-
PDF
- Split View
-
Views
-
Cite
Cite
Francisco J. Corpas, Ana Fernández-Ocaña, Alfonso Carreras, Raquel Valderrama, Francisco Luque, Francisco J. Esteban, María Rodríguez-Serrano, Mounira Chaki, José R. Pedrajas, Luisa M. Sandalio, Luis A. del Río, Juan B. Barroso, The Expression of Different Superoxide Dismutase Forms is Cell-type Dependent in Olive ( Olea europaea L.) Leaves , Plant and Cell Physiology, Volume 47, Issue 7, July 2006, Pages 984–994, https://doi.org/10.1093/pcp/pcj071
Close - Share Icon Share
Abstract
Superoxide dismutase (SOD) is a key antioxidant enzyme present in prokaryotic and eukaryotic cells as a first line of defense against the accumulation of superoxide radicals. In olive leaves, the SOD enzymatic system was characterized and was found to be comprised of three isozymes, an Mn-SOD, an Fe-SOD and a CuZn-SOD. Transcript expression analysis of whole leaves showed that the three isozymes represented 82, 17 and 0.8% of the total SOD expressed, respectively. Using the combination of laser capture microdissection (LCM) and real-time quantitative reverse transcription–PCR (RT–PCR), the expression of these SOD isozymes was studied in different cell types of olive leaves, including spongy mesophyll, palisade mesophyll, xylem and phloem. In spongy mesophyll cells, the isozyme proportion was similar to that in whole leaves, but in the other cells the proportion of expressed SOD isozymes was different. In palisade mesophyll cells, Fe-SOD was the most abundant, followed by Mn-SOD and CuZn-SOD, but in phloem cells Mn-SOD was the most prominent isozyme, and Fe-SOD was present in trace amounts. In xylem cells, only the Mn-SOD was detected. On the other hand, the highest accumulation of superoxide radicals was localized in vascular tissue which was the tissue with the lowest level of SOD transcripts. These data show that in olive leaves, each SOD isozyme has a different gene expression depending on the cell type of the leaf.
Introduction
Superoxide dismutases (SODs; EC 1.15.1.1) are a family of metalloenzymes that catalyze the disproportionation of superoxide ( 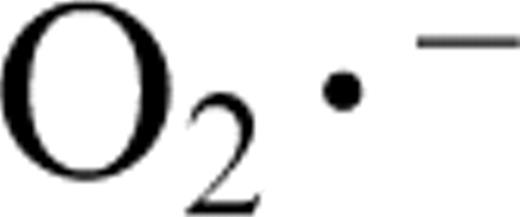 ) radicals into H 2 O 2 and O 2 , and are a first line of defense against the toxic effects of superoxide radicals produced in different cellular compartments (Fridovich 1986 , Halliwell and Gutteridge 2000 ). In general, there are three types of SOD, containing either Mn, Fe, or Cu plus Zn as prosthetic metals (Fridovich 1986 ).
) radicals into H 2 O 2 and O 2 , and are a first line of defense against the toxic effects of superoxide radicals produced in different cellular compartments (Fridovich 1986 , Halliwell and Gutteridge 2000 ). In general, there are three types of SOD, containing either Mn, Fe, or Cu plus Zn as prosthetic metals (Fridovich 1986 ).
In higher plants, SOD isozymes have been localized in different cell compartments. Mn-SOD is present in mitochondria and peroxisomes (Baum and Scandalios 1981 , del Río et al. 1983 , Palma et al. 1986 , Sandalio and del Rio 1987 , Bowler et al. 1994 , Corpas et al. 1998 , del Rio et al. 2003 ). Fe-SOD has been found mainly in chloroplasts (Salin 1988 , Asada 1994 ) but has also been detected in peroxisomes (Droillard and Paulin 1990 ), and CuZn-SOD has been localized in cytosol, chloroplasts, peroxisomes and apoplast (Sandalio and del Río 1987 , Sandalio and del Río 1988, Salin 1988 , Kanematsu and Asada 1991 , Ogawa et al. 1996 , Ogawa et al. 1997 , Sandalio et al. 1997 , Corpas et al. 1998 , del Río et al. 2002 ). The number and type of SOD isozymes can change depending on the plant species, age of development and environmental conditions (Bridges and Salin 1981 , Bowler et al. 1994 , Kliebenstein et al. 1998 , Alscher et al. 2002 ), and there are also cases of plants, such as sunflower, with only one type of isoform, a CuZn-SOD (Corpas et al. 1998 ).
Plant SODs have been studied under many aspects, including phylogenetic distribution, biochemical and molecular properties, structure and function, enzyme regulation, gene organization and expression, subcellular localization, role in abiotic and biotic stress, etc. (Bridges and Salin 1981 , del Río et al. 1983 , Tsang et al. 1991 , Bowler et al. 1994 , Bueno et al. 1995 , Allen et al. 1997 , Corpas et al. 1998 , Kliebenstein et al. 1998 , Alscher et al. 2002 , Fink and Scandalios 2002 ). However, in higher plants, there is still very little information on the specific function of each SOD isoenzyme (Mn-SOD, Fe-SOD and CuZn-SOD) in cells of different tissues. It is necessary to obtain deeper insights into the relationship between cellular localization and specific function of each SOD isoenzyme.
The olive tree ( Olea europaea L.) is an important crop in Southern Europe with a strong economic impact in the agricultural industry of these countries because the olive fruit is used to obtain the oil of choice for the Mediterranean diet (Owen et al. 2000 ). However, there are few studies on the metabolism of reactive oxygen species (ROS) in olive trees and, to our knowledge, there is no biochemical and molecular information on SODs and their subcellular localization in leaves of this plant species (Valderrama et al. 2006 ).
In this work, using the combination of laser capture microdissection (LCM) and quantitative reverse transcription–PCR (RT–PCR), the gene expression of the three SOD isozymes identified in olive leaves was analyzed. The existence of a differential transcript expression of SODs depending on the leaf cell type and the presence of Mn-SOD in the vascular tissue of leaves was demonstrated.
Results
Biochemical and molecular analysis of the SOD isozymes of olive leaves
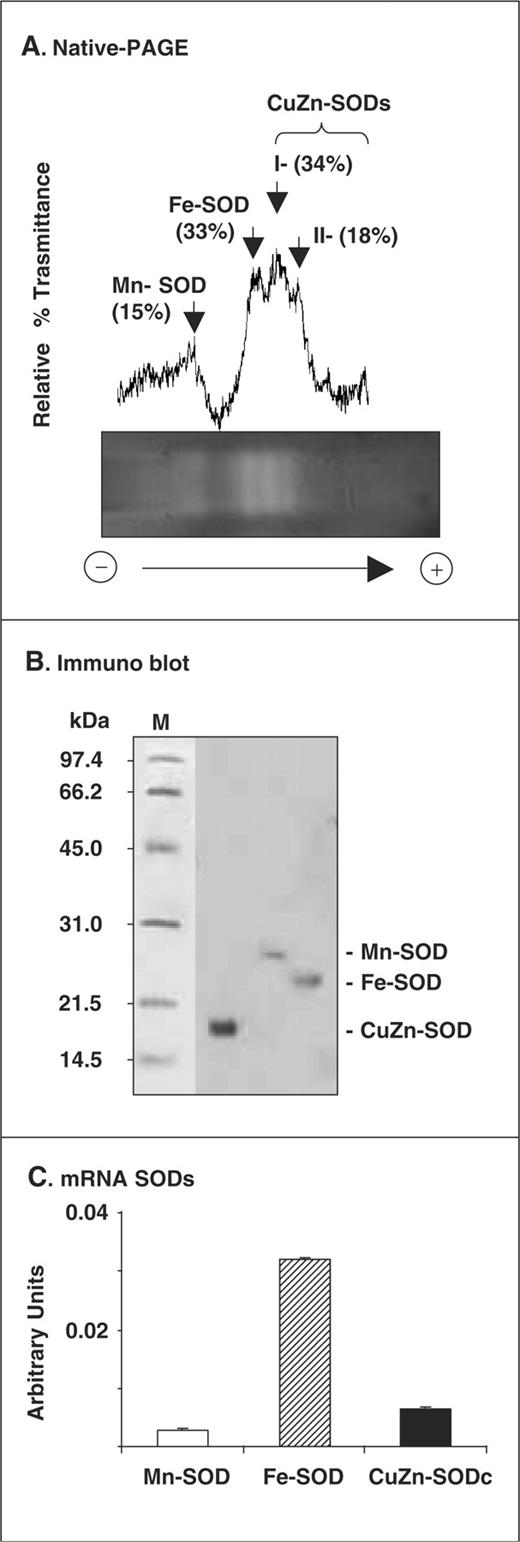
In crude extracts of olive leaves, the total SOD specific activity was 1.7 U mg −1 protein. The analysis of the SOD activity by native PAGE showed the presence of four isozymes which were identified with specific inhibitors (CN − and H 2 O 2 ) as a Mn-SOD, an Fe-SOD and two CuZn-SODs (I and II), which represented 15, 33 and 52% of the total SOD activity, respectively ( Fig. 1 A). The analysis by Western blot with specific antibodies against each type of SOD showed subunit molecular masses of 27 kDa for Mn-SOD, 25 kDa for Fe-SOD and 17 kDa for CuZn-SOD ( Fig. 1 B).
Identification of the SOD isozymes present in olive leaves. (A) Activity of SOD isozymes. SODs were separated by native PAGE on 10% (w/v) polyacrylamide gels, and gels were stained by the photochemical nitroblue tetrazolium method. Gels were analyzed using a Gel Doc system (BioRad) coupled with a highly sensitive CCD camera, and band intensities were expressed as relative transmittance (T) units. (B) Western blot of SODs of olive leaf extracts probed with antibodies against: pea Mn-SOD (1 : 2,000 dilution), Fe-SOD and spinach CuZn-SOD (1 : 3,000 dilution). Proteins (30 µg per lane) were separated by 15% SDS–PAGE and transferred onto a PVDF membrane. (C) Transcript analysis (arbitrary units) of the SOD isozymes by real-time quantitative RT–PCR. Data are the mean ± SEM of at least three different experiments.
On the basis of the information from the gene databank on the different plant SOD isozymes, oligonucleotides for conserved regions of each SOD isozyme were designed (see Table 1 ). Thus, a partial clone for each SOD isozyme was obtained. The olive leaf Mn-SOD (accession No. AF427107) showed an identity of 83% with the Mn-SOD of Glycine max , and 81% with the Mn-SODs of Lycopersicon esculentum, Prunus persica and Nicotiana plumbaginifolia . The olive leaf Fe-SOD (accession No. AY168776) showed an identity of about 80% with the Fe-SODs of Capsicum annuum, N. plumbaginifolia and L. esculentum . The olive leaf CuZn-SOD (accession No. AF426829) had identities of about 85% with the CuZn-SODs of C. annuum, L. esculentum, Solanum tuberosum and Populus tremuloides . As shown in Fig. 1 C, the mRNA expression analysis of SODs in olive leaves by quantitative RT–PCR using specific oligonucleotides ( Table 1 ) showed the maximum expression for Fe-SOD (82.5%), followed by CuZn-SOD (16.7%) and Mn-SOD (0.8%).
Oligonucleotides used for the cloning and real-time quantitative RT–PCR analysis of the three SOD isozymes
| Name . | Oligonucleotide sequence (5′ to 3′) . | Product size (bp) . |
|---|---|---|
| cDNA cloning | ||
| Mn-SOD-f | ACM MGA ARC ACC AYC ARACTTA | 435 |
| Mn-SOD-r | TGM ARG TAG TAG GCA TGY TCC CA | |
| CuZn-SOD-f | CCT GGA CTT CAT GGC TTC CAT | 312 |
| CuZn-SOD-r | TCT TCC GCC AGC GTT TCC AGT G | |
| Fe-SOD-f | TYC ACT GGG GKA AGC AYC A | 435 |
| Fe-SOD-r | TCM ARR TAG TAA GCA TGC TCC CA | |
| Quantitative-PCR | ||
| Mn-SOD-f1 | AGT CAA GTT GCA GAG TGC AAT CAA GTT C | 144 |
| Mn-SOD-r1 | CAA AGT GAT TGT CAA TAG CCC AAC CTA AAG | |
| CuZn-SOD-f1 | GGC TGT ATG TCA ACT GGA CCT CAT TTC A | 140 |
| CuZn-SOD-r1 | TGT CAA CAA TGT TGA TAG CAG CGG TG | |
| Fe-SOD-f1 | AAC AAG CAA ATA GCC GGA ACA GAA CTA AC | 128 |
| Fe-SOD-r1 | AGA AAT CGT GAT TCC AGA CCT GAG CAG | |
| RNA 18S-f1 | TTT GAT GGT ACC TGC TAC TCG GAT AAC C | 274 |
| RNA 18S-r1 | CTC TCC GGA ATC GAA CCC TAA TTC TCC |
| Name . | Oligonucleotide sequence (5′ to 3′) . | Product size (bp) . |
|---|---|---|
| cDNA cloning | ||
| Mn-SOD-f | ACM MGA ARC ACC AYC ARACTTA | 435 |
| Mn-SOD-r | TGM ARG TAG TAG GCA TGY TCC CA | |
| CuZn-SOD-f | CCT GGA CTT CAT GGC TTC CAT | 312 |
| CuZn-SOD-r | TCT TCC GCC AGC GTT TCC AGT G | |
| Fe-SOD-f | TYC ACT GGG GKA AGC AYC A | 435 |
| Fe-SOD-r | TCM ARR TAG TAA GCA TGC TCC CA | |
| Quantitative-PCR | ||
| Mn-SOD-f1 | AGT CAA GTT GCA GAG TGC AAT CAA GTT C | 144 |
| Mn-SOD-r1 | CAA AGT GAT TGT CAA TAG CCC AAC CTA AAG | |
| CuZn-SOD-f1 | GGC TGT ATG TCA ACT GGA CCT CAT TTC A | 140 |
| CuZn-SOD-r1 | TGT CAA CAA TGT TGA TAG CAG CGG TG | |
| Fe-SOD-f1 | AAC AAG CAA ATA GCC GGA ACA GAA CTA AC | 128 |
| Fe-SOD-r1 | AGA AAT CGT GAT TCC AGA CCT GAG CAG | |
| RNA 18S-f1 | TTT GAT GGT ACC TGC TAC TCG GAT AAC C | 274 |
| RNA 18S-r1 | CTC TCC GGA ATC GAA CCC TAA TTC TCC |
‘f’ and ‘r’ correspond to forward and reverse oligonucleotides, respectively. For the degenerated oligonucleotides: M = A,C; R = A,G; Y = C,T; K = G,T.
Oligonucleotides used for the cloning and real-time quantitative RT–PCR analysis of the three SOD isozymes
| Name . | Oligonucleotide sequence (5′ to 3′) . | Product size (bp) . |
|---|---|---|
| cDNA cloning | ||
| Mn-SOD-f | ACM MGA ARC ACC AYC ARACTTA | 435 |
| Mn-SOD-r | TGM ARG TAG TAG GCA TGY TCC CA | |
| CuZn-SOD-f | CCT GGA CTT CAT GGC TTC CAT | 312 |
| CuZn-SOD-r | TCT TCC GCC AGC GTT TCC AGT G | |
| Fe-SOD-f | TYC ACT GGG GKA AGC AYC A | 435 |
| Fe-SOD-r | TCM ARR TAG TAA GCA TGC TCC CA | |
| Quantitative-PCR | ||
| Mn-SOD-f1 | AGT CAA GTT GCA GAG TGC AAT CAA GTT C | 144 |
| Mn-SOD-r1 | CAA AGT GAT TGT CAA TAG CCC AAC CTA AAG | |
| CuZn-SOD-f1 | GGC TGT ATG TCA ACT GGA CCT CAT TTC A | 140 |
| CuZn-SOD-r1 | TGT CAA CAA TGT TGA TAG CAG CGG TG | |
| Fe-SOD-f1 | AAC AAG CAA ATA GCC GGA ACA GAA CTA AC | 128 |
| Fe-SOD-r1 | AGA AAT CGT GAT TCC AGA CCT GAG CAG | |
| RNA 18S-f1 | TTT GAT GGT ACC TGC TAC TCG GAT AAC C | 274 |
| RNA 18S-r1 | CTC TCC GGA ATC GAA CCC TAA TTC TCC |
| Name . | Oligonucleotide sequence (5′ to 3′) . | Product size (bp) . |
|---|---|---|
| cDNA cloning | ||
| Mn-SOD-f | ACM MGA ARC ACC AYC ARACTTA | 435 |
| Mn-SOD-r | TGM ARG TAG TAG GCA TGY TCC CA | |
| CuZn-SOD-f | CCT GGA CTT CAT GGC TTC CAT | 312 |
| CuZn-SOD-r | TCT TCC GCC AGC GTT TCC AGT G | |
| Fe-SOD-f | TYC ACT GGG GKA AGC AYC A | 435 |
| Fe-SOD-r | TCM ARR TAG TAA GCA TGC TCC CA | |
| Quantitative-PCR | ||
| Mn-SOD-f1 | AGT CAA GTT GCA GAG TGC AAT CAA GTT C | 144 |
| Mn-SOD-r1 | CAA AGT GAT TGT CAA TAG CCC AAC CTA AAG | |
| CuZn-SOD-f1 | GGC TGT ATG TCA ACT GGA CCT CAT TTC A | 140 |
| CuZn-SOD-r1 | TGT CAA CAA TGT TGA TAG CAG CGG TG | |
| Fe-SOD-f1 | AAC AAG CAA ATA GCC GGA ACA GAA CTA AC | 128 |
| Fe-SOD-r1 | AGA AAT CGT GAT TCC AGA CCT GAG CAG | |
| RNA 18S-f1 | TTT GAT GGT ACC TGC TAC TCG GAT AAC C | 274 |
| RNA 18S-r1 | CTC TCC GGA ATC GAA CCC TAA TTC TCC |
‘f’ and ‘r’ correspond to forward and reverse oligonucleotides, respectively. For the degenerated oligonucleotides: M = A,C; R = A,G; Y = C,T; K = G,T.
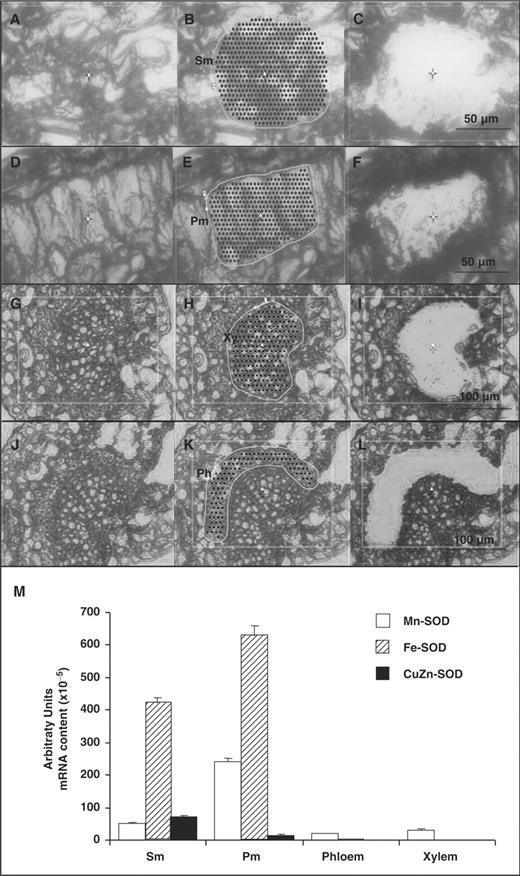
LCM of different cell types of olive leaves and gene expression of the SOD isozymes
Fig. 2 A–L shows representative pictures of the appearance of the leaf tissues before, during and after the use of the LCM method to obtain cell types from four olive leaf tissues, including spongy and palisade mesophyll, xylem and phloem. The selected cells were used as starting material to obtain the corresponding RNAs, which were used for the gene expression analysis of Fe-SOD, CuZn-SOD and Mn-SOD, by real-time quantitative PCR using specific primers (see Table 1 ). The mRNA content of each isozyme in the cells of spongy mesophyll, palisade mesophyll, phloem and xylem tissues is shown in Fig. 2 M. Mn-SOD was the only isoform which was present in all cell types. The highest expression of Mn-SOD was observed in palisade mesophyll cells followed by spongy mesophyll cells, xylem and phloem cells. On the other hand, Fe-SOD was only detected in palisade and spongy mesophyll. The highest expression of Fe-SOD was observed in palisade mesophyll cells, followed by spongy mesophyll and phloem cells. CuZn-SOD was only detected in spongy and palisade mesophyll cells.
Visualization of laser capture microdissection (LCM) of cell types from olive leaves and transcript analysis of the SOD isozymes. (A–C) Appearance of spongy mesophyll (Sm). (D–F) Appearance of palisade mesophyll (Pm). (G–I) Appearance of xylem (Xy). (J–L) Appearance of phloem (Ph). A, D, G and J show the olive leaf tissues before LCM analysis. B, E, H and K show the delimited target region. C, F, I and L show the appearance of the tissues after LCM analysis. (M) Real-time quantitative RT–PCR transcript analysis (arbitrary units) of the three SOD isozymes in olive leaf cells from spongy (Sm) and palisade mesophyll (Pm), phloem and xylem. Data are mean ± SEM of, at least, four independent RNA samples from leaf cells obtained by LCM.
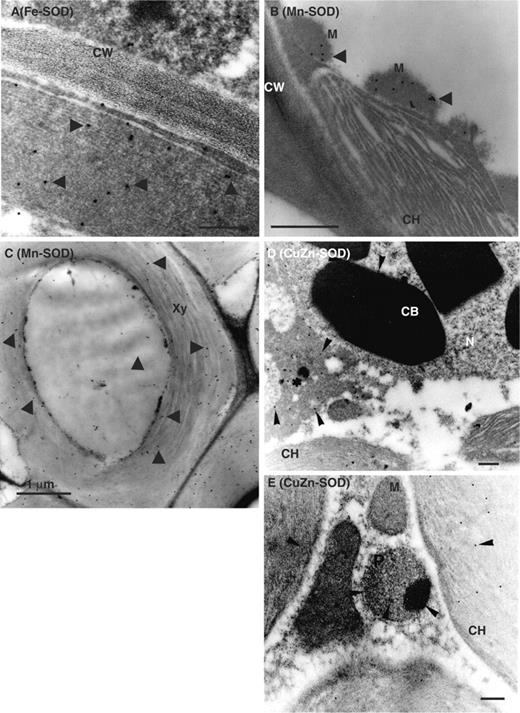
Immunocytochemical localization of Fe-SOD, Mn-SOD and CuZn-SOD in olive leaves
Electron micrographs of thin sections of olive leaves showing the specific subcellular localization of Fe-SOD, Mn-SOD and CuZn-SOD in spongy mesophyll and xylem cells are shown in Fig. 3 . Fe-SOD was localized in chloroplasts ( Fig. 3 A), but the immunogold labeling of Mn-SOD was present in mitochondria of spongy mesophyll cells ( Fig. 3 B) and in xylem cells ( Fig. 3 C). CuZn-SOD was present in different cell compartments, including amorphous electron-dense structures in the cytosol, chloroplasts and peroxisomes, and crystalline bodies in nuclei ( Fig. 3 D and E). The pre-immune serum did not show any significant labeling (data not shown).
Immunogold electron microscopy localization of SODs in spongy mesophyll and xylem cells of olive leaves. Representative electron micrographs of spongy mesophyll cells of olive leaves. The sections were incubated with antibodies against Fe-SOD (dilution 1 : 2,000), Mn-SOD (dilution 1 : 500) and CuZn-SOD (1 : 300 dilution). (A) Immunolocalization of Fe-SOD in spongy mesophyll cells. (B) Immunolocalization of Mn-SOD in spongy mesophyll cells. (C) Immunolocalization of Mn-SOD in xylem cells. (D and E) Immunolocalization of CuZn-SOD in spongy mesophyll cells. Arrows indicate 15 nm gold particles. *electron-dense structures in the cytosol; CB, crystalline body; CH, chloroplast; CW, cell wall; M, mitochondrion; N, nucleus; P, peroxisome; Xy, xylem. Bars represent 1.0 µm in A, C, D and E; and 0.5 µm in B.
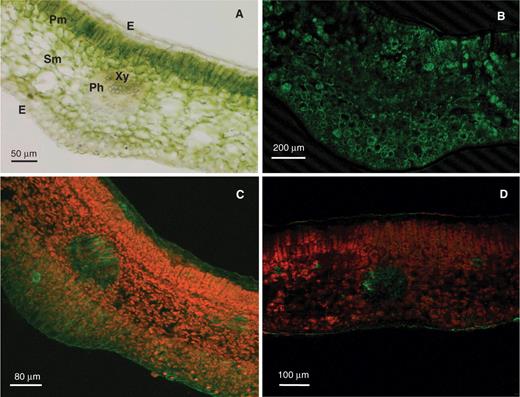
Immunohistochemical localization of Mn-SOD in olive leaves by CLSM
Considering that the mRNA of Mn-SOD was the only SOD transcript present in the four cell types, the protein expression of Mn-SOD was also analyzed by immunohistochemical analysis at the cellular level using an antibody against pea Mn-SOD which recognizes a single band of 27 kDa in crude extracts of olive leaves ( Fig. 1 B). The appearance under the optical microscope of an olive leaf section showing its different tissues is presented in Fig. 4 A. A representative picture of the immunolocalization of Mn-SOD in olive leaf sections analyzed by confocal laser scanning microsopy (CLSM) is shown in Fig. 4 B. The green fluorescence, which is attributable to the Mn-SOD, was observed in all cell types except in epidermis cells, and the strongest fluorescence was detected in the spongy mesophyll cells.
On the other hand, the accumulation of superoxide radicals ( 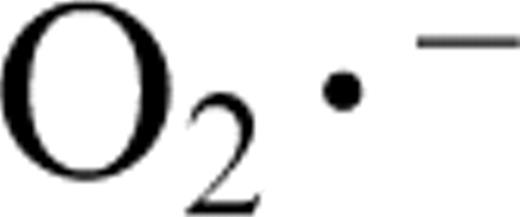 ) in olive leaf sections was analyzed by CLSM using the fluorescence probe dihydroethidium (DHE). The green fluorescence, due to
) in olive leaf sections was analyzed by CLSM using the fluorescence probe dihydroethidium (DHE). The green fluorescence, due to 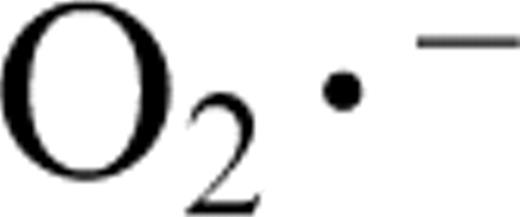 radicals, was mainly localized in vascular tissue and epidermal cells ( Fig. 4 C). The localization of superoxide accumulation in vascular tissue is consistent with the results showing that this tissue has the lowest level of SOD transcripts ( Fig. 2 M). When the olive leaf sections were pre-incubated with 1 mM TMP (a superoxide scavenger), a significant reduction of the green fluorescence was observed ( Fig. 4 D).
radicals, was mainly localized in vascular tissue and epidermal cells ( Fig. 4 C). The localization of superoxide accumulation in vascular tissue is consistent with the results showing that this tissue has the lowest level of SOD transcripts ( Fig. 2 M). When the olive leaf sections were pre-incubated with 1 mM TMP (a superoxide scavenger), a significant reduction of the green fluorescence was observed ( Fig. 4 D).
Immunohistochemical localization of Mn-SOD in olive leaves and detection of superoxide radicals ( 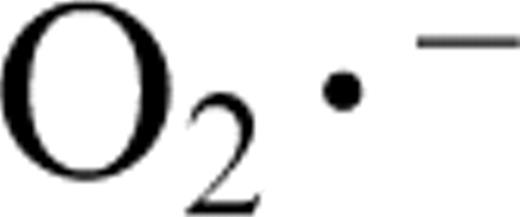 ). (A) Appearance of an olive leaf section under the optical microscope. (B) Cy2-streptavidin immunofluorescence (green color) attributable to the antibody against Mn-SOD (dilution 1 : 200). The olive leaf section was analyzed by confocal laser scanning microscopy (CLSM). (C) Representative image illustrating the CLSM detection of superoxide radicals (
). (A) Appearance of an olive leaf section under the optical microscope. (B) Cy2-streptavidin immunofluorescence (green color) attributable to the antibody against Mn-SOD (dilution 1 : 200). The olive leaf section was analyzed by confocal laser scanning microscopy (CLSM). (C) Representative image illustrating the CLSM detection of superoxide radicals ( 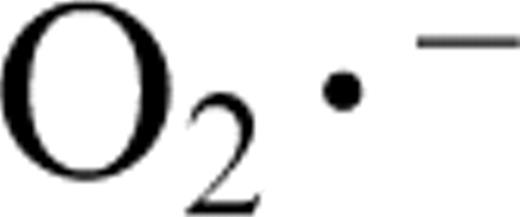 ) in olive leaf sections incubated for 1 h at 25°C, in darkness, with 10 µM DHE, where
) in olive leaf sections incubated for 1 h at 25°C, in darkness, with 10 µM DHE, where 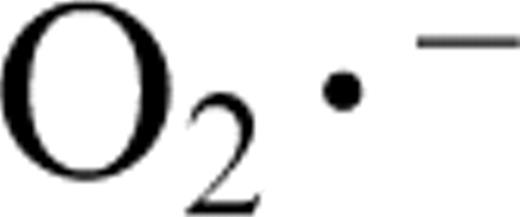 is detected by its bright green fluorescence. (D) Representative image of a leaf section pre-incubated with 1 mM TMP, a superoxide scavenger, and then with 10 µM DHE. The orange-yellow color corresponds to the Chl autofluorescence. E, epidermis. Ph, phloem. Pm, palisade mesophyll. Sm, spongy mesophyll. Xy, xylem.
is detected by its bright green fluorescence. (D) Representative image of a leaf section pre-incubated with 1 mM TMP, a superoxide scavenger, and then with 10 µM DHE. The orange-yellow color corresponds to the Chl autofluorescence. E, epidermis. Ph, phloem. Pm, palisade mesophyll. Sm, spongy mesophyll. Xy, xylem.
Discussion
In this work using leaves of olive plants as a model, the expression of the SOD genes in different cell types of leaf tissues, including phloem, xylem, and palisade and spongy mesophyll, was studied by LCM and quantitative RT–PCR. In olive leaves, three SOD isozymes were found, Mn-SOD, Fe-SOD and CuZn-SOD, and this isozyme pattern is different from that found in olive pollen tubes, where only four CuZn-SODs are present (Alché et al. 1998 ). The occurrence of the three types of SOD has also been described in other plant species such as Brassica campestris (Bridges and Salin 1981 ), Pisum sativum (Sandalio et al. 2001 , Gómez et al. 2004 ), Coffea arabica (Daza et al. 1993 ) and N. plumbaginifolia (Van Camp et al. 1997 ), among others. However, the existence of different patterns of SOD isozymes has been reported in other plant species. CuZn-SOD and Mn-SOD are present in Phaseolus vulgaris and Vigna unguiculata (Corpas et al. 1991 ), CuZn-SODs in Helianthus annuun and Hibiscus esculentus (Bridges and Salin 1981 , Corpas et al. 1998 ), CuZn-SODs and Fe-SODs in Ginkgo biloba , and Fe-SODs and Mn-SOD in Nuphar luteum (Bridges and Salin 1981 ). All these cases provide evidence of the heterogeneous distribution of SOD isozymes in higher plant species, and suggest that each SOD isoenzyme must have a specific function probably related to its cellular and subcellular localization.
The comparison of results of the percentile activity of SOD isozymes and their transcript expression showed clear discrepancies. Whilst the highest activity corresponded to CuZn-SODs (I + II), Fe-SOD was the isozyme showing the highest expression level. The reason for these differences could be due to the fact that the activity determinations were carried out in leaf crude extracts, and some isozyme(s) bound to the membranes of different cell compartments could have been lost in the pellet fraction after the centrifugation of homogenates, with the subsequent activity decrease. Some of these membrane-bound SODs could be the Fe-SOD of thylakoids, the CuZn-SOD of crystal bodies ( Fig. 3 ) and the xylem Mn-SOD ( Fig. 3 C). On the other hand, the mRNA level was evaluated by quantitative RT–PCR using specific primers of the cDNAs obtained, which corresponded to conserved regions of SOD isozymes from plant origin. However, perhaps some of the isozymes had different sequences in these regions and, therefore, their corresponding mRNAs could not be determined.
The subunit molecular mass of olive Mn-SOD is in the range 24–27 kDa reported for Mn-SODs from other higher plant species (Baum and Scandalios 1981 , Hayakawa et al. 1985 , Distefano et al. 1999 ), and is identical to the molecular mass determined for the mitochondrial and peroxisomal Mn-SOD of pea leaves (Palma et al. 1998 , del Río et al. 2003 ).
Fe-SOD is the main isoenzyme expressed in photosynthetic cells
LCM is a new tool in the study of cell type-specific expression. This technique was designed to be used in animal tissues (Emmert-Buck et al. 1996 ), and there are few reports on its application in plant cells (Kerk et al. 2003 , Nakazono et al. 2003 , Day et al. 2005 ). In this work, the combination of LCM with quantitative PCR has been used to establish the gene expression pattern of the SOD isozymes of olive leaves. The results obtained indicated that the Fe-SOD gene had the highest expression in whole leaves and isolated cells from spongy and palisade mesophyll. This could be correlated with the well known presence of Fe-SOD in chloroplasts, which are one of the most abundant organelles in photosynthetic cells. In chloroplasts of tobacco leaves, Fe-SOD is the most abundant isoenzyme and these organelles also contain a CuZn-SOD which is expressed in low amounts (Van Camp et al. 1997 ). This situation is similar to that reported in this work for Fe-SOD and CuZn-SOD which were immunolocalized in chloroplasts of spongy mesophyll cells ( Fig. 3 A, E). The chloroplastic CuZn-SOD of olive leaves could be involved in the response to oxidative stress in this plant species, such as has been described in Arabidosis whose chloroplasts contain one CuZn-SOD and three Fe-SODs with a differential regulation under environmental stimuli (Kliebenstein et al. 1998 ).
CuZn-SOD is mainly expressed in spongy mesophyll cells
CuZn-SOD is the most abundant SOD isozyme in many plant species (Asada et al. 1980 , Bridges and Salin 1981 , Bowler et al. 1994 , Schinkel et al. 2001 , Alscher et al. 2002 ). In crude extracts of olive leaves the activity of the two CuZn-SOD isozymes represented 52% of the total SOD activity ( Fig. 1 A) and this was not correlated with the mRNA expression data. The CuZn-SOD mRNA only represents 6% of the total mRNA in photosynthetic cells ( Fig. 2 M), and this SOD is not expressed in vascular tissues. However, it should be mentioned that the transcription analyses were done on the basis of the partial cDNA obtained for CuZn-SOD and, therefore, a strict relationship between the CuZn-SOD activity and its RNA expression cannot be established.
In olive leaves, CuZn-SOD was localized in chloroplasts, cytosol, nuclei and peroxisomes. These subcellular localizations have also been reported for CuZn-SODs in other plants species (Kanematsu and Asada 1991 , Bueno et al. 1995 , Ogawa et al. 1995 , Ogawa et al. 1996 , Sandalio et al. 1997 , Corpas et al. 1998 , Kernodle and Scandalios 2001 ). The occurrence of CuZn-SOD in the nucleus has been reported in spinach leaves (Ogawa et al. 1995 ), but the presence of CuZn-SOD in nuclear crystalline inclusions of olive leaves is most unusual in plant cells. The function of CuZn-SOD in the nucleus could be the protection of DNA against superoxide-derived oxidative damage. It is interesting to note the absence of CuZn-SOD in the vascular tissue and extracellular space of olive leaves, because in other plant species this is the only SOD isozyme present there (Ogawa et al. 1996 , Schinkel et al. 1998 , Karlsson et al. 2005 ).
Mn-SOD is the only SOD expressed in vascular tissues
The gene expression pattern of Mn-SOD in olive leaves was different from that of Fe-SOD. In photosynthetic cells (palisade and spongy tissues), transcripts of Mn-SOD, CuZn-SOD and Fe- SOD represent 21, 6 and 73% of the total SOD transcripts, respectively ( Fig. 2 M). However, Mn-SOD mRNA was the only SOD transcript which was present in all cell types analyzed, including phloem and xylem cells. At the cellular level, the immunolocalization of Mn-SOD was in agreement with the gene expression data because the green fluorescence was present in all cell types of leaves. At the subcellular level, Mn-SOD was localized in mitochondria where it could be involved in the control of superoxide radicals generated in the mitochondrial electron transport chain (Bowler et al. 1994 , Jiménez et al. 1997 , Alscher et al. 2002 ). However, in leaves from other plant species, such as peas, Mn-SOD is present in both mitochondria and peroxisomes, and the isozymes of both organelles are differentially expressed during leaf senescence (del Río et al. 2003 ).
On the other hand, the presence of Mn-SOD (gene and protein) in olive vascular tissue is new because so far only CuZn-SODs had been demonstrated to have an apoplastic or extracellular localization (Ogawa et al. 1996 , Schinkel et al. 1998 ). In this respect, the Mn-SOD of vascular tissue could be involved in the biosynthesis of lignin in olive leaves, as was proposed for CuZn-SOD in spinach hypocotyls (Ogawa et al. 1996 , Ogawa et al. 1997 ), but Mn-SOD could also participate in the antioxidant defense of vascular tissue. In phloem sap of cucumber, the presence of several antioxidative enzymes, including CuZn-SOD, monodehydroascorbate reductase and peroxidase, has been demonstrated recently (Walz et al. 2002 , Walz et al. 2004 ).
The presence of the gaseous radical nitric oxide (NO ·) in the vascular tissues of roots, stems and leaves has been reported (Corpas et al. 2004 , Corpas et al. 2006 ) and in these tissues NO could be involved in the cell wall differentiation and xylem lignification (Ferrer and Ros-Barceló 1999 , Gabaldón et al. 2005 ). It is known that NO· can react with 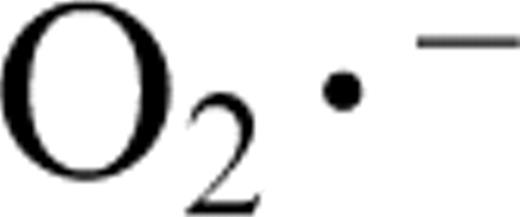 radicals to form the powerful oxidant peroxynitrite (ONOO − ) (Radi 2004 ). The accumulation of superoxide radicals in vascular tissue of olive leaves ( Fig. 4 C) suggests that the Mn-SOD present in that tissue could have a regulatory role in the formation of peroxynitrite that could be used in the programmed cell death (PCD) reactions of the xylogenesis process (Gabaldón et al. 2005 ).
radicals to form the powerful oxidant peroxynitrite (ONOO − ) (Radi 2004 ). The accumulation of superoxide radicals in vascular tissue of olive leaves ( Fig. 4 C) suggests that the Mn-SOD present in that tissue could have a regulatory role in the formation of peroxynitrite that could be used in the programmed cell death (PCD) reactions of the xylogenesis process (Gabaldón et al. 2005 ).
In summary, the results obtained in this work show that in olive leaves the Fe-SOD and CuZn-SOD genes were only expressed in photosynthetic cells, and the maximum expression corresponded to Fe-SOD. On the other hand, the Mn-SOD gene was expressed in all cell types and this was the only SOD present in vascular tissues where it could perform a specific function. This indicates that in olive leaves each SOD isozyme has a different gene expression pattern depending on the cell type, and strongly suggests that each isozyme could have a specific function depending on its cellular and subcellular localization.
Materials and Methods
Plant material and growth conditions
Experiments were carried out with olive seeds ( Olea europaea L., cv. Manzanillo) provided by the World Bank of Germoplasm, Departamento de Olivicultura y Arboricultura Frutal, CIFA, Córdoba. Seedlings were grown in the dark at 13°C for 15 d in an embryo medium and then were transferred to a DKW medium (Driver and Kumiyuki 1984). These cultures were grown in a temperature-controlled chamber at 25°C for another 51 d, with a 16 h photoperiod under Sylvania Gro-Lux (Sylvania, Westfield, IN, USA) lighting with a photon flux density of 130–140 µmol m −2 s −1 . Then, plants were harvested and leaves used for the preparation of crude extracts and RNA extraction.
Crude extracts of olive leaves
All operations were performed at 0–4°C. Leaves were ground to a powder in a mortar with liquid nitrogen, and were suspended in 100 mM Tris–HCl buffer, pH 8.0 (1/4; w/v) containing 1 mM EDTA, 1 mM EGTA, 0.1 M NaCl, 7% (w/v) polyvinyl polypyrrolidone (PVPP), 15 mM dithiothreitol (DTT), 15 mM phenylmethylsulfonyl fluoride (PMSF) and a commercial cocktail of protease inhibitors (AEBSF, 1,10-phenantroline, pepstatin A, leupeptin, bestatin and E-64) (Sigma, St. Louis, MO, USA). Homogenates were filtered through one layer of miracloth (Calbiochem, San Diego, CA, USA) and centrifuged at 3,000 g for 5 min (Valderrama et al. 2006 ). For SOD activity and Western blots, the supernatants were passed through NAP-10 columns (Amersham-Biosciences, Piscataway, NJ, USA) that were equilibrated with 10 mM Na-phosphate buffer, pH 6.8, and eluted with 10 mM K-phosphate buffer, pH 7.8.
Production of antibodies to Fe-SOD and Mn-SOD
The service of polyclonal antibody production from a selected peptide of Sigma-Genosys (Cambridge, UK) was used to obtain the antibody against Fe-SOD. A peptide of 14 amino acids from the C-terminus of the deduced amino acid sequence of the N. plumbaginifolia Fe-SOD (accession No. P22302) was selected. The peptide was SWEAVSSRLKAATA which corresponds to the residues between Ser189 and Ala202. This peptide is conserved among different plant Fe-SODs, is hydrophilic and contains one predicted β-turn. The selected peptide was conjugated to a carrier protein, the keyhole limpet hemocyanin (KLH) which is derived from marine molluscs via the thiol group of a cysteine residue added to the N-terminus of the selected peptide using MBS (maleimidobenzoyl- N -hydroxysuccinimide ester) chemistry. Thus the construction KLH-[C]-SWEAVSSRLKAATA was used for the immunization of two rabbits according to the protocol of six immunizations per rabbit (Sigma-Genosys, Cambridge, UK). For the preparation of the antibody to pea leaf Mn-SOD, the enzyme was purified to homogeneity from pea ( P. sativum L.) leaves, as described by del Río et al. ( 1983 ), and the antibodies were prepared in New Zealand rabbits by Immune Systems Ltd (Paignton, UK). The IgG fraction of serum was isolated using an Econ-Pac Serum IgG purification kit (Bio-Rad Laboratories, Hercules, CA, USA). Both antisera were evaluated by Western blot using the pre-immune sera as negative control.
Enzyme activity, electrophoretic methods and Western blot analyses
Total SOD activity (EC 1.15.1.1) was assayed according to the ferricytochrome c method of McCord and Fridovich ( 1969 ). SOD isozymes were separated by native PAGE on 10% acrylamide gels and visualized by a photochemical method (Beauchamp and Fridovich 1971 ). Quantification of the bands was performed using a Gel Doc system (Bio-Rad Laboratories, Hercules, CA, USA) coupled with a high sensitive CCD camera. Band intensity was expressed as relative transmittance units. Polypeptides were separated by 15% SDS–PAGE as described by Corpas et al. ( 1998 ). For immunodetection, polyclonal antibodies against cytosolic CuZn-SOD from spinach (1 : 3,000 dilution) (Kanematsu and Asada 1989), pea Mn-SOD (1 : 2,000 dilution) and Fe-SOD (1 : 2,000 dilution) were used with an enhanced chemiluminescence kit (ECL-PLUS, Amersham Pharmarcia Biotech) and were detected with a photographic film (Hyperfilm; Amersham Pharmarcia Biotech).
Other assays
The protein concentration of samples was determined by the method of Bradford ( 1976 ) with bovine serum albumin (BSA) as standard.
RNA isolation and partial cDNA cloning of the three SOD isozymes
Total RNA was isolated from olive leaves with the Trizol® reagent (Gibco-BRL, Life Technologies, Paisley, UK) as described in the manufacturer's manual, and RNA was quantified spectrophotometrically. First-strand cDNA was synthesized from 1 µg of total RNA primed with 3.2 µg of random primer (pdN) 6 and AMV reverse transcriptase, using the first-strand cDNA synthesis kit (Roche, Basel, Switzerland). Using SOD sequences from the data bank, specific oligonucleotides in conserved domains of each SOD isoform were designed (see Table 1 ) and by RT–PCR the following partial cDNAs were obtained: 435 bp for Mn-SOD (accession No. AF427107); 435 bp for Fe-SOD (accession No. AY168776); and 312 bp for CuZn-SOD (accession No. AF426829).
Laser capture microdissection (LCM)
An LCM system (P.A.L.M. Microlaser Technologies), connected to an Olympus IX-70 microscope, was used. By means of a cryostat (2800 Frigocut E, Reichert-Jung, Vienna, Austria), a series of olive leaf sections, 14–16 µm thick, were obtained. Cells from palisade and spongy mesophyll, and vascular tissue (xylem and phloem) were selected from 5–7 leaf sections. To confirm that the samples were representative, this procedure was repeated at least seven times for each tissue using 3–4 leaves from different plants each time. These microdissected cells were catapulted on an Eppendorf tube which contained 3 µl of mineral oil and 5 µl of 10 mM Tris–HCl buffer, pH 8.3, with 50 mM KCl and 50 U of RNase inhibitors (Roche). These samples were previously treated with a thermal shock of 95°C for 5 min, and cooled on ice, and then used directly for the first-strand cDNA synthesis, with the same kit mentioned above.
Real-time quantitative RT–PCR
Real-time quantitative RT–PCR was performed in 20 µl of reaction mixture, composed of 1 µl of different cDNAs and master mix IQ™ SYBR® Green Supermix with a final concentration of 0.5 U of hot-start iTaq™ DNA Polymerase (Bio-Rad Laboratories, Hercules, CA, USA), 16 mM Tris–HCl buffer, pH 8.4, 20 mM KCl, 0.16 mM each dNTPs, 2.4 mM MgCl 2 , 0.5 µM gene-specific primers (see Table 1 ) and SYBR Green I, 8 nM fluorescein, using a iCycler iQ system (Bio-Rad). Amplifications were performed under the following conditions: initial polymerase activation: 95°C, 4 min; then 40 cycles at 95°C, 30 s; 58°C, 30 s; 72°C, 1 min and a final extension at 72°C for 7 min. The primers (see Table 1 ) were designed to anneal at different exons, at distances large enough to avoid the appearance of false-positive bands caused by co-amplification of contaminating DNA, in the partial cDNA previously obtained. An internal control of 18S rRNA (accession No. L49289) was used for the normalization of results. For microdisected samples, identical conditions of real-time quantitative RT–PCR were used, but with 50 cycles. SOD mRNA contents were measured from at least four batches of cells, in three replicates each. In all experiments, controls without reverse transcriptase were included.
Electron microscopy and immunocytochemistry
Olive leaf segments (1 mm 2 ) were fixed, dehydrated and embedded in LR White resin as previously described by Corpas et al. ( 1998 ). Gold sections were mounted on nickel grids and were incubated for 1.5 h in blocking solution composed of 10 mM Tris–HCl buffer (pH 7.6), 0.9% (w/v) NaCl, 0.05% (v/v) Tween-20 and 0.02% (w/v) NaN 3 (TBST) containing 5% (w/v) fetal calf serum. The sections were then incubated for 2 h with antibodies against the following SODs: pea Mn-SOD (1 : 500 dilution), watermelon CuZn-SOD (1 : 300 dilution) (Bueno et al. 1995 ) and Fe-SOD (1 : 2,000 dilution). Pre-immune serum was used as control. The sections were then incubated for 1 h with goat anti-rabbit IgG conjugated to 15 nm gold particles diluted 1/40 in TBST buffer. Sections were post-stained in 2% (v/v) uranyl acetate for 3 min and examined in a Zeiss (Jena, Germany) EM 10C transmission electron microscope.
Immunohistochemical localization of Mn-SOD by CLSM
Olive leaves from plants grown under optimal conditions were cut into 4–5 mm pieces and fixed in 4% (w/v) p -formaldehyde in 0.1 M phosphate buffer, pH 7.4 (PB), for 3 h at room temperature. Then they were cryoprotected by immersion in 30% (w/v) sucrose in PB overnight at 4°C. Serial sections, 60 µm thick, were obtained by means of a cryostat (2800 Frigocut E, Reichert-Jung, Vienna, Austria). Free floating sections were incubated overnight at room temperature with an antibody to pea Mn-SOD diluted 1 : 200 in 5 mM Tris–HCl buffer, pH 7.6, 0.9% (w/v) NaCl, containing 0.05% (w/v) sodium azide, 0.1% (w/v) BSA and 0.1% (v/v) Triton X-100 (TBSA-BSAT). After several washes with TBSA-BSAT, sections were incubated with biotinylated goat anti-rabbit IgG (Pierce, Rockford, IL, USA), diluted 1 : 1,000 in TBSA-BSAT, for 1 h at room temperature. Then, sections were washed again and incubated with Cy2-streptavidin (Amersham Biosciences, Piscataway, NJ, USA), diluted 1 : 1,000 in TBSA-BSAT, for 1.5 h at room temperature. Controls for background staining, which was usually negligible, were performed by replacing the corresponding primary antiserum by pre-immune serum. Leaf sections were examined with a confocal laser scanning microscope (Leica TCS SL, Leica Microsystems, Wetzlar, Germany).
Detection of superoxide radicals by CLSM
Detection of superoxide radicals ( 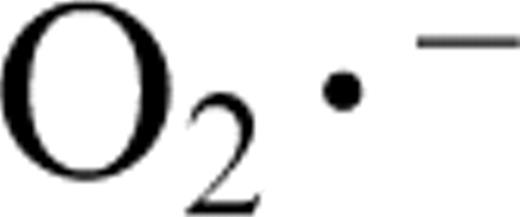 ) in olive leaf sections was carried out using the fluorophore DHE, according to the method described by Rodríguez-Serrano et al. ( 2006 ). Olive leaf segments of approximately 25 mm 2 were incubated for 1 h at 25°C, in darkness, with 10 µM DHE prepared in 5 mM Tris–HCl buffer, pH 7.4, and samples were washed twice with the same buffer for 15 min each. After washing, leaf sections were embedded in a mixture of 15% acrylamide–bisacrylamide stock solution, as described by Peinado et al. ( 2000 ), and 100 mm thick sections, as indicated by the vibratome scale, were cut under 10 mM phosphate-buffered saline (PBS). Sections were then soaked in glycerol : PBS (containing azide) (1 : 1 v/v) and mounted in the same medium for examination with a confocal laser scanning microscope system (Leica TCS SL, Leica Microsystems, Wetzlar, Germany), using standard filters and collection modalities for DHE green fluorescence (λ excitation 488 nm; λ emission 520 nm) and Chl autofluorescence (Chl a and b , λ excitation 429 and 450 nm, respectively; λ emission 650 and 670 nm, respectively) as orange. As a negative control, leaf sections were pre-incubated for 1 h at 25°C, in darkness, with 1 mM tetramethylpiperidinooxy (TMP), a scavenger of superoxide radicals, and then for 1 h at 25°C with 10 µM DHE (Rodríguez-Serrano et al. 2006 ).
) in olive leaf sections was carried out using the fluorophore DHE, according to the method described by Rodríguez-Serrano et al. ( 2006 ). Olive leaf segments of approximately 25 mm 2 were incubated for 1 h at 25°C, in darkness, with 10 µM DHE prepared in 5 mM Tris–HCl buffer, pH 7.4, and samples were washed twice with the same buffer for 15 min each. After washing, leaf sections were embedded in a mixture of 15% acrylamide–bisacrylamide stock solution, as described by Peinado et al. ( 2000 ), and 100 mm thick sections, as indicated by the vibratome scale, were cut under 10 mM phosphate-buffered saline (PBS). Sections were then soaked in glycerol : PBS (containing azide) (1 : 1 v/v) and mounted in the same medium for examination with a confocal laser scanning microscope system (Leica TCS SL, Leica Microsystems, Wetzlar, Germany), using standard filters and collection modalities for DHE green fluorescence (λ excitation 488 nm; λ emission 520 nm) and Chl autofluorescence (Chl a and b , λ excitation 429 and 450 nm, respectively; λ emission 650 and 670 nm, respectively) as orange. As a negative control, leaf sections were pre-incubated for 1 h at 25°C, in darkness, with 1 mM tetramethylpiperidinooxy (TMP), a scavenger of superoxide radicals, and then for 1 h at 25°C with 10 µM DHE (Rodríguez-Serrano et al. 2006 ).
Acknowledgments
M.R.-S. and M.C. acknowledge PhD fellowships from the Ministry of Education and Science and University of Jaén, respectively. This work was supported by a grant from the CICYT, Ministry of Science and Technology (AGL2003-05524), Universidad de Jaén (OA/2/2004) and Junta de Andalucía (groups CVI 0286 and CVI 0192). Olive seeds were kindly provided by the Departamento de Olivicultura y Arboricultura Frutal, Banco de Germoplasma Mundial, CIFA, Córdoba. The valuable help of Dr Araceli Barceló (CIFA, Churriana, Málaga) in setting up the in vitro culture conditions of olive plants is appreciated. We specially acknowledge Professor Kozi Asada (Fukuyama University, Japan) for his generous donation of the antibody against spinach CuZn-SOD. The valuable technical help of Miss Emperatriz Córdoba for the maintenance of in vitro plant cultures is also appreciated. Confocal laser scanning microscopy analyses were carried out at the Technical Services of the University of Jaén, and special thanks are given to Miss Nieves de la Casa-Adán for her technical assistance. The electron microscopy assays were carried out at the Centre of Scientific Instrumentation of the University of Granada.
References
Abbreviations:
- BSA
bovine serum albumin
- CLSM
confocal laser scanning microscopy
- DHE
dihydroethidium
- LCM
laser capture microdissection
- RT–PCR
reverse transcription–PCR
- SOD
superoxide dismutase
Sequence data from this article have been deposited in the EMBL/GenBank data libraries under accession numbers AF427107 for Mn-SOD, AY168776 for Fe-SOD and AF426829 for CuZn-SOD.