-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Goette, Kathleen Jentsch-Ullrich, Matthias Hammwöhner, Silke Trautmann, Astrid Franke, Helmut U. Klein, Angelo Auricchio, Cardiac uptake of progenitor cells in patients with moderate-to-severe left ventricular failure scheduled for cardiac resynchronization therapy, EP Europace, Volume 8, Issue 3, March 2006, Pages 157–160, https://doi.org/10.1093/europace/euj042
Close - Share Icon Share
Abstract
Aims Injury to the heart causes haematopoietic and endothelial progenitor cells (PCs) to migrate to the site of damage and to undergo PC differentiation, which may contribute to angiogenesis and myocardial tissue repair. We sought to determine the cardiac uptake of PC in patients with moderate-to-severe congestive heart failure (CHF) scheduled for cardiac resynchronization therapy.
Methods and results A total of 28 patients was included in the study. Fourteen patients had moderate-to-severe CHF with a mean left ventricular ejection fraction (LVEF) of 20±9%. The remaining patients had a normal LVEF and served as controls. PCs (CD34+ and CD34+/CD117+) were quantified using a fluorescence-activated cell sorter. In CHF patients, PCs were determined from whole blood samples taken from the aorta, the coronary sinus (CS), and the superior vena cava (SVC) during right and left heart catheterization. Cardiac PC uptake was determined as the difference in PC levels between the aorta and the CS. Differences in CD34+PC counts (Δ0.11±0.98×103 mL−1) and relative amount of CD34+/CD117+PC (Δ0.08±0.31%) between the aorta and the CS were not significant. PC levels were comparable between the SVC, CS, and aorta. CD34+ and PC levels did not correlate with New York Heart Association class (r2=0.22), LVEF (r2=0.01), LV diameter (r2=0.05), QRS complex duration (r2=0.1), or maximal O2 uptake during exercise (r2=0.08). There was no difference between patients with ischaemic cardiomyopathy (ICM) and non-ICM. Systemic PC levels were not different compared with age-matched controls without LV failure (CD34+: 4.61±1.83×103 mL−1 vs. control: 5.25±1.67×103 mL−1; P=n.s.).
Conclusion Moderate-to-severe chronic CHF is not associated with elevated PC levels in the systemic circulation. A measurable cardiac uptake of CD34+ and CD34+/CD117+PC cannot be demonstrated by FACS analysis in this cohort of patients.
Introduction
Recent studies suggest that acute injury to the heart causes haematopoietic and endothelial progenitor cells (PCs) to migrate to the site of damage and to undergo PC differentiation.1,2 Multipotent PCs, which are characterized by the cell-surface expression of CD34+ and CD117+, seem to be involved in neoangiogenesis and they may differentiate/transdifferentiate into cardiomyocytes.1–4 Thus, PCs appear to have the potential to improve cardiac performance after an acute cardiac injury.1,4 In contrast to acute cardiac injuries, the regulation of PC in patients with chronic stable cardiac abnormalities has not yet been analysed. Although it may be of great clinical relevance, the impact of moderate-to-severe stable congestive heart failure (CHF) on PC is unknown.
The purpose of the present study was to determine the amount of PC in patients with moderate-to-severe CHF. Therefore, venous and arterial PC levels were measured and the cardiac uptake of CD34+PC and CD34+/CD117+PC was assessed during cardiac catherization scheduled for cardiac resynchronization therapy.
Methods
Patients
A total of 28 patients was included in the study. Fourteen patients (59±14 years, 11 men, 3 women) had symptomatic moderate-to-severe left ventricular failure [New York Heart Association (NYHA) class≥II], sinus rhythm, and depressed left ventricular ejection fraction (LVEF: 20±9%) despite pharmacological therapy (Table 1). The patients with CHF were referred to our institution for evaluation of non-pharmacological therapy of heart failure. In patients with CHF, blood samples were drawn during diagnostic right and left heart catheterization. Venous CD34+ and CD34+/CD117+PC levels of these patients were compared with 14 age-matched patients (55±16 years; eight men, six women) without structural heart or coronary artery disease. Nine patients (65%) had a history of hypertension, treated with ACE-inhibitors (53%), beta-blockers (47%), and calcium channel antagonists (18%). None of the included patients was taking statins at the time of the study. All patients gave informed consent to the procedure, which was performed according to the institutional guidelines of good clinical practice.
CHF patients characteristics
| No. . | Age . | Sex . | Weight (kg)/Height (cm) . | DM . | RR . | NYHA . | ICM . | DCM . | LVEF . | MR . | LVd . | QRSd . | Medication . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 76 | m | 97/179 | 1 | 1 | III | 1 | — | 30 | I | 6.4 | 166 | A,B,D,Di |
| 2. | 39 | m | 110/176 | — | 1 | II | — | 1 | 30 | II | 6.3 | 98 | A,D,Di |
| 3. | 65 | w | 66/156 | 1 | 1 | III | — | 1 | 20 | III | 7.0 | 182 | A,D,Di |
| 4. | 76 | m | 66/173 | 1 | — | III | — | 1 | 20 | — | 5.8 | 110 | A,D,Di |
| 5. | 62 | m | 63/174 | — | — | III | — | 1 | 15 | III | 6.5 | 130 | A,D,Di,S |
| 6. | 57 | w | 79/168 | — | — | II | — | 1 | 40 | — | 4.7 | 100 | B |
| 7. | 64 | m | 70/173 | — | 1 | III | 1 | — | 20 | — | NA | 127 | A,B,D,Di |
| 8. | 67 | m | 89/169 | 1 | — | III | 1 | — | 15 | I | 6.3 | 135 | A,B,D,Di |
| 9. | 60 | w | 72/164 | — | 1 | III | — | 1 | 15 | III | 9.3 | 182 | A,B,D,Di |
| 10. | 63 | m | 74/179 | — | — | III | 1 | — | 10 | III | 7.3 | 148 | A,B,D,Di,S |
| 11. | 75 | m | 65/174 | — | — | III | 1 | — | 10 | II | 7.2 | 213 | A,B,D,Di,S |
| 12. | 60 | m | 105/183 | — | 1 | III | — | 1 | 30 | III | 6.0 | 134 | A,B.D,Di,S |
| 13. | 70 | m | 70/159 | — | — | III | 1 | — | 10 | I | 6.7 | 168 | A,B,D,N |
| 14. | 67 | m | 84/176 | 1 | 1 | III | 1 | — | 10 | IV | 6.8 | 186 | A,C,D,Di |
| 59±14 | 11/3 | 5(36) | 7(50) | 7(50) | 7(50) | 20±9 | 6.4±1 | 148±35 | |||||
| No. . | Age . | Sex . | Weight (kg)/Height (cm) . | DM . | RR . | NYHA . | ICM . | DCM . | LVEF . | MR . | LVd . | QRSd . | Medication . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 76 | m | 97/179 | 1 | 1 | III | 1 | — | 30 | I | 6.4 | 166 | A,B,D,Di |
| 2. | 39 | m | 110/176 | — | 1 | II | — | 1 | 30 | II | 6.3 | 98 | A,D,Di |
| 3. | 65 | w | 66/156 | 1 | 1 | III | — | 1 | 20 | III | 7.0 | 182 | A,D,Di |
| 4. | 76 | m | 66/173 | 1 | — | III | — | 1 | 20 | — | 5.8 | 110 | A,D,Di |
| 5. | 62 | m | 63/174 | — | — | III | — | 1 | 15 | III | 6.5 | 130 | A,D,Di,S |
| 6. | 57 | w | 79/168 | — | — | II | — | 1 | 40 | — | 4.7 | 100 | B |
| 7. | 64 | m | 70/173 | — | 1 | III | 1 | — | 20 | — | NA | 127 | A,B,D,Di |
| 8. | 67 | m | 89/169 | 1 | — | III | 1 | — | 15 | I | 6.3 | 135 | A,B,D,Di |
| 9. | 60 | w | 72/164 | — | 1 | III | — | 1 | 15 | III | 9.3 | 182 | A,B,D,Di |
| 10. | 63 | m | 74/179 | — | — | III | 1 | — | 10 | III | 7.3 | 148 | A,B,D,Di,S |
| 11. | 75 | m | 65/174 | — | — | III | 1 | — | 10 | II | 7.2 | 213 | A,B,D,Di,S |
| 12. | 60 | m | 105/183 | — | 1 | III | — | 1 | 30 | III | 6.0 | 134 | A,B.D,Di,S |
| 13. | 70 | m | 70/159 | — | — | III | 1 | — | 10 | I | 6.7 | 168 | A,B,D,N |
| 14. | 67 | m | 84/176 | 1 | 1 | III | 1 | — | 10 | IV | 6.8 | 186 | A,C,D,Di |
| 59±14 | 11/3 | 5(36) | 7(50) | 7(50) | 7(50) | 20±9 | 6.4±1 | 148±35 | |||||
m, male; w, women; DM, diabetes mellitus; RR, history of arterial hypertension; MR, degree of mitral regurgitation; LVd, LV diameter in diastole; QRSd, duration of the QRS complex on the surface ECG (ms); medication: A, ACE-inhibitor/angiotensin II receptor blocker; B, beta-blocker; C, amiodarone; D, diuretics; Di, digitalis; N, nitrates; S, spironolactone. NA, data not available. Numbers in parenthesis (%).
CHF patients characteristics
| No. . | Age . | Sex . | Weight (kg)/Height (cm) . | DM . | RR . | NYHA . | ICM . | DCM . | LVEF . | MR . | LVd . | QRSd . | Medication . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 76 | m | 97/179 | 1 | 1 | III | 1 | — | 30 | I | 6.4 | 166 | A,B,D,Di |
| 2. | 39 | m | 110/176 | — | 1 | II | — | 1 | 30 | II | 6.3 | 98 | A,D,Di |
| 3. | 65 | w | 66/156 | 1 | 1 | III | — | 1 | 20 | III | 7.0 | 182 | A,D,Di |
| 4. | 76 | m | 66/173 | 1 | — | III | — | 1 | 20 | — | 5.8 | 110 | A,D,Di |
| 5. | 62 | m | 63/174 | — | — | III | — | 1 | 15 | III | 6.5 | 130 | A,D,Di,S |
| 6. | 57 | w | 79/168 | — | — | II | — | 1 | 40 | — | 4.7 | 100 | B |
| 7. | 64 | m | 70/173 | — | 1 | III | 1 | — | 20 | — | NA | 127 | A,B,D,Di |
| 8. | 67 | m | 89/169 | 1 | — | III | 1 | — | 15 | I | 6.3 | 135 | A,B,D,Di |
| 9. | 60 | w | 72/164 | — | 1 | III | — | 1 | 15 | III | 9.3 | 182 | A,B,D,Di |
| 10. | 63 | m | 74/179 | — | — | III | 1 | — | 10 | III | 7.3 | 148 | A,B,D,Di,S |
| 11. | 75 | m | 65/174 | — | — | III | 1 | — | 10 | II | 7.2 | 213 | A,B,D,Di,S |
| 12. | 60 | m | 105/183 | — | 1 | III | — | 1 | 30 | III | 6.0 | 134 | A,B.D,Di,S |
| 13. | 70 | m | 70/159 | — | — | III | 1 | — | 10 | I | 6.7 | 168 | A,B,D,N |
| 14. | 67 | m | 84/176 | 1 | 1 | III | 1 | — | 10 | IV | 6.8 | 186 | A,C,D,Di |
| 59±14 | 11/3 | 5(36) | 7(50) | 7(50) | 7(50) | 20±9 | 6.4±1 | 148±35 | |||||
| No. . | Age . | Sex . | Weight (kg)/Height (cm) . | DM . | RR . | NYHA . | ICM . | DCM . | LVEF . | MR . | LVd . | QRSd . | Medication . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 76 | m | 97/179 | 1 | 1 | III | 1 | — | 30 | I | 6.4 | 166 | A,B,D,Di |
| 2. | 39 | m | 110/176 | — | 1 | II | — | 1 | 30 | II | 6.3 | 98 | A,D,Di |
| 3. | 65 | w | 66/156 | 1 | 1 | III | — | 1 | 20 | III | 7.0 | 182 | A,D,Di |
| 4. | 76 | m | 66/173 | 1 | — | III | — | 1 | 20 | — | 5.8 | 110 | A,D,Di |
| 5. | 62 | m | 63/174 | — | — | III | — | 1 | 15 | III | 6.5 | 130 | A,D,Di,S |
| 6. | 57 | w | 79/168 | — | — | II | — | 1 | 40 | — | 4.7 | 100 | B |
| 7. | 64 | m | 70/173 | — | 1 | III | 1 | — | 20 | — | NA | 127 | A,B,D,Di |
| 8. | 67 | m | 89/169 | 1 | — | III | 1 | — | 15 | I | 6.3 | 135 | A,B,D,Di |
| 9. | 60 | w | 72/164 | — | 1 | III | — | 1 | 15 | III | 9.3 | 182 | A,B,D,Di |
| 10. | 63 | m | 74/179 | — | — | III | 1 | — | 10 | III | 7.3 | 148 | A,B,D,Di,S |
| 11. | 75 | m | 65/174 | — | — | III | 1 | — | 10 | II | 7.2 | 213 | A,B,D,Di,S |
| 12. | 60 | m | 105/183 | — | 1 | III | — | 1 | 30 | III | 6.0 | 134 | A,B.D,Di,S |
| 13. | 70 | m | 70/159 | — | — | III | 1 | — | 10 | I | 6.7 | 168 | A,B,D,N |
| 14. | 67 | m | 84/176 | 1 | 1 | III | 1 | — | 10 | IV | 6.8 | 186 | A,C,D,Di |
| 59±14 | 11/3 | 5(36) | 7(50) | 7(50) | 7(50) | 20±9 | 6.4±1 | 148±35 | |||||
m, male; w, women; DM, diabetes mellitus; RR, history of arterial hypertension; MR, degree of mitral regurgitation; LVd, LV diameter in diastole; QRSd, duration of the QRS complex on the surface ECG (ms); medication: A, ACE-inhibitor/angiotensin II receptor blocker; B, beta-blocker; C, amiodarone; D, diuretics; Di, digitalis; N, nitrates; S, spironolactone. NA, data not available. Numbers in parenthesis (%).
Cardiac catheterization
Before performing a right and left cardiac catheterization as well as coronary angiography in a standard manner, in all patients, the coronary sinus (CS) was cannulated with an 8F CS-MP Easytrak guiding catheter (Guidant Corp., St Paul, MN, USA) inserted through the right jugular vein. While the guiding catheter was in the middle of the CS, 5 mL blood sample was withdrawn simultaneously with blood samples taken from the aortic root (via a 6F standard pigtail catheter) and from the superior vena cava (SVC) through the 8F sheath.
CD34+/CD117+ analysis
Analysis of PC was performed according to the recent publications.5–7 In brief, each of 50 µL EDTA–blood samples was incubated with a nucleic acid dye, CD45-PerCP and CD34-PE (ProCOUNT CD34 reagent, BD-Immunocytometry Systems, San Jose, CA, USA) in bead-containing TRUCOUNT tubes (BD). The control reagent (nucleic acid dye, γ1-PE and CD45-PerCP) was used to assess the amount of non-specific antibody binding. After red cell lysis with diluted fluorescence-activated cell sorter (FACS) lysing solution (BD), the samples were measured with an FACS Calibur flow cytometer (BD). The absolute number of CD34+ cells in the sample was determined by dividing the number of CD34+ cellular events by the number of fluorescent bead events and then multiplying with the bead concentration. The ProCOUNT software system (BD) was used to acquire and analyse data.
For CD34+/CD117+ quantification, 50 µL peripheral blood samples were labelled with FITC-CD34 and PE-CD117 (BD). After red cell lysis, flow cytometric analyses were performed on an FACS Calibur analytical flow cytometer (BD). List mode data were acquired by employing the CellQuest software (BD). Because of the low absolute number of CD34+/CD117+ cells, relative amounts of CD34+/CD117+ cells with regard to total leucocyte count were calculated. The coefficient of variation for CD34+PC measurements was <10%. All analyses regarding PC counting were performed in the Department of Haematology, University Hospital Magdeburg (certified laboratory by the German Accreditation Council and by the German Accreditation Body Chemistry), according to the European DIN EN ISO 15189.
Spiroergometry
Symptom-limited (fatigue or dyspnoea) metabolic stress testing was performed in eight patients with moderate-to-severe CHF, as previously described,8 on an upright bicycle ergometer, with a 10 W/min step protocol, starting with 2 min of unloaded cycling and recorded on a cardiopulmonary system (Oxycon Alpha, Jaeger, Wurzburg, Germany). Briefly, ventilation threshold was measured by the V-slope method if possible, otherwise, by inspection of ventilation equivalents.9 Peak VO2 was defined as the highest VO2 during any stage that could be sustained for 1 min; in most instances, this corresponded to the highest workload that was sustained for 1 min (i.e. peak workload). Peak VO2 is reported after correction of body weight (mL/min/kg) and as a percentage of predicted normal values accounting for age, weight, and sex.
Statistical analysis
All values are expressed as mean±standard deviation. The paired and unpaired Student's t-test was used to assess differences in cell levels. Pearson's correlation coefficient (r2) was used to demonstrate the correlation between variables. A value of P<0.05 was considered statistically significant.
Results
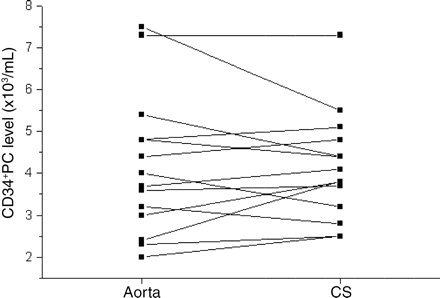
Baseline characteristics of the patients with CHF are shown in Table 1. In these patients, CD34+ and CD34+/CD117+PC levels were comparable at the different sample sites (Table 2). Importantly, no significant reduction of CD34+ and CD34+/CD117+PC could be detected in the CS compared with the aorta (Figure 1, Table 2). The relative amounts of CD34+/CD117+PC were very low in all patients. There was no difference between patients with ischaemic cardiomyopathy (ICM) and non-ICM (CD34+ ICM: 3.6±1.2×103 mL−1 vs. dilated cardiomyopathy (DCM): 4.4±0.9×103 mL−1 and CD34+/CD117+ ICM: 0.37±0.27% vs. DCM: 0.28±0.23%; P=n.s.). No relationship was found between medical therapy and CD34+ and CD34+/CD117+PC levels.
CD34+PC levels in the aorta and CS (differences were not significant; n=14).
Cardiac PC level at different sites
| . | SVC . | CS . | Aorta . | ΔAorta–CS . |
|---|---|---|---|---|
| CD 34+ | 4.61±1.83 | 4.02±1.04 | 4.19±1.72 | 0.11±0.98 |
| CD34/CD117+ | 0.36±0.35 | 0.31±0.25 | 0.40±0.32 | 0.08±0,31 |
| Leucocytes | 7.74±2.25 | 7.04±2.04 | 7.26±1.88 | 0.14±0.58 |
| . | SVC . | CS . | Aorta . | ΔAorta–CS . |
|---|---|---|---|---|
| CD 34+ | 4.61±1.83 | 4.02±1.04 | 4.19±1.72 | 0.11±0.98 |
| CD34/CD117+ | 0.36±0.35 | 0.31±0.25 | 0.40±0.32 | 0.08±0,31 |
| Leucocytes | 7.74±2.25 | 7.04±2.04 | 7.26±1.88 | 0.14±0.58 |
CD34+ values (×103 mL−1). CD34+/117+ (%) of leucocytes. Leucocytes ×103 mL−1 from whole blood. All differences are not significant (n=14).
Cardiac PC level at different sites
| . | SVC . | CS . | Aorta . | ΔAorta–CS . |
|---|---|---|---|---|
| CD 34+ | 4.61±1.83 | 4.02±1.04 | 4.19±1.72 | 0.11±0.98 |
| CD34/CD117+ | 0.36±0.35 | 0.31±0.25 | 0.40±0.32 | 0.08±0,31 |
| Leucocytes | 7.74±2.25 | 7.04±2.04 | 7.26±1.88 | 0.14±0.58 |
| . | SVC . | CS . | Aorta . | ΔAorta–CS . |
|---|---|---|---|---|
| CD 34+ | 4.61±1.83 | 4.02±1.04 | 4.19±1.72 | 0.11±0.98 |
| CD34/CD117+ | 0.36±0.35 | 0.31±0.25 | 0.40±0.32 | 0.08±0,31 |
| Leucocytes | 7.74±2.25 | 7.04±2.04 | 7.26±1.88 | 0.14±0.58 |
CD34+ values (×103 mL−1). CD34+/117+ (%) of leucocytes. Leucocytes ×103 mL−1 from whole blood. All differences are not significant (n=14).
Levels of CD34+ and CD34+/CD117+PC did not correlate significantly with clinical parameters such as age (CD34+: r2=0.14; CD34+/CD117+: r2=0.02; P=n.s.), height (CD34+: r2=0.03; CD34+/CD117+: r2=0.02; P=n.s.), NYHA class (CD34+: r2=0.22; CD34+/CD117+: r2=0.05; P=n.s.), LVEF (CD34+: r2=0.01; CD34+/CD117+: r2=0.12; P=n.s.), LV diameter (CD34+: r2=0.05; CD34+/CD117+: r2=0.1; P=n.s.), mean heart rate (CD34+: r2=0.08; CD34+/CD117+: r2=0.002; P=n.s.), systolic blood pressure (CD34+: r2=0.03; CD34+/CD117+: r2=0.01; P=n.s.), maximal O2-uptake on spiroergometry (CD34+: r2=0.08; CD34+/CD117+: r2=0.28; P=n.s.), and QRS duration (CD34+: r2=0.1; CD34+/CD117+: r2=0.08; P=n.s.). Mean maximal O2-uptake during exercise was 13±2 mL/min/kg in this group of patients (n=8).
Venous CD34+ and CD34+/CD117+PC levels were not different in patients with CHF compared with age-matched controls (CD34+level: 4.61±1.83×103 mL−1 vs. control: 5.25±1.67×103 mL−1; CD34+/CD117+: 0.36±0.35% vs. control: 0.28±0.21%; P=n.s.).
Discussion
To the best of our knowledge, this is the first study analysing the potential cardiac uptake of CD34+ and CD34+/CD117+PC in patients with moderate-to-severe CHF. The main finding of our study is that CD34+ and CD34+/CD117+PC levels are not significantly reduced in the CS compared with the aorta, which demonstrates that there is no measurable cardiac uptake of CD34+ and CD34+/CD117+PC. In addition, systemic levels of CD34+ and CD34+/CD117+PC are not significantly altered in patients with chronic stable CHF compared with age-matched controls. Importantly, venous and arterial levels of CD34+ and CD34+/CD117+PC are not significantly different, which shows that venous blood samples are a robust measure for systemic PC levels.
Recent reports suggest that blood levels of CD34+PC increase in response to cardiac injury.1,2 Chemokines such as the stromal cell-derived factor-1α and the vascular endothelium growth factor modulate the homing of PC in the bone marrow, and overexpression of these factors results in mobilization of PC in vivo.1,10 Especially during myocardial ischaemia, CD34+/CD117+cells are increased.4 In this setting, the PC population seems to be involved in neoangiogenesis.1 Kocher et al.1 have demonstrated that venous injections of CD34+/CD117+PC result in an increased LVEF and increased amounts of myocardial capillaries in a myocardial infarction model. Besides ventricular ischaemia, vascular trauma, application of direct current shocks, etc. also influence systemic PC levels.6,11,12 Furthermore, CD34+PC levels are increased in the blood of patients with persistent atrial fibrillation.6 The importance of atrial abnormalities on systemic PC was introduced by Quaini et al.2 They have demonstrated a high proportion of PC in atrial tissue compared with ventricular myocardium.2 However, the source of the demonstrated primitive cells remained unclear and early indicators of bone marrow cell differentiation were not found. Thus, myocardial uptake of CD34+PC from the systemic circulation may occur to only a very limited extent in the ventricles. This may help to explain the negative results of the present study, because the potential uptake of PC at the atrial level might be missed in blood samples taken from the CS. Furthermore, recent studies have assessed cardiac PC uptake using 111indium oxide-labelled PC in a rodent infarct model.13,14 Importantly, the amount of radioactivity in the whole heart was only 1% of the injected activity, which correlated to 1×104PC. In contrast to the low cardiac uptake, scintigraphic images showed a significant lung uptake (17%) and a very high uptake by the liver and spleen (57%).13 This study suggests that left ventricular PC uptake is <1% even after application of high amounts of PC.
A recent study by Hofmann et al.15 showed that intravenous injections of unselected bone marrow cells (5% of these cells were radiolabelled with 18F-FDG) does not cause increased PC activity in the infarcted human heart. Even after intracoronary injection of unselected bone marrow cells, 1.3–2.6% of radiolabelled cells became detectable in the infarcted myocardium after 60 min. Thus, these results also suggest that the intrinsic capacity for cardiac PC uptake from the blood is limited. In a clinical setting, FACS analysis appears as the most relevant technique for CD34+PC counting. Nevertheless and in contrast to more sensitive 18F-FDG-labelling techniques, relative differences in PC levels ≤1% are not detectable using FACS analysis, although an absolute reduction of 1×104PC would reliably be detected.
In the present study, absolute amounts of CD34+ and CD34+/CD117+PC were relatively low (∼4×103 mL−1) in patients with CHF with no difference from patients with normal LV function. Therefore, chronic moderate-to-severe CHF appears to be an insufficient stimulus to increase systemic PC levels and to induce a measurable cardiac PC uptake. Interestingly, clinical variables such as NYHA class, LVEF, LV diameter, etc. did not predict PC levels in our cohort of patients. This is further supported by the recent finding that amounts of CD34+ cells are significantly reduced in patients with decompensated heart failure (NYHA class IV).16 Experimental and clinical studies have revealed so far that large amounts of PC have to be injected/infused to induce modest improvements in ventricular performance.1,13,15,17 Thus, spontaneous uptake of very small amounts of circulating PC by the left ventricle, even if present, might have no clinical benefit. Our study demonstrates by using FACS analysis the absence of a relevant cardiac uptake of circulating PC in patients with stable CHF. Thus, the combination of reduced amounts of circulating PC in severe heart failure and no or minimal PC uptake by the left ventricle supports the clinical knowledge that left ventricular failure is a progressive disease with no spontaneous recovery. Nevertheless, PC may reside in the adult heart and co-localize with small capillary vessels, which questions the relevance of circulating PC.
The injection of PC, however, into the myocardium or into the coronary arteries has been shown to improve cardiac performance.17 Perin et al.17 have shown in a prospective non-randomized open-label study including 21 patients with chronic ischaemic heart failure that myocardial injections of bone marrow mononuclear cells improve cardiac function and reduce detectable ischaemia. This underlines that PCs have beneficial effects in patients with heart failure and especially in patients with ischaemic heart disease. However, to induce such beneficial effects in vivo, higher concentrations of PC have to be generated ex vivo.
Limitations
Because of the extensive instrumentation used in our study, we included a limited number of patients only. However, even a large cohort of patients (more than 100 patients) may be not enough to detect slight differences (≤1%) in PC levels by FACS analysis due to the intrinsic variability of the methodology. More sensitive techniques for PC labelling and detection may lead to different results.
The method used to determine ‘cardiac uptake’ in the present study (ΔPC aorta–CS) has technical limitations. Nevertheless, the approach has been used in multiple studies regarding cardiac uptake or secretion of neurohormones, etc. in vivo.18,19 The results obtained from aspirated blood from the aorta and the CS may vary with time and coronary blood flow. Differences in coronary blood flow between groups could have a substantial impact on PC uptake measured at a particular time. In the present study, a ‘steady state’ in PC levels and blood flow measurements were not performed.
We cannot comment on potential differences in PC differentiation in patients with and without heart failure. The pure quantification of PC may miss such differences.
We have not determined the uptake of CD34+/CD117+PC in patients without cardiac disease. Thus, no comment can be made about the physiological situation with regard to CD34+/CD117+PC uptake. However, such a study would have ethical limitations and was therefore not performed.
Conclusions
Moderate-to-severe CHF is not associated with elevated PC levels in the systemic circulation. In addition, a measurable cardiac uptake of CD34+ and CD34+/CD117+PC cannot be demonstrated by FACS analysis in this setting.
Acknowledgements
This work was supported by a grant from ‘Bundesministerium für Bildung und Forschung, Germany’ (BMBF 01 ZZ 0107 and 01 GI 0204).
References
- angiogenesis
- aorta
- myocardium
- heart failure, left-sided
- left ventricular ejection fraction
- catheterization of left heart
- endothelial progenitor cells
- congestive heart failure
- ischemic cardiomyopathy
- superior vena cava
- stem cells
- exercise
- cd34 antigens
- fluorescence
- hematopoietic system
- portacaval shunt, surgical
- proto-oncogene protein c-kit
- wound healing
- heart
- cardiac resynchronization therapy
- whole blood
- systemic circulatory system
- coronary sinus
- qrs complex duration
- precordial catch syndrome
- diameter
- photon correlation spectroscopy