ||
| Death, cardiovascular hospitalization or myocardial infarction | 299 (34) | 55 (25) |
| Individual endpoints | ||
| Death | 37 (4) | 5 (2) |
| Cardiovascular hospitalization | 247 (28) | 47 (22) |
| Myocardial infarction | 15 (2) | 3 (1) |
The composite includes only the first event. A patient may have more than one event. Only first events are included in the individual endpoint frequencies.
Incidence of composite endpoint and its components
| . | AFFIRM (n=874) . | RACE (n=217) . |
|---|---|---|
| N (%) | N (%) | |
| Composite endpoint | ||
| Death, cardiovascular hospitalization or myocardial infarction | 299 (34) | 55 (25) |
| Individual endpoints | ||
| Death | 37 (4) | 5 (2) |
| Cardiovascular hospitalization | 247 (28) | 47 (22) |
| Myocardial infarction | 15 (2) | 3 (1) |
| . | AFFIRM (n=874) . | RACE (n=217) . |
|---|---|---|
| N (%) | N (%) | |
| Composite endpoint | ||
| Death, cardiovascular hospitalization or myocardial infarction | 299 (34) | 55 (25) |
| Individual endpoints | ||
| Death | 37 (4) | 5 (2) |
| Cardiovascular hospitalization | 247 (28) | 47 (22) |
| Myocardial infarction | 15 (2) | 3 (1) |
The composite includes only the first event. A patient may have more than one event. Only first events are included in the individual endpoint frequencies.
Adjusted analysis of composite endpoint: AFFIRM vs. RACE
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| RACE group | 0.92 | 0.60–1.43 | 0.72 | 0.88 | 0.62–1.25 | 0.48 |
| In AF | 0.69 | 0.50–0.93 | 0.02 | 0.67 | 0.54–0.85 | 0.0006 |
| Age≥65 years | 1.02 | 0.73–1.42 | 0.90 | |||
| Female gender | 0.89 | 0.66–1.21 | 0.46 | |||
| History of | ||||||
| Coronary artery disease | 1.53 | 1.41–2.06 | 0.005 | 1.39 | 1.11–1.73 | 0.004 |
| NYHA class II or III | 1.89 | 1.28–2.78 | 0.001 | 1.45 | 1.07–1.97 | 0.02 |
| Stroke | 1.58 | 1.11–2.24 | 0.01 | |||
| Hypertension | 1.31 | 0.95–1.82 | 0.10 | |||
| Diabetes | 1.28 | 0.90–1.82 | 0.17 | 1.29 | 1.00–1.68 | 0.05 |
| Smoking | 1.47 | 1.00–2.15 | 0.05 | |||
| Valve disease | 1.36 | 0.95–1.95 | 0.09 | 1.48 | 1.14 –1.93 | 0.003 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.99–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.98–1.01 | 0.27 | 0.99 | 0.98 –1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.02 | 0.34 | |||
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| RACE group | 0.92 | 0.60–1.43 | 0.72 | 0.88 | 0.62–1.25 | 0.48 |
| In AF | 0.69 | 0.50–0.93 | 0.02 | 0.67 | 0.54–0.85 | 0.0006 |
| Age≥65 years | 1.02 | 0.73–1.42 | 0.90 | |||
| Female gender | 0.89 | 0.66–1.21 | 0.46 | |||
| History of | ||||||
| Coronary artery disease | 1.53 | 1.41–2.06 | 0.005 | 1.39 | 1.11–1.73 | 0.004 |
| NYHA class II or III | 1.89 | 1.28–2.78 | 0.001 | 1.45 | 1.07–1.97 | 0.02 |
| Stroke | 1.58 | 1.11–2.24 | 0.01 | |||
| Hypertension | 1.31 | 0.95–1.82 | 0.10 | |||
| Diabetes | 1.28 | 0.90–1.82 | 0.17 | 1.29 | 1.00–1.68 | 0.05 |
| Smoking | 1.47 | 1.00–2.15 | 0.05 | |||
| Valve disease | 1.36 | 0.95–1.95 | 0.09 | 1.48 | 1.14 –1.93 | 0.003 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.99–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.98–1.01 | 0.27 | 0.99 | 0.98 –1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.02 | 0.34 | |||
NYHA New York Heart Association.
Adjusted analysis of composite endpoint: AFFIRM vs. RACE
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| RACE group | 0.92 | 0.60–1.43 | 0.72 | 0.88 | 0.62–1.25 | 0.48 |
| In AF | 0.69 | 0.50–0.93 | 0.02 | 0.67 | 0.54–0.85 | 0.0006 |
| Age≥65 years | 1.02 | 0.73–1.42 | 0.90 | |||
| Female gender | 0.89 | 0.66–1.21 | 0.46 | |||
| History of | ||||||
| Coronary artery disease | 1.53 | 1.41–2.06 | 0.005 | 1.39 | 1.11–1.73 | 0.004 |
| NYHA class II or III | 1.89 | 1.28–2.78 | 0.001 | 1.45 | 1.07–1.97 | 0.02 |
| Stroke | 1.58 | 1.11–2.24 | 0.01 | |||
| Hypertension | 1.31 | 0.95–1.82 | 0.10 | |||
| Diabetes | 1.28 | 0.90–1.82 | 0.17 | 1.29 | 1.00–1.68 | 0.05 |
| Smoking | 1.47 | 1.00–2.15 | 0.05 | |||
| Valve disease | 1.36 | 0.95–1.95 | 0.09 | 1.48 | 1.14 –1.93 | 0.003 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.99–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.98–1.01 | 0.27 | 0.99 | 0.98 –1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.02 | 0.34 | |||
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| RACE group | 0.92 | 0.60–1.43 | 0.72 | 0.88 | 0.62–1.25 | 0.48 |
| In AF | 0.69 | 0.50–0.93 | 0.02 | 0.67 | 0.54–0.85 | 0.0006 |
| Age≥65 years | 1.02 | 0.73–1.42 | 0.90 | |||
| Female gender | 0.89 | 0.66–1.21 | 0.46 | |||
| History of | ||||||
| Coronary artery disease | 1.53 | 1.41–2.06 | 0.005 | 1.39 | 1.11–1.73 | 0.004 |
| NYHA class II or III | 1.89 | 1.28–2.78 | 0.001 | 1.45 | 1.07–1.97 | 0.02 |
| Stroke | 1.58 | 1.11–2.24 | 0.01 | |||
| Hypertension | 1.31 | 0.95–1.82 | 0.10 | |||
| Diabetes | 1.28 | 0.90–1.82 | 0.17 | 1.29 | 1.00–1.68 | 0.05 |
| Smoking | 1.47 | 1.00–2.15 | 0.05 | |||
| Valve disease | 1.36 | 0.95–1.95 | 0.09 | 1.48 | 1.14 –1.93 | 0.003 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.99–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.98–1.01 | 0.27 | 0.99 | 0.98 –1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.02 | 0.34 | |||
NYHA New York Heart Association.
In order to assess whether resting heart rate during AF is associated with outcome, we performed a sub-analysis in all patients who had, in addition to the inclusion criteria as depicted in Figure 1, AF at all of their visits (314 patients in AFFIRM and 177 in RACE, respectively). Table 4 indicates that there were significant differences among the three heart rate groups (P=0.004), with decreased probability of a composite event in the two lower heart rate groups (hazard ratios of 0.69 and 0.58, respectively, for ≤80 and <100 bpm compared with ≥100 bpm). Additional covariates associated with composite outcome included a history of coronary artery disease (P=0.01), smoking (P=0.01), valve disease (P=0.03), and lower diastolic blood pressure (P=0.02).
Adjusted analysis of composite endpoint in patients with AF at all visits: lenient vs. stringent heart rate criteria
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| Heart rate≤80 bpm | 0.70 | 0.42–1.15 | 0.08 | 0.69 | 0.45–1.05 | 0.004 |
| Heart rate <100 bpm | 0.66 | 0.31–1.38 | 0.58 | 0.32–1.04 | ||
| Age≥65 years | 1.53 | 0.85–2.78 | 0.16 | |||
| Female gender | 0.58 | 0.34–1.00 | 0.05 | |||
| History of | ||||||
| Coronary artery disease | 1.85 | 1.15–2.97 | 0.01 | 1.63 | 1.12–2.38 | 0.01 |
| NYHA class II or III | 1.87 | 1.11–3.15 | 0.02 | |||
| Stroke | 1.65 | 0.90–3.01 | 0.11 | |||
| Hypertension | 1.24 | 0.73–2.10 | 0.44 | |||
| Diabetes | 1.79 | 0.97–3.30 | 0.06 | |||
| Smoking | 1.86 | 1.02–3.38 | 0.04 | 1.79 | 1.13–2.83 | 0.01 |
| Valve disease | 1.41 | 0.84–2.38 | 0.20 | 1.59 | 1.05–2.41 | 0.03 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.98–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.96–1.02 | 0.39 | 0.98 | 0.96–1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.03 | 0.28 | |||
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| Heart rate≤80 bpm | 0.70 | 0.42–1.15 | 0.08 | 0.69 | 0.45–1.05 | 0.004 |
| Heart rate <100 bpm | 0.66 | 0.31–1.38 | 0.58 | 0.32–1.04 | ||
| Age≥65 years | 1.53 | 0.85–2.78 | 0.16 | |||
| Female gender | 0.58 | 0.34–1.00 | 0.05 | |||
| History of | ||||||
| Coronary artery disease | 1.85 | 1.15–2.97 | 0.01 | 1.63 | 1.12–2.38 | 0.01 |
| NYHA class II or III | 1.87 | 1.11–3.15 | 0.02 | |||
| Stroke | 1.65 | 0.90–3.01 | 0.11 | |||
| Hypertension | 1.24 | 0.73–2.10 | 0.44 | |||
| Diabetes | 1.79 | 0.97–3.30 | 0.06 | |||
| Smoking | 1.86 | 1.02–3.38 | 0.04 | 1.79 | 1.13–2.83 | 0.01 |
| Valve disease | 1.41 | 0.84–2.38 | 0.20 | 1.59 | 1.05–2.41 | 0.03 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.98–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.96–1.02 | 0.39 | 0.98 | 0.96–1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.03 | 0.28 | |||
HR, hazard ration; NYHA, New York Heart Association.
Adjusted analysis of composite endpoint in patients with AF at all visits: lenient vs. stringent heart rate criteria
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| Heart rate≤80 bpm | 0.70 | 0.42–1.15 | 0.08 | 0.69 | 0.45–1.05 | 0.004 |
| Heart rate <100 bpm | 0.66 | 0.31–1.38 | 0.58 | 0.32–1.04 | ||
| Age≥65 years | 1.53 | 0.85–2.78 | 0.16 | |||
| Female gender | 0.58 | 0.34–1.00 | 0.05 | |||
| History of | ||||||
| Coronary artery disease | 1.85 | 1.15–2.97 | 0.01 | 1.63 | 1.12–2.38 | 0.01 |
| NYHA class II or III | 1.87 | 1.11–3.15 | 0.02 | |||
| Stroke | 1.65 | 0.90–3.01 | 0.11 | |||
| Hypertension | 1.24 | 0.73–2.10 | 0.44 | |||
| Diabetes | 1.79 | 0.97–3.30 | 0.06 | |||
| Smoking | 1.86 | 1.02–3.38 | 0.04 | 1.79 | 1.13–2.83 | 0.01 |
| Valve disease | 1.41 | 0.84–2.38 | 0.20 | 1.59 | 1.05–2.41 | 0.03 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.98–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.96–1.02 | 0.39 | 0.98 | 0.96–1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.03 | 0.28 | |||
| . | Full model . | Reduced model . | P-value . | |||
|---|---|---|---|---|---|---|
| . | . | . | ||||
| . | HR . | 95% CI . | P-value . | HR . | 95% CI . | |
| Heart rate≤80 bpm | 0.70 | 0.42–1.15 | 0.08 | 0.69 | 0.45–1.05 | 0.004 |
| Heart rate <100 bpm | 0.66 | 0.31–1.38 | 0.58 | 0.32–1.04 | ||
| Age≥65 years | 1.53 | 0.85–2.78 | 0.16 | |||
| Female gender | 0.58 | 0.34–1.00 | 0.05 | |||
| History of | ||||||
| Coronary artery disease | 1.85 | 1.15–2.97 | 0.01 | 1.63 | 1.12–2.38 | 0.01 |
| NYHA class II or III | 1.87 | 1.11–3.15 | 0.02 | |||
| Stroke | 1.65 | 0.90–3.01 | 0.11 | |||
| Hypertension | 1.24 | 0.73–2.10 | 0.44 | |||
| Diabetes | 1.79 | 0.97–3.30 | 0.06 | |||
| Smoking | 1.86 | 1.02–3.38 | 0.04 | 1.79 | 1.13–2.83 | 0.01 |
| Valve disease | 1.41 | 0.84–2.38 | 0.20 | 1.59 | 1.05–2.41 | 0.03 |
| Blood pressure | ||||||
| Systolic | 1.00 | 0.98–1.01 | 0.54 | |||
| Diastolic | 0.99 | 0.96–1.02 | 0.39 | 0.98 | 0.96–1.00 | 0.02 |
| Shortening fraction, % | 1.01 | 0.99–1.03 | 0.28 | |||
HR, hazard ration; NYHA, New York Heart Association.
Secondary endpoints
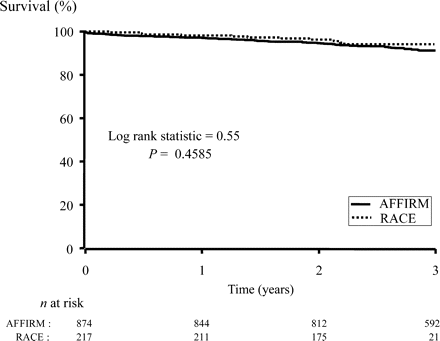
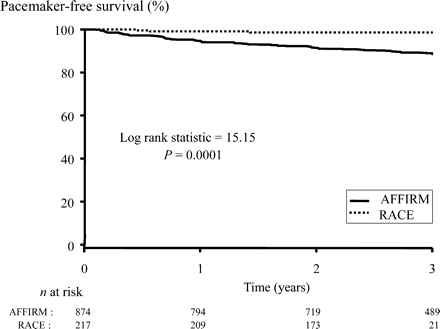
There was no difference in all-cause mortality between the rate-control strategies of the two trials (Figure 5). This finding was also true when only patients with AF at all visits were included (data not shown). Adjusted proportional hazards models of all-cause mortality looking at resting heart rate among patients in AF at all follow-ups were not run because the number of events in this subgroup was too small to produce stable estimates. Pacemaker implantations were more frequent in the AFFIRM patients (11 vs. 1% in AFFIRM and RACE, respectively, P=0.0001, Figure 6). Pacemakers were mainly implanted in the setting of atrioventricular junctional ablation or bradycardia induced by rate-control drugs.
Overall survival in AFFIRM and RACE. All causes of death are included.
Freedom from pacemaker implantation survival in AFFIRM and RACE.
Discussion
The data of the present retrospective analysis of two large multicentre randomized clinical trials of an elderly population with mostly persistent AF show that there were no differences in mortality or cardiovascular morbidity observed in the two trials. Stringent heart rate control resulted in a heart rate difference over the course of the two studies averaging 7 bpm. Stringent or lenient rate-control criteria analysed based on the observed resting heart rate were associated with decreased rates of a composite endpoint of major clinical events compared with heart rates over 100 bpm. The patients in AFFIRM had increased incidence of pacemaker implantations.
Strict or lenient rate control during AF
Rate control is an acceptable treatment strategy for AF.2,3 The optimal target heart rate during AF, however, is still unknown. The question remains whether strict rate control is associated with an improved prognosis compared with a more lenient approach. The data of the present study comparing patients in RACE vs. patients in AFFIRM suggest that the stringency of rate control within the limits achieved in these two trials does not influence outcome.
There are several explanations why patients with lower heart rates in AFFIRM did not show a more favourable outcome, compared with patients in RACE (with higher heart rates). First, the greater use of pacemaker implantations in the stringent rate-control group (AFFIRM) might have caused cardiovascular hospitalizations, a component of the primary endpoint of the present study. Second, a more aggressive rate-control approach that included higher dosages and combinations of negative chronotropic drugs may have reduced the incidence of endpoints in some patients, but at the same time, may have produced avoidable cardiovascular hospitalizations and bradycardia-related sudden death in others. Third, it may be argued that in spite of different criteria in the two trials, the actual resting heart rates achieved in the two studies were not markedly different. This last point is certainly debatable. Nevertheless, an average difference of this magnitude (7.3 bpm) is probably substantial. Although the target resting heart rate in RACE was <100, the actual average rate was approximately 83 bpm, and a minority of patients in both studies had resting heart rates over 90 bpm. Therefore, it should not be assumed on the basis of these results that resting heart rates over 90 bpm are a suitable long-term goal. In the sub-analysis of those in AF throughout the duration of follow-up, rates over 100 bpm were associated with poor outcome.
We observed more pacemaker implantations in the strict rate-control group. This observation could be another important finding in favour of a lenient rate-control strategy. In the setting of AF, pacemaker implantation is frequently needed for underlying intrinsic sick sinus syndrome that is unmasked especially by a rhythm control strategy. Bradycardia may also be due to conduction system disease. Obviously, the more negative chronotropic drugs are given to obtain a certain heart rate, the greater the risk of producing symptomatic bradycardia. In addition, if the target heart rate is not reached, atrioventricular junctional ablation is performed. Although a strict rate control may have been beneficial in some patients, this may also have come at the price of iatrogenic bradycardia and excess implantation of pacemakers in others. As long as the benefit of a strict rate-control strategy is not clear, and given the potential for the deleterious effect of pacing at the right ventricular apex on ventricular function in some patients, iatrogenic drug-induced bradycardia or atrioventricular junctional ablation with a pacemaker should be avoided. However, the possibility that the different thresholds that exist in Europe and North America for pacemaker insertion, to treat bradycardia, contributed to this observation cannot be excluded without a randomized trial.
Previous data from the AFFIRM study group showed that strict rate control is often difficult to achieve in a substantial minority of patients and requires frequent medication changes. This may favour the more-easily attainable lenient rate-control approach, at least for some patients.4
Clinical outcome was not associated with being in the RACE or AFFIRM study in analyses comparing these two groups of patients. This result is confirmed by analyses based on resting heart rate at each visit which indicate that in those with AF throughout the duration of observation, increased risk of a clinical outcome is observed in patients with heart rates greater than the rate-control criteria for both studies (≥100 bpm).
Two other post hoc analyses studied the influence of the level of rate control during AF on morbidity and mortality. The detailed analysis of the AFFIRM rate-control population showed that after 2 months of drug titration, neither resting heart rate nor exercise heart rate was related to overall survival, cardiac hospitalization, quality of life, or functional status.5 However, in that AFFIRM rate-control population only 7.3% had a resting heart rate >100 bpm. In another post hoc analysis of patients with AF in the setting of advanced heart failure, we observed that higher heart rates at baseline were not associated with a worse survival.6 In contrast to the latter findings, Khand et al.7 observed that in patients with an impaired left ventricular function and AF, a more strict rate-control approach may be beneficial. They randomized patients with heart failure (left ventricular ejection fraction averaging 24%) and AF to carvedilol plus digoxin or to digoxin alone. After a follow-up of 4 months, heart rate was significantly lower in the patients treated with the combination drugs, compared with the patients who were treated with digoxin alone (65±15 vs. 75±11%, P<0.0001). Compared with placebo, the addition of carvedilol to digoxin significantly improved left ventricular ejection fraction (24±7% to 31±10%, P<0.05). Whether this observation is due to heart rate control itself or a salutary effect of beta-blockade in patients with congestive heart failure cannot be determined. Furthermore, whether this translates into a survival benefit and a reduced morbidity remains to be seen. A strategy producing a higher ejection fraction does not necessarily guarantee improved overall morbidity and mortality, especially if the eventual ejection fraction is still low. This is particularly pertinent given that the Cardiac Insufficiency Bisoprolol Study (CIBIS) did not show a survival benefit with beta-blockade in the subgroup of heart failure patients with AF.8
Small-scale studies have demonstrated that a heart rate above 100 bpm is associated with the development of heart failure.9–12 Still other studies suggested that it is the irregularity of the rhythm that contributed to the heart failure.13,14 Although hospitalization for heart failure was not an endpoint for our comparison because those data were not specifically collected prospectively in a uniform manner in both trials, all cardiovascular hospitalizations were included in the composite primary endpoint. Serious aggravation of heart failure requiring hospitalization would have been counted in this endpoint. Clearly, this was not affected by stringency of rate control. Furthermore, when the data were analysed by the actual heart rate achieved, only heart rates ≥100 bpm were associated with adverse outcome.
Prognostic significance of severity of underlying heart disease on outcome
As could be expected, the composite endpoint was predominantly influenced by severity of the underlying heart disease rather than heart rate control.
Time-dependent presence of AF during follow-up was associated with a favourable prognosis for the composite endpoint. In this respect, permanent AF with continuous oral anticoagulation may have lowered the risk of bleeding or thromboembolic events; thus, may have reduced the number of cardiovascular hospitalizations.15,16 In a previous sub-analysis of the AFFIRM trial data, the presence of sinus rhythm over time was associated with reduced risk of death, however. It is important to point out, therefore, that the present study included only patients randomized to rate control and therefore use of antiarrhythmic drugs, an important co-variable in the previous sub-analysis, was minimal in the present cohort.15
Limitations
The two studies were not entirely comparable. The enrolment criteria for the two studies were quite different, requiring exclusion of many patients in this comparison of selected patients with similar clinical characteristics. Furthermore, our comparison of AFFIRM and RACE was a retrospective, post hoc analysis. The number of AFFIRM and RACE patients eligible for this analysis was quite different, limiting the power for the statistical comparisons. In AFFIRM, not all patients had persistent AF. More patients in AFFIRM had a history of hypertension. However, AFFIRM patients' actual blood pressures were lower, compared with the patients in RACE. It is possible that the treatment in the AFFIRM study for underlying conditions such as hypertension was more aggressive than in the RACE study. In the analyses of pacemaker implantation, it cannot be determined how much of the association was due to stringent heart rate control and how much was due to differences in the threshold for pacemaker implantation between Europe and North America. There could also be geographical differences in the measurement of outcomes or covariates that biased the results.
Finally, these data apply only to patients with AF as defined in these studies.
Conclusions
Rate control with anticoagulation is an acceptable treatment strategy in patients with AF who have risk factors for stroke or death. The stringency of the approach to rate control, based on this comparison of the AFFIRM and the RACE studies, is not associated with a substantial difference in clinical outcome, although patients with mean heart rates during AF within the study heart rate criteria for AFFIRM (≤80) or RACE (<100) had better outcome than patients whose mean heart rates were ≥100.
Analysis of a combined endpoint of morbidity and mortality does not favour either the lenient or the strict rate-control strategy. However, the lenient strategy can be more easily applied because it does not require assessment of rate control during activity, except when symptoms are present. Furthermore, the more lenient approach may lead to less pacemaker implantations. Nevertheless, we must await randomized studies before recommending a particular approach.
Acknowledgements
The RACE Study is supported by the Center for Health Care Insurance (OG96-047), the Interuniversity Cardiology Institute, The Netherlands, and an unrestricted grant from 3M Pharma, The Netherlands. The AFFIRM Study is supported by contract No. N01-HC-55139 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland, MD 20892, USA.
References
The participants in the RACE Study have been listed elsewhere (N Engl J Med 2002;347:1834–40)
The participants in the AFFIRM Study have been listed elsewhere (Am Heart J 2002;143:991–1001)