-
PDF
- Split View
-
Views
-
Cite
Cite
Stephan R. Orth, Torsten Schroeder, Eberhard Ritz, Paolo Ferrari, Effects of smoking on renal function in patients with type 1 and type 2 diabetes mellitus, Nephrology Dialysis Transplantation, Volume 20, Issue 11, November 2005, Pages 2414–2419, https://doi.org/10.1093/ndt/gfi022
Close - Share Icon Share
Abstract
Background. Smoking increases the risk of end-stage renal failure in patients with primary renal disease. Whether and to what extent smoking affects the kidneys in diabetic patients with normal renal function and variable degrees of proteinuria has not been fully studied.
Methods. We followed 185 patients with type 1 or 2 diabetes mellitus and with or without signs of overt renal disease for at least 3 years, median 5.1 (3–6.8) years. Each patient had a baseline visit and at least four follow-up visits (average 4.8±0.3). Cases were patients who were smoking (n = 44) at the time the survey was started. Controls were patients who had never smoked (n = 141). Glomerular filtration rate (GFR) was estimated using the MDRD formula. Multiple logistic regression was used to correct for confounding factors.
Results. At baseline, smokers were younger (47±14 vs 54±16 years, P<0.01), and had a lower GFR (95±26 ml/min) than non-smokers (107±33 ml/min, P<0.05). Mean GFR remained constant during follow-up in non-smokers (106±31 ml/min), but decreased significantly in smokers (83±22 ml/min, P<0.0001), and this relationship persisted when adjusted for retinopathy, glycaemic control, age, body habitus, ACE-inhibitor treatment, blood pressure control or severity of proteinuria. The effect of smoking on GFR decline was stronger in patients with type 1 diabetes or male gender.
Conclusions. Cigarette smoking causes a decrease in GFR in diabetic patients with normal or near-normal renal function, independent of confounding factors including severity of proteinuria. The latter finding suggests a mechanism independent of glomerular damage.
Introduction
The role of smoking as a risk factor for renal disease is increasingly appreciated [1]. In retrospective case-control studies, smoking has been associated with renal impairment in subjects with biopsy-proven primary kidney diseases with or without proteinuria [2,3]. According to some studies, smoking is a risk factor for the development and progression of diabetic nephropathy (see [1] for references), despite angiotensin-converting enzyme inhibition for blood pressure control [4]. However, these adverse renal effects of cigarette smoking in diabetic patients have not been reported in all studies [5,6], suggesting that the relationship between smoking and nephropathy is more complex. Possible differences between type 1 and type 2 diabetes mellitus, small sample size, stage of diabetic nephropathy (with more advanced stages presumably progressing rapidly independently of a noticeable contributory effect of smoking), quantity of smoking or insufficient follow-up may account for some of the inconsistencies (see [1] for references). Some studies found a significant effect of smoking on the rate of decline in glomerular filtration rate (GFR) only with heavier cigarette smoking [4,7]. However, the study with the most valid determination of GFR (using 51Cr-EDTA), the largest population of patients with overt diabetic nephropathy and longest follow-up showed no effect of the quantity of smoking when adjusted for blood pressure or glycaemic control in young patients with type 1 diabetes [6].
The effect of smoking on renal structure may also be somewhat heterogeneous. Morphometric analysis of kidney biopsies showed that cigarette smoking has an adverse effect on glomerular structure in type 2 diabetes [7]. Other studies found, however, that the major effect of cigarette smoking concerned the vessels with pronounced luminal narrowing [8].
The aim of the present observational study in a diabetes outpatient clinic was to further examine the relation between cigarette smoking and GFR as well as proteinuria in a population of patients with type 1 and type 2 diabetes, taking into consideration a number of known confounding factors.
Subjects and methods
In 1998 a cohort of 289 patients with either type 1 or type 2 diabetes mellitus attending the outpatient clinic of the Department of Internal Medicine, Ruperto Carola University, Heidelberg, was screened for the purpose of longitudinally assessing the effect of cigarette smoking on renal function. Recruitment of patients was done during a screening phase of 4 months including all patients with micro- or macroalbuminuria attending the outpatient clinic. No intervention was performed and recommendations to discontinue smoking were at the discretion of the treating physicians. Patients with renal impairment corresponding to a GFR <60 ml/min at screening were excluded from follow-up. From the remaining patients clinical and biochemical data from their yearly clinic reviews were collected. In the interval, patient management was under the supervision of their usual primary care physician. Each patient had a baseline visit and at least four follow-up visits (average 4.8±0.3).
The screening visit involved an initial interview, followed by a physical examination that included standard anthropometry and blood pressure measurements, collection of a fasting blood specimen and spot urine specimen, and an administered questionnaire with questions related to smoking habits. Smoking status was self-reported as current smoker (smoking at least daily), ex-smoker or never-smoker. Because definition and impact of smoking in patients who quit days to years before the inclusion in this survey would be difficult to assess, ex-smokers were not included in the analysis. Lifetime consumption was estimated in pack-years.
Estimated GFR was calculated using the Modification of Diet in Renal Disease (MDRD) study equation [9]. No adjustment for race was necessary as all subjects were Caucasians. Microalbuminuria was not tested for systematically. Proteinuria (>0.15 g/24 h) was categorized as either absence or presence of macroproteinuria (1.0–4.5 g/24 h) and absence or presence of severe proteinuria (>4.5 g/day).
Blood pressure was measured in a seated position after the subject had rested for at least 5 min, using the first and fifth Korotkoff sounds recorded to the nearest 2 mmHg. Blood pressure was measured using a standard mercury sphygmomanometer. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg or the use of medication for hypertension regardless of the blood pressure. Body mass index was calculated from weight and height measurements.
Serum creatinine and urine protein and creatinine levels were measured using the Olympus AU600 autoanalyzer (Olympus Optica Co., Shizuoka, Japan). Total cholesterol, and triglyceride levels were determined on the blood specimen collected after an overnight fast and measured on the Olympus AU 600 analyzer.
The study was approved by the Ethical Committee of the Medical Faculty of Heidelberg University, Germany.
Statistical methods
Statistical analysis was performed with the Systat for Windows software package version 10 (SPSS Inc, Chicago, IL). The last visit was defined as the last observation carried forward. Results are in mean±standard deviation for continuous variables and number and percentage for categorical variables. Differences between subjects were tested by non-parametric Mann–Whitney U-test statistic for continuous data and Fisher's exact test for categorical data. The effect of potential confounding factors was examined. These included demographic (age and sex), clinical (body mass index, systolic and diastolic blood pressure, diabetes type, proteinuria, retinopathy, use of ACE inhibitors or insulin), and biochemical (HbA1c, fasting total cholesterol and triglyceride levels) covariates. Evidence of effect modification by covariates on smoking was examined for both renal impairment and proteinuria and considered to exist if P for the interaction term was <0.10. Final estimates of the association between smoking and renal impairment were computed and adjusted for all potential confounding covariates. The odds ratios (OR) and 95% confidence interval (95%CI) for a 20% decrease in estimated GFR at final visit compared to baseline in smokers vs non-smokers were analysed for the categorical variables gender, retinopathy, diabetes type, proteinuria and ACE inhibitor therapy.
Results
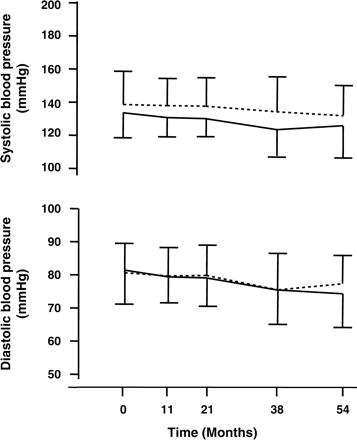
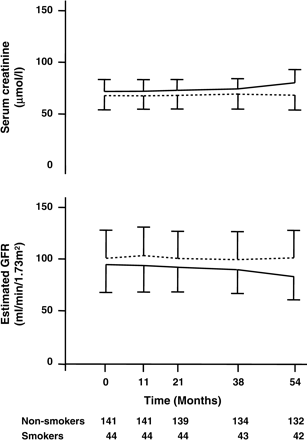
Of the 289 patients screened initially, 37 had a GFR<60 ml/min and 67 were previous smokers who quit before the screening date. Thus, follow-up data were collected on 185 diabetic patients. Table 1 summarizes the main baseline characteristics of these diabetic patients with normal or mildly decreased GFR according to smoking status. Approximately 60% of the patients had type 1 diabetes. Younger patients with diabetes were more likely to be active smokers (P<0.01). Estimated baseline GFR was lower in smokers (95±26 ml/min) than non-smokers (107±33 ml/min, P<0.05). There were no differences with regard to duration of diabetes, other micro- or macrovascular complications, insulin treatment or metabolic control (as assessed by HbA1c). Blood pressure control at baseline was good (135/80±14/10 vs 138/79±22/11 mmHg, smokers vs non-smokers, respectively) and improved (P<0.0001) throughout the entire study period, with non-smokers showing a tendency for higher values than smokers (P = NS; Figure 1). From baseline to last visit systolic and diastolic blood pressure decreased by −8±10 and −7±5 mmHg in smokers (P<0.01 systolic and P<0.05 diastolic) and by −6±9 and −7±6 mmHg in non-smokers (P<0.05 systolic and P<0.001 diastolic), respectively. This difference was stronger for systolic (P<0.01) but not diastolic (P = NS) blood pressure in smokers compared to non-smokers. During the observation period HbA1c did not change significantly from baseline (Table 1) and was comparable up to the last visit in smokers (7.6±0.9%) and non-smokers (7.6±1.3%). Proteinuria increased from baseline to the end of the study from 0.47±0.94 to 0.54±1.82 g/24 h in non-smokers and from 0.36±0.51 to 0.44±0.48 g/24 h in smokers (P = NS for differences between groups). GFR remained stable during follow-up in non-smokers, but decreased significantly in smokers (F-ratio = 45.1, P<0.0001 for the effect of time in smokers versus non-smokers) (Figure 2). At the final visit non-smokers had a GFR of 106±31 ml/min, while in smokers it was 83±22 ml/min. Over the observation period estimated GFR declined by 5.2±9.8 ml/min in female smokers and 2.2±11.3 ml/min in women who never smoked, but this difference did not reach statistical significance. In addition, serum creatinine values were used to evaluate the effect of smoking on renal function. The increase in serum creatinine was also significantly higher in smokers than non-smokers (F-ratio = 35.4, P<0.0001), although general linear model analysis also revealed a time-effect on this variable (F-ratio = 4.5, P = 0.034) (Figure 2).
Baseline characteristics of the 185 diabetic patients by smoking status
| . | Never smokers . | Current smokers . | P-value . |
|---|---|---|---|
| N | 141 | 44 | |
| Age | 54±16 | 47±14 | <0.01 |
| Gender (M/F) | 54/87 | 26/18 | <0.01 |
| BMI (kg/m2) | 26.0±3.3 | 25.3±3.8 | 0.27 |
| Diabetes type 1 | 83 (59) | 31 (70) | 0.21 |
| Insulin therapy | 126 (89) | 42 (95) | 0.25 |
| Duration (years) | 18±4 | 17±9 | 0.52 |
| HbA1c (%) | 7.4±1.0 | 7.7±1.2 | 0.17 |
| Retinopathy | 53 (38) | 13 (30) | 0.59 |
| Macroproteinuria (g/24 h) | 102 (72) | 38 (86) | 0.09 |
| 0.15–1.0 | 60 (42) | 22 (50) | 0.49 |
| 1.0–4.5 | 40 (28) | 16 (36) | 0.57 |
| >4.5 | 2 (1) | 0 | 0.68 |
| MDRD GFR (ml/min) | 107±33 | 95±26 | <0.05 |
| BP >140/90 mmHg | 87 (62) | 29 (66) | 0.56 |
| Blood pressure (mmHg) | |||
| Systolic | 138±22 | 135±14 | 0.14 |
| Diastolic | 79±11 | 80±10 | 0.98 |
| ACE-inhibitors | 61 (43) | 14 (32) | 0.16 |
| CAD | 13 (9) | 2 (4) | 0.54 |
| Stroke | 0 | 0 | 1.00 |
| PVD | 13 (9) | 5 (11) | 0.56 |
| . | Never smokers . | Current smokers . | P-value . |
|---|---|---|---|
| N | 141 | 44 | |
| Age | 54±16 | 47±14 | <0.01 |
| Gender (M/F) | 54/87 | 26/18 | <0.01 |
| BMI (kg/m2) | 26.0±3.3 | 25.3±3.8 | 0.27 |
| Diabetes type 1 | 83 (59) | 31 (70) | 0.21 |
| Insulin therapy | 126 (89) | 42 (95) | 0.25 |
| Duration (years) | 18±4 | 17±9 | 0.52 |
| HbA1c (%) | 7.4±1.0 | 7.7±1.2 | 0.17 |
| Retinopathy | 53 (38) | 13 (30) | 0.59 |
| Macroproteinuria (g/24 h) | 102 (72) | 38 (86) | 0.09 |
| 0.15–1.0 | 60 (42) | 22 (50) | 0.49 |
| 1.0–4.5 | 40 (28) | 16 (36) | 0.57 |
| >4.5 | 2 (1) | 0 | 0.68 |
| MDRD GFR (ml/min) | 107±33 | 95±26 | <0.05 |
| BP >140/90 mmHg | 87 (62) | 29 (66) | 0.56 |
| Blood pressure (mmHg) | |||
| Systolic | 138±22 | 135±14 | 0.14 |
| Diastolic | 79±11 | 80±10 | 0.98 |
| ACE-inhibitors | 61 (43) | 14 (32) | 0.16 |
| CAD | 13 (9) | 2 (4) | 0.54 |
| Stroke | 0 | 0 | 1.00 |
| PVD | 13 (9) | 5 (11) | 0.56 |
Results are given as mean±SD or numbers and (%). P-values are for comparison between current smokers and non-smokers. BMI = body mass index, CAD = coronary artery disease (myocardial infarction, PTCA or CABG), PVD = peripheral vascular disease.
Baseline characteristics of the 185 diabetic patients by smoking status
| . | Never smokers . | Current smokers . | P-value . |
|---|---|---|---|
| N | 141 | 44 | |
| Age | 54±16 | 47±14 | <0.01 |
| Gender (M/F) | 54/87 | 26/18 | <0.01 |
| BMI (kg/m2) | 26.0±3.3 | 25.3±3.8 | 0.27 |
| Diabetes type 1 | 83 (59) | 31 (70) | 0.21 |
| Insulin therapy | 126 (89) | 42 (95) | 0.25 |
| Duration (years) | 18±4 | 17±9 | 0.52 |
| HbA1c (%) | 7.4±1.0 | 7.7±1.2 | 0.17 |
| Retinopathy | 53 (38) | 13 (30) | 0.59 |
| Macroproteinuria (g/24 h) | 102 (72) | 38 (86) | 0.09 |
| 0.15–1.0 | 60 (42) | 22 (50) | 0.49 |
| 1.0–4.5 | 40 (28) | 16 (36) | 0.57 |
| >4.5 | 2 (1) | 0 | 0.68 |
| MDRD GFR (ml/min) | 107±33 | 95±26 | <0.05 |
| BP >140/90 mmHg | 87 (62) | 29 (66) | 0.56 |
| Blood pressure (mmHg) | |||
| Systolic | 138±22 | 135±14 | 0.14 |
| Diastolic | 79±11 | 80±10 | 0.98 |
| ACE-inhibitors | 61 (43) | 14 (32) | 0.16 |
| CAD | 13 (9) | 2 (4) | 0.54 |
| Stroke | 0 | 0 | 1.00 |
| PVD | 13 (9) | 5 (11) | 0.56 |
| . | Never smokers . | Current smokers . | P-value . |
|---|---|---|---|
| N | 141 | 44 | |
| Age | 54±16 | 47±14 | <0.01 |
| Gender (M/F) | 54/87 | 26/18 | <0.01 |
| BMI (kg/m2) | 26.0±3.3 | 25.3±3.8 | 0.27 |
| Diabetes type 1 | 83 (59) | 31 (70) | 0.21 |
| Insulin therapy | 126 (89) | 42 (95) | 0.25 |
| Duration (years) | 18±4 | 17±9 | 0.52 |
| HbA1c (%) | 7.4±1.0 | 7.7±1.2 | 0.17 |
| Retinopathy | 53 (38) | 13 (30) | 0.59 |
| Macroproteinuria (g/24 h) | 102 (72) | 38 (86) | 0.09 |
| 0.15–1.0 | 60 (42) | 22 (50) | 0.49 |
| 1.0–4.5 | 40 (28) | 16 (36) | 0.57 |
| >4.5 | 2 (1) | 0 | 0.68 |
| MDRD GFR (ml/min) | 107±33 | 95±26 | <0.05 |
| BP >140/90 mmHg | 87 (62) | 29 (66) | 0.56 |
| Blood pressure (mmHg) | |||
| Systolic | 138±22 | 135±14 | 0.14 |
| Diastolic | 79±11 | 80±10 | 0.98 |
| ACE-inhibitors | 61 (43) | 14 (32) | 0.16 |
| CAD | 13 (9) | 2 (4) | 0.54 |
| Stroke | 0 | 0 | 1.00 |
| PVD | 13 (9) | 5 (11) | 0.56 |
Results are given as mean±SD or numbers and (%). P-values are for comparison between current smokers and non-smokers. BMI = body mass index, CAD = coronary artery disease (myocardial infarction, PTCA or CABG), PVD = peripheral vascular disease.
Mean (±SD) systolic and diastolic blood pressure in diabetic smokers (solid line, n = 44) and non-smokers (dotted line, n = 141) during the 4.5 year follow-up.
Mean (±SD) serum creatinine and estimated GFR in diabetic smokers (solid line) and non-smokers (dotted line) during the 4.5 year follow-up. Smokers vs non-smokers F-ratio 35.5 (P<0.0001) for serum creatinine and F-ratio 45.1 (P<0.0001) for estimated GFR.
At the final visit a decrease in GFR from baseline of 20% or greater was found in 16 (11%) of non-smokers and in 10 (23%) of smokers (P<0.01). The OR for a 20% decrease in estimated GFR in smokers was 2.52 (95%CI 1.06–5.99, P<0.01). An estimate of renal function by the Cockcroft–Gault formula equation (data not shown) did not significantly alter our observations using the MDRD formula.
When adjustment for diabetes type or control, retinopathy, age, body mass index, ACE-inhibitor treatment, blood pressure control or the severity of proteinuria was performed, this relation persisted (F-ratio = 65.9, P<0.0001). The number of pack years strongly influenced the loss of GFR (F-ratio 16.1, P<0.001) thereby reducing the effect of time on the loss of renal function (F-ratio = 2.8, P<0.01). Gender and diabetes type independently influenced the course of renal function in smokers compared to non-smokers. The odds ratio for a 20% decrease in estimated GFR in smokers compared to non-smokers of male gender was 5.32 (95% CI 1.49–18.9, P<0.05) and in type 1 diabetes it was 4.49 (95%CI 1.36–14.7, P<0.05), while retinopathy, severity of proteinuria or ACE inhibitor therapy did not affect the risk of progression (Table 2).
Odds ratios (OR) and 95% confidence interval (95%CI) for a 20% decrease in renal function over the observation period according to several categorical variables
| . | OR . | 95%CI . | P . |
|---|---|---|---|
| Diabetes type | |||
| 1 | 4.49 | 1.36–14.7 | <0.05 |
| 2 | 1.25 | 0.31–4.94 | 0.93 |
| Gender | |||
| Male | 5.32 | 1.49–18.9 | <0.05 |
| Female | 0.91 | 0.29–3.02 | 0.82 |
| Retinopathy | |||
| No | 1.92 | 0.69–5.28 | 0.33 |
| Yes | 3.00 | 0.64–13.9 | 0.37 |
| Proteinuria | |||
| No | 1.14 | 0.53–5.07 | 0.60 |
| Yes | 3.66 | 0.97–13.7 | 0.12 |
| ACE inhibitor | |||
| No | 2.66 | 0.81–7.80 | 0.14 |
| Yes | 2.48 | 0.61–10.1 | 0.42 |
| . | OR . | 95%CI . | P . |
|---|---|---|---|
| Diabetes type | |||
| 1 | 4.49 | 1.36–14.7 | <0.05 |
| 2 | 1.25 | 0.31–4.94 | 0.93 |
| Gender | |||
| Male | 5.32 | 1.49–18.9 | <0.05 |
| Female | 0.91 | 0.29–3.02 | 0.82 |
| Retinopathy | |||
| No | 1.92 | 0.69–5.28 | 0.33 |
| Yes | 3.00 | 0.64–13.9 | 0.37 |
| Proteinuria | |||
| No | 1.14 | 0.53–5.07 | 0.60 |
| Yes | 3.66 | 0.97–13.7 | 0.12 |
| ACE inhibitor | |||
| No | 2.66 | 0.81–7.80 | 0.14 |
| Yes | 2.48 | 0.61–10.1 | 0.42 |
Odds ratios (OR) and 95% confidence interval (95%CI) for a 20% decrease in renal function over the observation period according to several categorical variables
| . | OR . | 95%CI . | P . |
|---|---|---|---|
| Diabetes type | |||
| 1 | 4.49 | 1.36–14.7 | <0.05 |
| 2 | 1.25 | 0.31–4.94 | 0.93 |
| Gender | |||
| Male | 5.32 | 1.49–18.9 | <0.05 |
| Female | 0.91 | 0.29–3.02 | 0.82 |
| Retinopathy | |||
| No | 1.92 | 0.69–5.28 | 0.33 |
| Yes | 3.00 | 0.64–13.9 | 0.37 |
| Proteinuria | |||
| No | 1.14 | 0.53–5.07 | 0.60 |
| Yes | 3.66 | 0.97–13.7 | 0.12 |
| ACE inhibitor | |||
| No | 2.66 | 0.81–7.80 | 0.14 |
| Yes | 2.48 | 0.61–10.1 | 0.42 |
| . | OR . | 95%CI . | P . |
|---|---|---|---|
| Diabetes type | |||
| 1 | 4.49 | 1.36–14.7 | <0.05 |
| 2 | 1.25 | 0.31–4.94 | 0.93 |
| Gender | |||
| Male | 5.32 | 1.49–18.9 | <0.05 |
| Female | 0.91 | 0.29–3.02 | 0.82 |
| Retinopathy | |||
| No | 1.92 | 0.69–5.28 | 0.33 |
| Yes | 3.00 | 0.64–13.9 | 0.37 |
| Proteinuria | |||
| No | 1.14 | 0.53–5.07 | 0.60 |
| Yes | 3.66 | 0.97–13.7 | 0.12 |
| ACE inhibitor | |||
| No | 2.66 | 0.81–7.80 | 0.14 |
| Yes | 2.48 | 0.61–10.1 | 0.42 |
Discussion
The salient feature of the present study is the demonstration that in this cohort including patients with type 1 or type 2 diabetes, with or without overt nephropathy, cigarette smoking causes a decrease in the estimated GFR independent of proteinuria even after correction for a number of confounding factors. The effect increased with increasing exposure to cigarette smoking (pack years). This finding is in good agreement with some previous cross-sectional [10,11] and some longitudinal [4,12,13] studies investigating patients with diabetes with or without renal involvement. In particular the finding is in line with the observation of Chuahirun and Wesson [4] who found that in smokers, as compared to non-smokers, with type 2 diabetes mellitus and diabetic nephropathy initially normal serum creatinine values increased despite ACE inhibition and well controlled blood pressure. It has to be acknowledged, however, that our study also included patients with type 1 diabetes and patients with microalbuminuria. Our findings that smoking had a negative impact on GFR decline, independent of diabetes type parallels the data of a small prospective study by Biesenbach et al. [12]. However, it should be emphasized that our study was not performed in patients exclusively with diabetic nephropathy, because many patients did not have overt proteinuria and only one-third actually had retinopathy.
Our findings are in apparent contrast with the observation by Hovind et al. [6] in a large population of patients with type 1 diabetes with overt diabetic nephropathy and with a median follow-up of 7 years (range 3–14 years). These authors found that smoking was not associated with a decline in measured GFR, even when the quantity of smoking, adjusted for blood pressure or HbA1c, was analysed [6]. Possible explanations for this difference between the two studies could be age, severity of nephropathy and glycaemic control. In the study by Hovind et al. [6] patients were younger (by ∼15 years), had lower baseline GFR (by ∼14 ml/min) and higher HbA1c (by ∼2%). Also, the two studies differ in the methodology of reporting renal function, with measured 51Cr-EDTA GFR in the study of Hovind et al. [6] and estimated MDRD-GFR in ours. It is possible that the effect of smoking becomes less apparent with poorer glycaemic control and when nephropathy is already advanced. Older patients might also be more susceptible to the effect of smoking than younger ones. Alternatively, age might reflect the effect of a longer exposure to smoking. In fact, the effect of the quantity of smoking in the study by Hovind et al. [6] was analysed by evaluating the numbers of cigarettes smoked per day, while our analysis used the number of pack-years estimated from the questionnaire.
It has recently been found that in diabetes GFR may be low despite the absence of proteinuria [14,15] indicating that deterioration of renal function may also occur through pathways other than glomerular damage associated with proteinuria. We do not have histological documentation, but vascular damage associated with cigarette smoking—as found in non-diabetic patients with non-diabetic primary renal disease [8]—might provide a potential explanation.
Irrespective of the exact pathogenetic pathway the finding of relatively rapid loss of GFR in diabetic patients who smoke cigarettes, ∼2.4 ml/min/year compared to no significant loss in non-smokers in early stages of diabetic nephropathy, is clinically important. It also calls for intensive efforts to motivate patients with diabetes to stop smoking. The finding of an adverse effect of smoking on renal function is not unique to diabetes. In a population-based study Haroun et al. [16] recently found that smoking explained no less than 31% (attributable risk) of impaired renal function in the general population, illustrating the epidemiological magnitude of the problem. The finding that younger diabetic patients were more likely to be active smokers than older patients in the present study illustrates the success of tobacco companies’ media campaigns addressing mainly younger subjects and underscores the need to fully implement evidence-based strategies that are effective in preventing youth tobacco use.
The MDRD equation was used to estimate GFR in the present study. The accuracy of this tool as an estimate of renal function in various populations has been debated. MDRD estimates have correlated well with radioisotope measures of GFR in several studies in non-diabetic and diabetic subjects [9,17]. Using a single laboratory for all testing also minimized inaccuracy caused by variation in serum creatinine levels. Accepting these considerations, MDRD estimations recently have been recommended as a valid tool to assess renal function in large population-based studies [18].
It is interesting to observe that blood pressure control in our cohort of diabetic patients improved over the course of the years. This might reflect the introduction of new hypertension guidelines, which became common knowledge around the time or after the initial screening visit, suggesting tighter blood pressure control in patients with diabetes [19]. In our cohort, however, smokers showed a decline in renal function despite better blood pressure control than non-smokers. It could be argued that the lower rate of treatment of smokers with an ACE inhibitor might have negatively affected this group. However, Chuahirun et al. [20] recently showed that cigarette smoking exacerbates renal injury in type 2 diabetes, despite improved blood pressure control and ACE inhibitor therapy. On the other hand it was encouraging to see that smoking cessation in those patients with microalbuminuria ameliorates the progressive renal injury caused by continued smoking [20].
Smoking does not seem to affect the decline of GFR in our female population, although as suggested in previous studies the problem might lie in the small sample size (see [1] for references). Despite the fact that the number of women enrolled in this and other studies was limited, which is particularly critical because women smoke less than men, these data indicate that cigarette smoking confers a higher renal risk in men than in women.
The demonstration that in patients with both type 1 and type 2 diabetes and normal or near normal renal function cigarette smoking causes a decrease in the estimated GFR independent of proteinuria, even after correcting for confounding factors, argues in favour of smoking cessation as a strategy to reduce ESRD risk even in patients with diabetes without overt nephropathy.
Conflict of interest statement. None declared.
References
Orth SR, Stockmann A, Conradt C et al. Smoking as a risk factor for end-stage renal failure in men with primary renal disease.
Stengel B, Couchoud C, Cenee S, Hemon D. Age, blood pressure and smoking effects on chronic renal failure in primary glomerular nephropathies.
Chuahirun T, Wesson DE. Cigarette smoking predicts faster progression of type 2 established diabetic nephropathy despite ACE inhibition.
Hovind P, Rossing P, Tarnow L, Parving HH. Smoking and progression of diabetic nephropathy in type 1 diabetes.
Baggio B, Budakovic A, Dalla Vestra M, Saller A, Bruseghin M, Fioretto P. Effects of cigarette smoking on glomerular structure and function in type 2 diabetic patients.
Lhotta K, Rumpelt HJ, König P, Mayer G, Kronenberg F. Cigarette smoking and vascular pathology in renal biopsies.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group.
Mühlhauser I, Sawicki P, Berger M. Cigarette-smoking as a risk factor for macroproteinuria and proliferative retinopathy in type 1 (insulin-dependent) diabetes.
Keller CK, Bergis KH, Fliser D, Ritz E. Renal findings in patients with short-term type 2 diabetes.
Biesenbach G, Janko O, Zazgornik J. Similar rate of progression in the predialysis phase in type I and type II diabetes mellitus.
Klein R, Klein BE, Moss SE. Incidence of gross proteinuria in older-onset diabetes. A population-based perspective.
Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus.
MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, Smith TJ, McNeil KJ, Jerums G. Nonalbuminuric renal insufficiency in type 2 diabetes.
Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23534 men and women in Washington County, Maryland.
Vervoort G, Willems HL, Wetzels JF. Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation.
K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification.
1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee.
Author notes
1Klinik und Poliklinik für Innere Medizin II – Nephrologie, University of Regensburg, Regensburg, Germany, 2Department of Internal Medicine, Ruperto Carola University, Heidelberg, Germany and 3Department of Nephrology, Fremantle Hospital, University of Western Australia, Perth, Australia





Comments