-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Wolf, Tobias Heidelmann, Irene Marten, ABA Regulation of K + -Permeable Channels in Maize Subsidiary Cells , Plant and Cell Physiology, Volume 47, Issue 10, October 2006, Pages 1372–1380, https://doi.org/10.1093/pcp/pcl007
Close - Share Icon Share
Abstract
An antiparallel-directed potassium transport between subsidiary cells and guard cells which form the graminean stomatal complex has been proposed to drive stomatal movements in maize. To gain insights into the coordinated shuttling of K + ions between these cell types during stomatal closure, the effect of ABA on the time-dependent K + uptake and K + release channels as well as on the instantaneously activating non-selective cation channels (MgC) was examined in subsidiary cells. Patch-clamp studies revealed that ABA did not affect the MgC channels but differentially regulated the time-dependent K + channels. ABA caused a pronounced rise in time-dependent outward-rectifying K + currents (K out ) at alkaline pH and decreased inward-rectifying K + currents (K in ) in a Ca 2+ -dependent manner. Our results show that the ABA-induced changes in time-dependent K in and K out currents from subsidiary cells are very similar to those previously described for guard cells. Thus, the direction of K + transport in subsidiary cells and guard cells during ABA-induced closure does not seem to be grounded solely on the cell type-specific ABA regulation of K + channels.
Introduction
In grasses, the stomatal complex is formed by two guard cells and two flanking subsidiary cells (Raschke and Fellows 1971 , Willmer and Pallas 1973 , Willmer and Pallas 1974 , Wille and Lucas 1984 ). According to previous studies, K + ions are shuttling between these cell types during stomatal movement (Raschke and Fellows 1971 , Willmer and Pallas 1974 , Penny and Bowling 1974 , Penny et al. 1976 ). This short-distance K + shuttle transport seems to account for the efficiency of stomatal movement in grasses compared with dicots and other monocots lacking subsidiary cells. Besides light and CO 2 , the phytohormone ABA represents a physiological trigger for stomatal closure (for reviews, see Blatt and Grabov 1997 , Assmann and Shimazaki 1999 , Schroeder et al. 2001 , Roelfsema and Hedrich 2005 ). Via an as yet unknown signaling chain, including Ca 2+ -dependent and -independent pathways, ABA stimulates anion channels and outward-rectifying K + channels in the physically isolated guard cells (Wille and Lucas 1984 , Allan et al. 1994 , Anderson et al. 1994 , Gilroy and Jones 1994 , Schwartz et al. 1994 , Knox et al. 1995 , Assmann and Shimazaki 1999 , Jeannette et al. 1999 , Schroeder et al. 2001 , Levchenko et al. 2005 ). Thereby, anions and K + ions are released to drive cell volume shrinkage for stomatal closure. In addition to membrane depolarization, ABA induces a cytosolic alkalinization in guard cells known to assist the net loss of K + from depolarized guard cells (Blatt 1990 , Blatt and Armstrong 1993 , Miedema and Assmann 1996 ). Once osmolytes are released from the guard cells, they do not seem to accumulate in the apoplast and rather enter the surrounding epidermal and mesophyll cells (Raschke and Fellows 1971 , Penny and Bowling 1974 , Willmer and Pallas 1974 , Penny et al. 1976 , Itai and Meidner 1978 , Felle et al. 2000 ). Thus, subsidiary cells may serve as a sink for osmolytes during stomatal closure.
Despite the physiological importance of subsidiary cells for stomatal movement (Pallaghy 1971 , Raschke and Fellows 1971 , Squire and Mansfield 1972 , Penny and Bowling 1974 , Squire and Mansfield 1974 , Willmer and Pallas 1974 , Penny et al. 1976 ), ABA action on this cell type remained as yet unexplored. Like guard cells (Fairley-Grenot and Assmann 1992 ), subsidiary cells from maize are equipped with hyperpolarization- and depolarization-activated K + -selective channels (K in and K out channels, respectively) (Majore et al. 2002 ). Interestingly, genes encoding K in channels are differentially expressed within the maize stomatal complex. While KZM1 and KZM2 transcripts were found in guard cells, the ZMK1 gene was expressed in subsidiary cells only (Phillipar et al. 1999, Phillipar et al. 2003, Büchsenschütz et al. 2005 ). Expression of the K out channel gene ZORK , however, was verified in both cell types (Büchsenschütz et al. 2005 ). Apart from these K + -selective channels, recently a non-selective cation channel type (MgC) has been electrophysiologically identified in maize subsidiary cells and guard cells (Wolf et al. 2005 ). MgC activates very rapidly upon depolarization, giving rise to instantaneous outward-rectifying cation currents. In contrast to K in and K out channels, the MgC channel activity does not depend on cytosolic nucleotides and is down-regulated by Mg 2+ ions. With respect to their voltage dependence and selectivity, the K in channels are suited to mediate K + uptake, while K out and MgC channels could release K + ions during stomatal movement. In order to gain first insights into the ABA signal transduction events in subsidiary cells, here we studied the ABA-dependent regulation of the K + -permeable channels in maize subsidiary cells. In this context, we investigated the role of intracellular Ca 2+ ions and protons as possible transmitters of the ABA signal in these cells.
Results
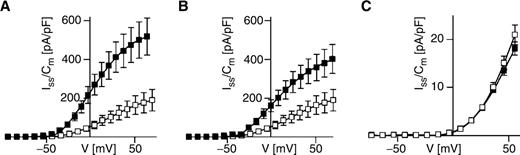
To examine the regulation of the K + transport from maize subsidiary cells during ABA-induced stomatal closure, we monitored plasma membrane K + currents from subsidiary cell protoplasts in the presence and absence of ABA with the patch-clamp technique. Instantaneously activating outward-rectifying K + currents (K inst ) as well as time-dependent outward- and inward-rectifying K + currents (K out and K in , respectively) were evoked in protoplasts at depolarizing and hyperpolarizing voltages ( Fig. 1 A–C). When ABA was added to both sides of the plasma membrane, K inst currents remained unaltered ( Fig. 1 A, D), and K out currents were not or only slightly increased ( Fig. 1 B, E). In contrast, K in currents were reduced in the presence of ABA ( Fig. 1 C, F).
Differential effect of ABA on time-dependent and instantaneous K + currents from subsidiary cells at high intracellular free Ca 2+ concentrations. Representative outward-rectifying K + current traces (A, B) evoked upon voltage pulses in the range of −54 to +96 mV in 30 mV steps, and time-dependent inward-rectifying K + current traces (C) elicited upon voltage steps to −184, −164, −144 and −124 mV are shown. In (A) and (D), the instantaneous outward-rectifying K + currents, and in (B) and (E) the time-dependent K out currents are presented. (D–F) current densities determined in the steady state (I ss /C m ) are given as a function of membrane voltage. The currents in A–F were recorded in the absence (control, open squares) or presence of 50 μM ABA (filled squares) on both membrane sides by using standard bath and standard pipet solutions. Note that EGTA was not present in the standard pipet medium adjusted to pH 7.4. (D) ABA, n = 6; control, n = 7. (E) ABA, n = 6; control, n = 5–6. (F) ABA, n = 8; control, n = 7.
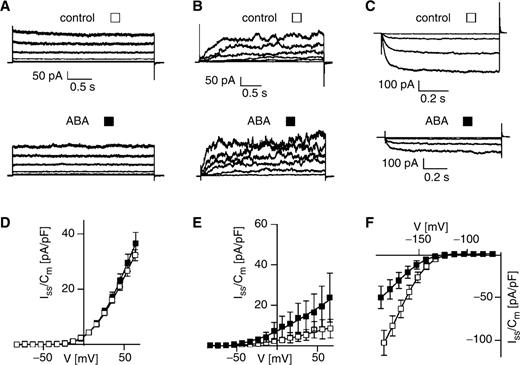
In other plant cell systems, control of ion channel activities by ABA was reported to require Ca 2+ ions (Lemtiri-Chlieh and MacRobbie 1994 , Assmann and Shimazaki 1999 , Schroeder et al. 2001 , Gilliham and Tester 2005 , Levchenko et al. 2005 ). In line with the previous experiments performed at high intracellular Ca 2+ concentrations ( Fig. 1 A, B), an ABA effect on the K inst and K out currents still was not observed at an intracellular free Ca 2+ concentration of 110 nM ( Table 1 ). Increasing the Ca 2+ buffering capacity of the pipet solution did not result in different K inst current amplitudes in the absence or presence of ABA ( Fig. 2 A). This finding corresponds well to the insensitivity of the K inst currents towards changes in the intracellular free Ca 2+ concentration ( Fig. 3 A). Likewise, no or only a minor ABA effect on the K out currents by ABA was recorded at low and high intracellular Ca 2+ concentrations ( Figs. 1 E, 2 B, Table 1 ). These results suggest that a potential ABA regulation of the slowly activating K out channels and the fast activating MgC channels in subsidiary cells does not depend on intracellular Ca 2+ ions. In contrast to the K + release channels, however, the ABA-dependent repression of K in currents as observed in Fig. 1 C was abolished at a low intracellular Ca 2+ concentration ( Fig. 2 C). Since ABA was able to reduce K in currents at an intracellular free Ca 2+ concentration of 110 nM ( Table 1 ), Ca 2+ concentrations around the resting Ca 2+ level of the cell seem to be sufficient to mediate the ABA effect.
ABA effect on time-dependent and instantaneous K + currents from subsidiary cells at low intracellular free Ca 2+ concentrations. (A) Instantaneous K + currents were measured in the absence (open triangles) or presence of 50 μM ABA (filled triangles) in standard pipet medium. The bath solution in (C) was composed of 10 mM K-gluconate, 10 mM Ca-gluconate 2 and 10 mM MES (pH 5.6/Tris). (B, C) Time-dependent K + currents were recorded in the absence (open squares) or presence of 50 μM ABA (filled squares) on both membrane sides by using standard bath and pipet solutions. Note that the standard pipet medium was used in (A–C) and thus contained 10 mM EGTA and was adjusted to pH 7.4. (A) ABA, n = 6; control, n = 4. (B) ABA, n = 6–7; control, n = 7. (C) ABA, n = 7–8; control, n = 7.
Intracellular Ca 2+ and pH sensitivity of the MgC channels from maize subsidiary cells. (A) Instantaneous K + currents were recorded at an intracellular Ca 2+ concentration of 0 nM (circles, n = 6), 110 nM (squares, n = 12) and 410 nM (triangles, n = 5). The standard bath medium was applied, and the standard pipet medium contained 0 mM, 6 mM or 8.5 mM Ca-gluconate 2 to adjust the free Ca 2+ concentration to 0, 110 or 410 nM, respectively. The determined current densities were shown as a function of membrane voltage. (B) Instantaneous K + current densities at +36 mV were determined at intracellular pH values as indicated. The number of experiments is given in parentheses. The standard pipet medium was adjusted to pH 7.4, 6.7 or 8.2, and the bath medium contained 10 mM K-gluconate, 10 mM Ca-gluconate 2 and 10 mM MES pH 5.6/Tris. In (A) and (B), isopropanol was not added to the pipet solution.
Differential ABA effect on K + currents (K in , K out and K inst ) at an intracellular free Ca 2+ concentration of 110 nM
| . | K in (pA/pF) at −198 mV . | K out (pA/pF) at +73 mV . | K inst (pA/pF) at +74 mV . |
|---|---|---|---|
| Control | −96.0 ± 10.0 (9) | 74.6 ± 26.1 (9) | 31.0 ± 1.5(6) |
| 50 μM ABA | −61.6 ± 11.1 (8) | 77.4 ± 31.6 (8) | 33.5 ± 2.6 (5) |
| . | K in (pA/pF) at −198 mV . | K out (pA/pF) at +73 mV . | K inst (pA/pF) at +74 mV . |
|---|---|---|---|
| Control | −96.0 ± 10.0 (9) | 74.6 ± 26.1 (9) | 31.0 ± 1.5(6) |
| 50 μM ABA | −61.6 ± 11.1 (8) | 77.4 ± 31.6 (8) | 33.5 ± 2.6 (5) |
To adjust the intracellular free Ca 2+ concentration to 110 nM, the standard pipet solution additionally contained 6 mM Ca-gluconate 2 . For recording of K in and K out currents, 5 mM ATP was added, while K inst currents were evoked in the presence of 5 mM GTP. The number of experiments is given in parentheses.
Differential ABA effect on K + currents (K in , K out and K inst ) at an intracellular free Ca 2+ concentration of 110 nM
| . | K in (pA/pF) at −198 mV . | K out (pA/pF) at +73 mV . | K inst (pA/pF) at +74 mV . |
|---|---|---|---|
| Control | −96.0 ± 10.0 (9) | 74.6 ± 26.1 (9) | 31.0 ± 1.5(6) |
| 50 μM ABA | −61.6 ± 11.1 (8) | 77.4 ± 31.6 (8) | 33.5 ± 2.6 (5) |
| . | K in (pA/pF) at −198 mV . | K out (pA/pF) at +73 mV . | K inst (pA/pF) at +74 mV . |
|---|---|---|---|
| Control | −96.0 ± 10.0 (9) | 74.6 ± 26.1 (9) | 31.0 ± 1.5(6) |
| 50 μM ABA | −61.6 ± 11.1 (8) | 77.4 ± 31.6 (8) | 33.5 ± 2.6 (5) |
To adjust the intracellular free Ca 2+ concentration to 110 nM, the standard pipet solution additionally contained 6 mM Ca-gluconate 2 . For recording of K in and K out currents, 5 mM ATP was added, while K inst currents were evoked in the presence of 5 mM GTP. The number of experiments is given in parentheses.
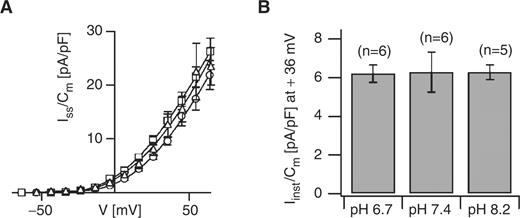
Since ABA is known to alter the intracellular pH in guard cells (Irving et al. 1992 , Blatt and Armstrong, 1993 ), we tested whether protons besides calcium ions might play a role in transmitting the ABA signal on the K + -permeable channels in subsidiary cells. When examining the pH sensitivity of these channels, the K inst current amplitudes were found to be similar at an intracellular pH of 8.2, 7.4 and 6.7 ( Fig. 3 B), indicating that the MgC channels from subsidiary cells are insensitive towards the intracellular pH. In contrast, the K in currents increased ( Fig. 4 A, B) when the pH value was lowered from 8.2 to 7.4. This effect was accompanied by an 18 mV shift in the half-maximal activation voltage (V 1/2 ) ( Fig. 4 C, Table 2 ), showing that the voltage dependence of the corresponding K in channels is altered by intracellular protons. A pH change from 7.4 to 6.7, however, did not increase the K in current amplitude further ( Fig. 4 B), and the half-maximal activation voltage only slightly shifted by 6 mV towards more positive voltages ( Fig. 4 C, Table 2 ). Thus K in channels in subsidiary cells may sense an alkalinization far better than an acidification of the subsidiary cell cytoplasm.
Intracellular pH sensitivity of the K + -selective channels from maize subsidiary cells. Macroscopic K in (A–C) and K out (D–F) currents were monitored and analyzed for different intracellular pH values. (A) Representative K in current traces elicited upon voltage steps to −184, −164, −144 and −124 mV are shown. (D) Representative K out currents evoked upon voltage pulses to −24, +6, +36 and +66 mV are given. Dashed lines give the zero current level. (B, E) The respective steady-state current densities were plotted against the membrane voltage. (C, F) The intracellular pH effect on the voltage-dependent relative open probability is reflected by normalized macroscopic conductance–voltage curves [G/G max (V)]. The solid curves give the mean of the individual fits describing the data values according to the Boltzmann distribution. (B) pH 8.2, filled squares, n = 11; pH 7.4, filled circles, n = 6; pH 6.7, filled triangles, n = 7. (C) pH 8.2, open squares, n = 8–11; pH 7.4, open circles, n = 6; pH 6.7: open triangles, n = 7. (E) pH 8.2, filled squares, n = 5; pH 7.4, filled circles, n = 4; pH 6.7, filled triangles, n = 6. (F) pH 8.2, open squares, n = 5; pH 7.4: open circles, n = 4. In A–F, standard pipet solution was used without isopropanol, contained only ATP (no GTP) and was adjusted to the pH values as indicated. The bath solution was composed of 10 mM K-gluconate, 1 mM Ca-gluconate 2 and 10 mM MES pH 5.6/Tris.
Effect of intracellular pH on gating properties of K in and K out channels
| . | K in channels . | K out channels . | |||
|---|---|---|---|---|---|
| . | . | . | |||
| . | pH 8.2 . | pH 7.4 . | pH 6.7 . | pH 8.2 . | pH 7.4 . |
| V 1/2 (mV) | −171.8 ± 2.3 (8–11) | −153.6 ± 2.3 (6) | −147.7 ± 3.0 (7) | 4.2 ± 3.1 (5) | −5.1 ± 4.1 (4) |
| |z| | 2.09 ± 0.12 (8–11) | 2.3 ± 0.09(6) | 2.07 ± 0.01(7) | 2.07 ± 0.09 (5) | 1.76 ± 0.14 (4) |
| . | K in channels . | K out channels . | |||
|---|---|---|---|---|---|
| . | . | . | |||
| . | pH 8.2 . | pH 7.4 . | pH 6.7 . | pH 8.2 . | pH 7.4 . |
| V 1/2 (mV) | −171.8 ± 2.3 (8–11) | −153.6 ± 2.3 (6) | −147.7 ± 3.0 (7) | 4.2 ± 3.1 (5) | −5.1 ± 4.1 (4) |
| |z| | 2.09 ± 0.12 (8–11) | 2.3 ± 0.09(6) | 2.07 ± 0.01(7) | 2.07 ± 0.09 (5) | 1.76 ± 0.14 (4) |
V 1/2 and |z| represent the half-maximal activation voltage and the apparent equivalent gating charges, respectively. The number of experiments is given in parentheses.
Effect of intracellular pH on gating properties of K in and K out channels
| . | K in channels . | K out channels . | |||
|---|---|---|---|---|---|
| . | . | . | |||
| . | pH 8.2 . | pH 7.4 . | pH 6.7 . | pH 8.2 . | pH 7.4 . |
| V 1/2 (mV) | −171.8 ± 2.3 (8–11) | −153.6 ± 2.3 (6) | −147.7 ± 3.0 (7) | 4.2 ± 3.1 (5) | −5.1 ± 4.1 (4) |
| |z| | 2.09 ± 0.12 (8–11) | 2.3 ± 0.09(6) | 2.07 ± 0.01(7) | 2.07 ± 0.09 (5) | 1.76 ± 0.14 (4) |
| . | K in channels . | K out channels . | |||
|---|---|---|---|---|---|
| . | . | . | |||
| . | pH 8.2 . | pH 7.4 . | pH 6.7 . | pH 8.2 . | pH 7.4 . |
| V 1/2 (mV) | −171.8 ± 2.3 (8–11) | −153.6 ± 2.3 (6) | −147.7 ± 3.0 (7) | 4.2 ± 3.1 (5) | −5.1 ± 4.1 (4) |
| |z| | 2.09 ± 0.12 (8–11) | 2.3 ± 0.09(6) | 2.07 ± 0.01(7) | 2.07 ± 0.09 (5) | 1.76 ± 0.14 (4) |
V 1/2 and |z| represent the half-maximal activation voltage and the apparent equivalent gating charges, respectively. The number of experiments is given in parentheses.
With respect to the K in currents, the slowly activating K out currents were characterized by inverted pH sensitivity. K out current amplitudes were higher at an intracellular pH of 8.2 compared with pH 7.4 or 6.7 ( Fig. 4 D, E). A positive-going shift of the half-maximal activation voltage by 9 mV upon alkalinization from 7.4 to pH 8.2 was observed ( Fig. 4 F, Table 2 ). Since this shift did not explain the 3-fold increase in the K out current amplitude ( Fig. 4 E), we hypothesized that intracellular protons may additionally alter the single channel conductance and/or the number of channels available for activation. To test these possibilities, single channel fluctuations were recorded from subsidiary cell protoplasts in the outside-out configuration. Under experimental conditions where only macroscopic K out but no K inst currents were monitored (Wolf et al. 2005 ), single K out channel openings were evoked upon depolarization and could be reversibly blocked by extracellular application of the potassium channel blocker TEA + ( Fig. 5 A). Channel activity increased with depolarization ( Fig. 5 B) and was characterized by a dynamic voltage threshold for channel activation positive of E K (data not shown). A 10-fold change in the K + gradient shifted the reversal potential by about 48 mV, indicating that these channels predominantly mediate transport of K + ions and to a minor extent of some other cations as well ( Fig. 5 C). When single channel fluctuations were monitored at different intracellular pH values (pH 6.7 and 8.2), channel activity decreased at lower pH ( Fig. 5 D). The single channel conductance γ, however, was not markedly affected by changes in the intracellular pH ( Fig. 5 E; pH 6.7, γ = 20.8 ± 1.7 pS; pH 7.4, γ = 21.9 ± 0.7 pS; pH 8.2, γ = 23.5 ± 1.3; n = 3–4). Just as for intracellular acidification, a rise in the intracellular free Ca 2+ concentration resulted in a decrease in the macroscopic outward-rectifying current amplitude (Majore et al. 2002 ) and in the number of channel openings (data not shown). Nevertheless, changes in the free Ca 2+ level did not alter the single channel conductance of the K + release channels (2 nM Ca 2+ , γ = 21.9 ± 0.6 pS; 350 nM Ca 2+ , γ = 22.9 ± 1.8 pS; 430 nM Ca 2+ , γ = 23.3 ± 1.8 pS; n = 3). Taken together, these data suggest that intracellular protons and Ca 2+ ions affect the voltage-dependent open probability as well as the number of K out channels available for voltage-dependent activation in subsidiary cells.
Recording of single K out channels from subsidiary cell protoplasts in the outside-out configuration. (A) Single channel fluctuations were observed at −14 mV under control conditions (upper trace), then disappeared with 30 mM TEA + in the bath solution (middle trace). After wash out of the inhibitor (lower trace), open–closed transitions occurred again. C indicates the current baseline where all channels are in the closed state. Upward deflections represent channel openings with i simultaneously opened channels (O1, O2, …, O i ). (B) The response of single channels to different voltage pulses is shown. (C) Reversal potentials (V rev ) of single channel outward currents were determined for different extracellular K + activities. The straight line is the linear regression to the experimental data with a slope of 48.2 mV, while the dashed line gives the Nernst potentials for K + . The data points represent the mean of five, three and seven independent experiments determined in the presence of 10, 30 or 100 mM K-gluconate in the extracellular solution, respectively. (D) Representative channel fluctuations evoked upon a voltage pulse to +16 mV were recorded at different intracellular pH values as indicated. (E) The single channel conductance was determined for different intracellular pH values from single channel current–voltage [i(V)] curves. The solid (pH 7.4) and dotted (pH 8.2) lines are the means of the individual linear regressions to the experimental data. Each symbol represents the data points obtained from one independent experiment (open symbols, pH 7.4, n = 3; filled symbols, pH 8.2, n = 4). The same pipet and bath solutions were used as in Fig. 4 . The bath medium in (C) was varied concerning the K-gluconate concentration as indicated, and in (D) and (E) contained 30 mM K-gluconate.
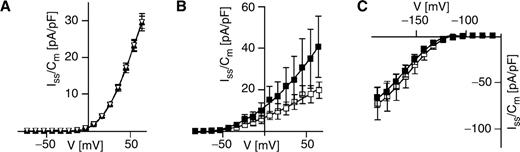
In Vicia faba guard cells, ABA was shown to stimulate the slowly activating pH-sensitive K out channels through an alkalinization of the cytoplasm (Blatt and Armstrong 1993 ). To mimic an ABA-induced alkalinization, we adjusted the pipet solution to pH 8.2 ( Fig. 6 ). Due to this alkaline pH stimulation, the K out current amplitudes were considerably higher at pH 8.2 ( Fig. 6 A, B) compared with pH 7.4 ( Fig. 2 B). A further pronounced rise in the K out currents at pH 8.2, however, could be achieved by extra- and intracellular ABA application during the patch-clamp experiments ( Fig. 6 A). When ABA was present only in the pipet solution adjusted to pH 8.2, an increase in K out currents, although attenuated, was still observed ( Fig. 6 B). This points to the presence of an intracellular site for perception of the ABA signal in subsidiary cells. Despite this fact but in line with the revealed pH insensitivity ( Fig. 3 B), intracellular ABA did not affect the K inst currents even at an intracellular alkaline pH ( Fig. 6 C).
The ABA effect on K out currents and K inst currents from subsidiary cells at an intracellular alkaline pH. Steady-state outward-rectifying currents were recorded in the absence (open squares) or presence of ABA (50 μM, filled squares) at an intracellular pH of 8.2 and plotted as a function of membrane voltage. ABA was applied to both the standard bath and standard pipet solution in (A) but only to the standard pipet solution in (B) and (C). The number of experiments were in (A) n = 6 (ABA), n = 7 (control), in (B) n = 8 (ABA), n = 7 (control), and in (C) n = 6–9 (ABA), n = 9–11 (control). In (A) and (B), the standard bath solution was used containing 3 mM K-gluconate, 1 mM Ca-gluconate 2 and 10 mM MES (pH 5.6/Tris), while in (C) the bath solution was composed of 10 mM K-gluconate, 10 mM Ca-gluconate 2 and 10 mM MES (pH 5.6/Tris). Note that the standard pipet solution in (A–C) was adjusted to pH 8.2.
Discussion
Stomatal closure is associated with K + efflux from guard cells probably mediated through depolarization-activated K + -permeable channels such as K out or MgC channels (Hirsch et al. 1998 , Wolf et al. 2005 ). The apoplastic K + content around closing guard cells, however, is below the value expected from K + loss during ABA-induced stomatal closure (Willmer and Fricker 1996 , Felle et al. 2000 ). Maize subsidiary cells flanking the guard cells were shown to accumulate K + ions in closed stomata (Raschke and Fellows 1971 ). Thus, K + ions released from maize guard cells by, for example, ZORK (Büchsenschütz et al. 2005 ) or MgC channels (Wolf et al. 2005 ) are supposed to be taken up by the neighboring subsidiary cells through hyperpolarization-activated K + -selective channels such as ZMK1 (Philippar et al. 1999 , Büchsenschütz et al. 2005 ). Since subsidiary cells and guard cells are equipped with a similar set of voltage-regulated K + -permeable channels such as K in , K out and MgC channels (Wolf et al. 2005 ), ABA is supposed to act differentially on these channels, thereby allowing K + release from guard cells on one hand and K + uptake into subsidiary cells on the other during stomatal closure. Such an inverse ABA regulation was described for K + release channels which were inhibited in Zea mays stelar cells but stimulated by ABA in V. faba guard cells (Blatt 1990 , Blatt and Armstrong 1993 , Lemtiri-Chlieh and MacRobbie 1994 , Roberts 1998 ). The K + -selective channels (K in , K out ) from subsidiary cells, however, responded towards ABA in a similar manner to that reported for guard cells ( Figs. 1 , 2 , 6 ). ABA caused a reduction in K in currents and an elevation in K out currents in maize subsidiary cells. ABA applied from the intracellular membrane side only was sufficient to increase K out currents ( Fig. 6 B), suggesting an intracellular perception site for ABA in maize subsidiary cells as proposed for guard cells (Allan et al. 1994 , Schwartz et al. 1994 , Levchenko et al. 2005 ). Extra- and intracellular ABA application reinforced the rise in K out currents ( Fig. 6 A, B), implying that like guard cells (Anderson et al. 1994 , Gilroy and Jones 1994 , Knox et al. 1995 , Jeannette et al. 1999 ), subsidiary cells may possess an additional external perception site for this phytohormone. In contrast, K inst currents from subsidiary cells were not affected by ABA irrespective of different intracellular Ca 2+ and proton levels ( Figs. 1 D, 2 A, 6 C). This suggests that other factors (e.g. membrane voltage) control the activity of MgC channels in subsidiary cells during ABA-dependent stomatal closure (see below).
Transmission of the ABA signal towards K in and K out channels in subsidiary cells is likely to be mediated via protons and/or Ca 2+ ions ( Figs. 2 , 6 ). A strong ABA-dependent stimulation of K out currents was observed only at alkaline pH ( Fig. 6 A, B). As described for guard cells (Lemtiri-Chlieh and MacRobbie 1994 ), the ABA-induced stimulation of K out channels in subsidiary cells occurred in a Ca 2+ -independent manner ( Figs. 1 E, 2 B, Table 1 ) but inhibition of K in channels occurred in a Ca 2+ -dependent manner ( Figs. 1 F, 2 C). Ca 2+ concentrations around the resting Ca 2+ level of the cell (110 nM) were already sufficient to transmit the ABA signal towards the K in channels ( Table 1 ). Furthermore, Figs. 4 and 5 and a former study (Majore et al. 2002 ) demonstrate that these K + -selective channels in subsidiary cells were sensitive towards intracellular Ca 2+ ions and protons in a similar manner to those from guard cells (Fairley-Grenot and Assmann 1992 , Blatt and Armstrong 1993 , Hoshi 1995 , Miedema and Assmann 1996 , Grabov and Blatt 1997 , Philippar et al. 2003 ).
K out currents declined with both increasing Ca 2+ and H + concentrations. The maintenance of the intracellular pH and Ca 2+ sensitivity on the single K out channel level ( Fig. 5 D) further suggests that as in V. faba guard cells (Miedema and Assmann 1996 ), protons and Ca 2+ ions interact directly with the K out channels or closely associated protein(s). K in currents were reduced at higher intracellular Ca 2+ concentrations (Majore et al. 2002 ) and alkaline pH ( Fig. 4 A–C). Compared with guard cell K in channels (Hoshi 1995 , Philippar et al. 2003 ), K in channels from subsidiary cells seem to exhibit a different pH sensitivity ( Fig. 4 B, C). While guard cell K in channels are activated upon cytosolic acidification, subsidiary cell K in channels showed a pronounced pH sensitivity in the alkaline pH range ( Fig. 4 C). This observation might be linked to different intracellular proton-sensing residues in the K in channel proteins of both cell types. In fact, guard cells express the K in channel genes KZM1 and KZM2 while subsidiary cells express ZMK1 (Büchsenschütz et al. 2005 ). The intracellular pH sensitivity of K + uptake channels has been assigned to a histidine residue in the linker region between the second and third transmembrane helices (S2–S3) of the channel protein (Tang et al. 2000 ). While KZM1 harbors a histidine in the S2–S3 linker (H149), KZM2 and ZMK1 possess a tyrosine at the H149-equivalent position. This substitution might account for the reduced intracellular proton sensitivity of the K in channels in subsidiary cells vs. the K in channels in guard cells in the acidic pH range.
At first view, our findings seem to contradict the hypothesis for an ABA-dependent mechanism regulating the shuttling of K + ions from guard cells to subsidiary cells. This is based on the fact that the nature of the ABA-dependent regulation of K + -permeable channels provides the basis to influence the current magnitude only but not the direction of K + flow in these cell types during stomatal movement. It is, however, tempting to speculate that ABA could trigger different signal transduction pathways in guard cells and subsidiary cells, leading to the proposed antiparallel K + transport within these cells during stomatal closure. With respect to the comparable pH dependence of K in and K out channels in guard cells and subsidiary cells, ABA hence would have to affect the cytosolic pH value of these cell types in vivo differentially. In guard cells, ABA causes an intracellular alkalinization that deactivates K in channels and stimulates K out channels, favoring K + efflux during stomatal closure (Irving et al. 1992 , Blatt and Armstrong 1993 , Grabov and Blatt 1997 ). In subsidiary cells during ABA action, K in channels should be maintained in an active gating mode to mediate K + uptake, while at the same time K out channels should be in an inactive channel state. Accordingly, one would claim that ABA should not alter the intracellular pH value or should even acidify the cytoplasm of subsidiary cells. The ABA-induced signaling cascade in guard cells can also involve a rise in the intracellular free Ca 2+ level (McAinsh et al. 1990 , Irving et al. 1992 , Allan et al. 1994 , Webb et al. 2001 ). In a previous study, we showed that K in currents decreased with increasing intracellular free Ca 2+ concentrations (Majore et al. 2002 ). Hence a potential ABA-triggered Ca 2+ signal in subsidiary cells would serve as an amplification mechanism reinforcing the inhibition of the K in currents already observed at resting Ca 2+ level ( Table 1 ). Thus, as discussed above for the pH regulation of the subsidiary cell K + channels, ABA should not induce a Ca 2+ signal in subsidiary cells. Under these conditions, ABA might suppress only a part of the K in channel population at the resting Ca 2+ level, still leaving a substantial number of K in channels in an active state to enable a sufficient K + influx into subsidiary cells during stomatal closure. Since different pH and Ca 2+ signals would still affect the magnitude of the K + flow only in both stomatal cell types, it would be desirable that ABA induces an opposite membrane potential polarization such as a hyperpolarization in subsidiary cells and a depolarization in guard cells finally to achieve an inverted direction in K + flow in subsidiary cells and guard cells. This would consequently result in an inverse regulation of the respective voltage-dependent K in , K out and MgC channels, and in turn drive the K + ions from guard cells into the adjacent subsidiary cells. Membrane potential measurements on intact cells would be well suited to obtain evidence for such an opposite membrane potential polarization in subsidiary cells and guard cells during stomatal closure (cf. Roelfsema et al. 2004 ).
Materials and Methods
Protoplast isolation
Zea mays L. var. Caraibe (Saaten Union, Hannover, Germany) was grown in a climate chamber at 70% humidity and a 12.5 h photoperiod with a photon flux density of about 130 μmol m −2 s −1 . The air temperature was 26°C during the day and 16°C during the night. Subsidiary cell protoplasts were enzymatically isolated from lower epidermal strips of 8-day-old leaves as described by Wolf et al. ( 2005 ).
Electrophysiology
Whole-cell patch-clamp experiments were performed as previously described (Hamill et al. 1981 , Majore et al. 2002 ). The data acquisition rate was set between 0.3 and 1 ms in the whole-cell and to 0.1 ms in the outside-out configuration. Whole-cell currents were low-pass filtered between 0.2 and 1 kHz, and single channel currents at 1 kHz. The voltages clamped in the whole-cell as well as in the outside-out mode were adjusted for the liquid junction potential (Neher 1992 ). Additionally, the clamped voltages in the whole-cell configuration were corrected by the error related to the access resistance.
For whole-cell current recordings, voltage pulses between +96 and −214 mV were applied for 1–3 s in 10 mV voltage steps. Steady-state currents (I ss ) were measured at the end of the voltage pulses and corrected for background currents that were determined in a voltage range of high membrane resistance. To allow the comparison of the current magnitude among different protoplasts, the current densities (I ss /C m ) were determined upon dividing the macroscopic ionic currents through the whole-cell capacitance C m of the individual protoplasts and plotted vs. the membrane voltages (V). To describe the relative voltage-dependent open probability, the macroscopic conductance was calculated from the steady-state current amplitude under consideration of the ion driving force (V − V rev ). The derived conductance values were plotted as a function of the membrane voltage V and fitted with a Boltzmann distribution as given by the following function: G(V) = 1/[1 + exp(V − V 1/2 )zF/RT]. The individual G(V) curves were normalized to the corresponding fits by setting the maximal macroscopic conductance G max = 1. R, T and F have their usual meanings, V 1/2 represents the half-maximal activation voltage at which 50% of the maximal conductance is reached, and z represents the number of apparent equivalent gating charges.
For single current measurements in the outside-out configuration, the glass bottom of the patch-clamp chamber was covered with poly- l -lysine at a dilution of 1 : 185, dried at 60°C for 90 min and sonicated for 3 min. As a result, the attachment of the protoplasts to the patch-clamp chamber was improved such that the outside-out configuration could be established. Voltage pulses in 5 or 10 mV steps were applied for up to 60 s. Single channel current amplitudes (i) were determined for different voltages from all-point histograms and plotted against the corresponding voltage. The single channel conductance was derived from the slope of the linear regression fitted to the data points. The reversal potential V rev was determined upon the intersection of the extrapolated linear regression line to the x -axis. The K + Nernst potential for the different solutions was calculated by using K + activities (Robinson and Stokes 1965).
Data values are given as mean ± SEM, and n denotes the number of independent experiments.
Patch-clamp solutions
The osmolality of the pipet (intracellular) and bath (extracellular) solutions was adapted to 560 and 530 mosmol kg −1 , respectively, with d -mannitol. The standard bath medium consisted of (in mM) 3 K-gluconate, 1 Ca-gluconate 2 , 10 MES and 0.5% isopropanol (pH 5.6/Tris). The standard pipet solution contained (in mM) 150 K-gluconate, 2 MgCl 2 , 10 EGTA, 0.5% isopropanol, 10 HEPES (pH 7.4/Tris) and either 1 GTP plus 5 ATP for monitoring the time-dependent K + currents, or only 5 GTP for recording the instantaneous outward-rectifying K + currents (Wolf et al. 2005 ). To adjust the standard pipet solution to an intracellular pH of 8.2 or 6.7, HEPES was replaced by Trizma or MES, respectively. ABA was added to the experimental solutions from a stock solution that was prepared with isopropanol and stored at –18°C. Further modifications of the standard solutions are mentioned in the figure legends. To examine the effect of intracellular Ca 2+ ions on the level of single ion channels, pipet solutions previously described by Majore et al. ( 2002 ) were applied without GTP. These Ca 2+ experiments were performed in the presence of 30 mM K-gluconate, 1 mM Ca-gluconate 2 and 10 mM MES (pH 5.6/Tris) in the bath solution.
Chemicals
Cis / trans -ABA, TEA-Cl (tetraethylammonium chloride), ATP (Mg salt), GTP (Tris salt) and BAPTA-K 4 were from Sigma-Aldrich (Deisenhofen, Germany).
Acknowledgments
We thank D. Becker, R. Hedrich and M.R.G. Roelfsema for helpful comments on the manuscript. This work was funded by grants of the Deutsche Forschungsgemeinschaft (SPP1108) to I.M.
References
Author notes
These authors contributed equally to this work.



![Intracellular pH sensitivity of the K + -selective channels from maize subsidiary cells. Macroscopic K in (A–C) and K out (D–F) currents were monitored and analyzed for different intracellular pH values. (A) Representative K in current traces elicited upon voltage steps to −184, −164, −144 and −124 mV are shown. (D) Representative K out currents evoked upon voltage pulses to −24, +6, +36 and +66 mV are given. Dashed lines give the zero current level. (B, E) The respective steady-state current densities were plotted against the membrane voltage. (C, F) The intracellular pH effect on the voltage-dependent relative open probability is reflected by normalized macroscopic conductance–voltage curves [G/G max (V)]. The solid curves give the mean of the individual fits describing the data values according to the Boltzmann distribution. (B) pH 8.2, filled squares, n = 11; pH 7.4, filled circles, n = 6; pH 6.7, filled triangles, n = 7. (C) pH 8.2, open squares, n = 8–11; pH 7.4, open circles, n = 6; pH 6.7: open triangles, n = 7. (E) pH 8.2, filled squares, n = 5; pH 7.4, filled circles, n = 4; pH 6.7, filled triangles, n = 6. (F) pH 8.2, open squares, n = 5; pH 7.4: open circles, n = 4. In A–F, standard pipet solution was used without isopropanol, contained only ATP (no GTP) and was adjusted to the pH values as indicated. The bath solution was composed of 10 mM K-gluconate, 1 mM Ca-gluconate 2 and 10 mM MES pH 5.6/Tris.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/47/10/10.1093_pcp_pcl007/3/m_pcl007f4.jpeg?Expires=1716604439&Signature=4x~uHlkXbizkjl6zAyWGytRFTEy4tWA9IvkOJpBU3T01mqPf8jdl16wj3JM712CeUAZQTohdAU74bCgiWG9L02i8C6lfbKFee~dmH7AB6J0YHrcBYI~IC3bSeuPJUzSU2tZN1qwfKVId06vKLI1fr4hrXBS0EA1NJdPLZIN0vYCoyJyzG8NredBq-pFDUrTHh11iSJ3O2h5mXdapCMiWpEo7Ww58sHSHr94QchSc2I1XmHPCkgqvhEh7Zg6adMQ0Zj7~xcP2G5KzGHmUu1Wfb81I8~5DV-mZo4phQ8rJiUuqWpakE85oac8lQYt0ql7OfODBq9Xiwt0fY5d9Ib6DSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Recording of single K out channels from subsidiary cell protoplasts in the outside-out configuration. (A) Single channel fluctuations were observed at −14 mV under control conditions (upper trace), then disappeared with 30 mM TEA + in the bath solution (middle trace). After wash out of the inhibitor (lower trace), open–closed transitions occurred again. C indicates the current baseline where all channels are in the closed state. Upward deflections represent channel openings with i simultaneously opened channels (O1, O2, …, O i ). (B) The response of single channels to different voltage pulses is shown. (C) Reversal potentials (V rev ) of single channel outward currents were determined for different extracellular K + activities. The straight line is the linear regression to the experimental data with a slope of 48.2 mV, while the dashed line gives the Nernst potentials for K + . The data points represent the mean of five, three and seven independent experiments determined in the presence of 10, 30 or 100 mM K-gluconate in the extracellular solution, respectively. (D) Representative channel fluctuations evoked upon a voltage pulse to +16 mV were recorded at different intracellular pH values as indicated. (E) The single channel conductance was determined for different intracellular pH values from single channel current–voltage [i(V)] curves. The solid (pH 7.4) and dotted (pH 8.2) lines are the means of the individual linear regressions to the experimental data. Each symbol represents the data points obtained from one independent experiment (open symbols, pH 7.4, n = 3; filled symbols, pH 8.2, n = 4). The same pipet and bath solutions were used as in Fig. 4 . The bath medium in (C) was varied concerning the K-gluconate concentration as indicated, and in (D) and (E) contained 30 mM K-gluconate.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/pcp/47/10/10.1093_pcp_pcl007/3/m_pcl007f5.jpeg?Expires=1716604439&Signature=A1BXE51PYo5Keo9BVvXLzHFT7rAR8w-wM5y85vPfldbth15WqiecKDKfUtcZBOgyDIIS6kvJHZR6xMQjZZ9ee6ItEbYcZIG7jkt-fBfWtBhxOTyPZdDAfvfR3k0~XZV-X9IVJ2N6XaUkjexCDthN68qycP0wd1fWJQenoNnywo2v-WD85v-Bkfoqi0LiKTQwC0E2pXw3FubZ27afpWPAP8kdCpsTTcCoo7JfGg1gYI749ojhrThnMQ1eHkvIwp1hV8iHSl6ENqkaePezqMSIZ80hnSA4Hquj8bNlAJSFfctdWtCNraenm-1HZVUy-vhSsOTAN9w7GSObRzBIeKWsgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)