-
PDF
- Split View
-
Views
-
Cite
Cite
Toshiaki Tanaka, Hiroshi Kitamura, Atsushi Takahashi, Naoya Masumori, Naoki Itoh, Taiji Tsukamoto, Long-term Functional Outcome and Late Complications of Studer's Ileal Neobladder, Japanese Journal of Clinical Oncology, Volume 35, Issue 7, July 2005, Pages 391–394, https://doi.org/10.1093/jjco/hyi112
Close - Share Icon Share
Abstract
Objective: The purpose of this study was to evaluate the long-term functional outcome and late complications of Studer's ileal neobladder.
Methods: The study included 57 patients who underwent radical cystectomy and bladder reconstruction with Studer's ileal neobladder, and were followed-up for at least 3 months after surgery. The voiding and storage function, and late complications were evaluated. The times of evaluation after surgery were categorized into periods I (3–23 months), II (24–59 months), III (60–95 months) and IV (≥96 months).
Results: Daytime and night-time continence rates were 95.6 and 88.6%, respectively. The averages of functional capacity (439 ml), maximum flow rate (15.7 ml/s) and residual urine (35 ml) evaluated in period I were maintained in period IV. Of the 57 patients, intermittent self-catheterization was needed in five (8.8%) due to incomplete emptying or urinary retention. Urethroileal anastomotic stricture was found in two patients (3.5%), who were successfully treated by transurethral intervention. Inguinal hernia was found in seven patients (12.8%), five of whom developed it within 2 years after surgery.
Conclusions: Our results indicate that Studer's ileal neobladder had a favorable long-term functional outcome. Although late complication rates were low, the incidence of inguinal hernia was relatively high, and this was considered as a definite late complication in our study.
INTRODUCTION
Orthotopic bladder substitutions have become standard for urinary reconstruction after radical cystectomy in patients who do not have neoplastic lesions of the urethra. Several types of orthotopic bladder substitutions have been developed, of which Studer's ileal neobladder is one of the most common procedures (1).
Studer's ileal neobladder is easily constructed and provides unchanged voiding habits with good continence and upper urinary tract preservation, with relatively low rates of complication (2,3), even compared with the intermediate-term results of an ileal conduit (4). However, only a few reports are available on the long-term results of this operation. In this study, we reviewed the clinical outcomes of patients who underwent Studer's ileal neobladder operation and were followed-up for a long time to elucidate whether the voiding function was maintained and to clarify what complications developed in the late period.
PATIENTS AND METHODS
Between February 1991 and September 2003, 62 patients underwent bladder reconstruction with a Studer's ileal neobladder after radical cystectomy for high risk T1 or Tis and invasive bladder cancer. Indications for this procedure consisted of no evidence of neoplastic lesions of the prostatic urethra of male patients and bladder neck of female patients, which was histopathologically confirmed by biopsy before cystectomy.
We used the original operative procedures for construction of the ileal neobladder reported by Studer et al. (2). However, ureters were implanted in the afferent limb of the ileum with the Le Duc–Camey technique, as previously reported, in all but five patients (3).
Of the 62 patients who received Studer's ileal neobladder, 57 patients who were followed-up for at least 3 months after the operation were analyzed retrospectively. All complications in the periods were reviewed. Continence rates were estimated by the Kaplan–Meier method. The follow-up period was categorized into four groups, depending on the period after surgery: period I consisting of 57 patients who were followed-up from 3 months to 2 years; period II, 40 with follow-up for 2–5 years; period III, 23 patients, for 5–8 years; and period IV, comprising 13 patients for ≥8 years. In each period, we evaluated the functional capacity of the neobladder with a frequency/volume chart, the maximum flow rate (Qmax) with conventional uroflowmetry and the post-void residual urine volume (PVR) with catheterization. Changes in these parameters over the periods were statistically examined with the Kruskal–Wallis test. P-values <0.05 were considered to be statistically significant.
RESULTS
Patient Characteristics
A total of 53 males (93.0%) and four females (7.0%) were included in this study (Table 1). The mean follow-up period was 57.0 months with a range of 5–136. Patients who were followed-up for ≥5 years accounted for 40% of the total. More than 90% of patients had invasive disease.
Patients characteristics (n = 57)
| Characteristics . | . | |
|---|---|---|
| Sex | ||
| Male (%) | 53 (93.0) | |
| Female (%) | 4 (7.0) | |
| Mean age: years (range) | 60.1 (34–75) | |
| Mean follow-up period: months (range) | 57.0 (5–136) | |
| Clinical stage: no. of patients (%) | ||
| T0 | 1 (1.8) | |
| T1 | 1 (1.8) | |
| Tis | 2 (3.5) | |
| T2 | 35 (61.4) | |
| T3 | 18 (31.5) | |
| Characteristics . | . | |
|---|---|---|
| Sex | ||
| Male (%) | 53 (93.0) | |
| Female (%) | 4 (7.0) | |
| Mean age: years (range) | 60.1 (34–75) | |
| Mean follow-up period: months (range) | 57.0 (5–136) | |
| Clinical stage: no. of patients (%) | ||
| T0 | 1 (1.8) | |
| T1 | 1 (1.8) | |
| Tis | 2 (3.5) | |
| T2 | 35 (61.4) | |
| T3 | 18 (31.5) | |
Patients characteristics (n = 57)
| Characteristics . | . | |
|---|---|---|
| Sex | ||
| Male (%) | 53 (93.0) | |
| Female (%) | 4 (7.0) | |
| Mean age: years (range) | 60.1 (34–75) | |
| Mean follow-up period: months (range) | 57.0 (5–136) | |
| Clinical stage: no. of patients (%) | ||
| T0 | 1 (1.8) | |
| T1 | 1 (1.8) | |
| Tis | 2 (3.5) | |
| T2 | 35 (61.4) | |
| T3 | 18 (31.5) | |
| Characteristics . | . | |
|---|---|---|
| Sex | ||
| Male (%) | 53 (93.0) | |
| Female (%) | 4 (7.0) | |
| Mean age: years (range) | 60.1 (34–75) | |
| Mean follow-up period: months (range) | 57.0 (5–136) | |
| Clinical stage: no. of patients (%) | ||
| T0 | 1 (1.8) | |
| T1 | 1 (1.8) | |
| Tis | 2 (3.5) | |
| T2 | 35 (61.4) | |
| T3 | 18 (31.5) | |
Storage and Voiding Function
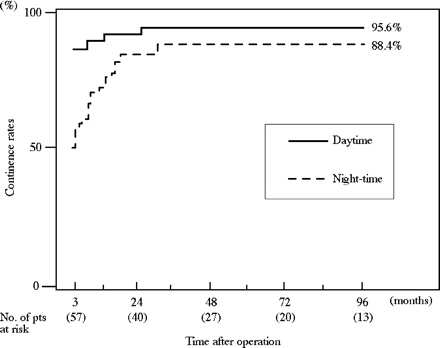
Patients achieved 95.6% daytime continence and 88.6% for the night-time, when continence was defined as that with no use of a pad (Fig. 1). Most patients achieved daytime continence within 6 months after the operation. Night-time continence recovered more slowly than that in the daytime. Functional capacity was maintained at 400–500 ml for each period, with no significant change (Table 2). However, two patients developed a neobladder capacity >1000 ml 5 years after the operation. There were no significant changes of Qmax and PVR during the follow-up periods, with the mean rates being maintained at 10–20 ml/s and the mean PVR at <60 ml.
Changes in functional capacity, maximum flow rate and post-void residual urine volume after surgery
| Periods (months) . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | P-value . |
|---|---|---|---|---|---|
| No. of patients | 57 | 40 | 23 | 13 | |
| Functional capacity (ml) | 439 (109) | 447 (115) | 509 (270) | 405 (153) | 0.741 |
| Qmax (ml/s) | 15.7 (8.2) | 13.7 (7.7) | 16.8 (8.0) | 16.7 (7.9) | 0.636 |
| PVR (ml) | 35 (60) | 36 (83) | 60 (115) | 34 (71) | 0.386 |
| Periods (months) . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | P-value . |
|---|---|---|---|---|---|
| No. of patients | 57 | 40 | 23 | 13 | |
| Functional capacity (ml) | 439 (109) | 447 (115) | 509 (270) | 405 (153) | 0.741 |
| Qmax (ml/s) | 15.7 (8.2) | 13.7 (7.7) | 16.8 (8.0) | 16.7 (7.9) | 0.636 |
| PVR (ml) | 35 (60) | 36 (83) | 60 (115) | 34 (71) | 0.386 |
Values in parentheses are the SD.
Qmax, maximum flow rate; PVR, post-void residual urine volume.
The P-value was determined with the Kruskal–Wallis test.
Changes in functional capacity, maximum flow rate and post-void residual urine volume after surgery
| Periods (months) . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | P-value . |
|---|---|---|---|---|---|
| No. of patients | 57 | 40 | 23 | 13 | |
| Functional capacity (ml) | 439 (109) | 447 (115) | 509 (270) | 405 (153) | 0.741 |
| Qmax (ml/s) | 15.7 (8.2) | 13.7 (7.7) | 16.8 (8.0) | 16.7 (7.9) | 0.636 |
| PVR (ml) | 35 (60) | 36 (83) | 60 (115) | 34 (71) | 0.386 |
| Periods (months) . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | P-value . |
|---|---|---|---|---|---|
| No. of patients | 57 | 40 | 23 | 13 | |
| Functional capacity (ml) | 439 (109) | 447 (115) | 509 (270) | 405 (153) | 0.741 |
| Qmax (ml/s) | 15.7 (8.2) | 13.7 (7.7) | 16.8 (8.0) | 16.7 (7.9) | 0.636 |
| PVR (ml) | 35 (60) | 36 (83) | 60 (115) | 34 (71) | 0.386 |
Values in parentheses are the SD.
Qmax, maximum flow rate; PVR, post-void residual urine volume.
The P-value was determined with the Kruskal–Wallis test.
Late Complications
The most frequent complication was transient or long-lasting metabolic acidosis, which had to be continuously treated with potassium/sodium citrate in nine patients (Table 3). Neobladder stones, which were found in seven patients, were successfully treated with endoscopic lithotripsy in all but one patient who had spontaneous passage of a stone. All stones seemed to be formed with a nucleus consisting of mucus and debris from the intestine. A unique late complication was inguinal hernia, which seven patients developed in our study. Most of these patients developed the condition within 2 years after the operation. Because incomplete emptying and urinary retention that resulted in a large amount of PVR (>150 ml) developed during follow-up, five patients needed to undergo clean intermittent catheterization (CIC). No patients had urethroileal anastomotic stricture when starting CIC. Of these patients, two had a poor voiding condition in the early period after the operation. Two other patients gradually developed a poor voiding condition without apparent cause. Urinary retention occurred in one female patient a year after operation, though she had achieved better voiding and continence before this episode. Urethroilael anastomotic stricture was seen in two patients in period I. Balloon dilation or internal urethrotomy under direct vision was effective for management of the stricture. Febrile urinary tract infection occurred in one patient (1.8%) who received Le Duc–Camey ureterointestinal anastomosis. No ureteroileal anastomotic stricture and impaired renal function was observed during follow-up in our series.
Late complications
| . | Overall no. of patients (%) . | No. of onsets in each period (months) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
. | . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | |||
| Metabolic acidosis | 9 (15.8) | 4 | 4 | 1 | 0 | |||
| Inguinal hernia | 7 (12.3) | 5 | 1 | 1 | 0 | |||
| Need for intermittent catheterization | 5 (8.8) | 4 | 0 | 1 | 0 | |||
| Neobladder calculi | 4 (7.0) | 1 | 3 | 0 | 0 | |||
| Upper urinary tract calculi | 3 (5.3) | 0 | 1 | 2 | 0 | |||
| Urethroileal anastomotic stricture | 2 (3.5) | 2 | 0 | 0 | 0 | |||
| Febrile urinary tract infection | 1 (1.8) | 1 | 0 | 0 | 0 | |||
| . | Overall no. of patients (%) . | No. of onsets in each period (months) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
. | . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | |||
| Metabolic acidosis | 9 (15.8) | 4 | 4 | 1 | 0 | |||
| Inguinal hernia | 7 (12.3) | 5 | 1 | 1 | 0 | |||
| Need for intermittent catheterization | 5 (8.8) | 4 | 0 | 1 | 0 | |||
| Neobladder calculi | 4 (7.0) | 1 | 3 | 0 | 0 | |||
| Upper urinary tract calculi | 3 (5.3) | 0 | 1 | 2 | 0 | |||
| Urethroileal anastomotic stricture | 2 (3.5) | 2 | 0 | 0 | 0 | |||
| Febrile urinary tract infection | 1 (1.8) | 1 | 0 | 0 | 0 | |||
Late complications
| . | Overall no. of patients (%) . | No. of onsets in each period (months) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
. | . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | |||
| Metabolic acidosis | 9 (15.8) | 4 | 4 | 1 | 0 | |||
| Inguinal hernia | 7 (12.3) | 5 | 1 | 1 | 0 | |||
| Need for intermittent catheterization | 5 (8.8) | 4 | 0 | 1 | 0 | |||
| Neobladder calculi | 4 (7.0) | 1 | 3 | 0 | 0 | |||
| Upper urinary tract calculi | 3 (5.3) | 0 | 1 | 2 | 0 | |||
| Urethroileal anastomotic stricture | 2 (3.5) | 2 | 0 | 0 | 0 | |||
| Febrile urinary tract infection | 1 (1.8) | 1 | 0 | 0 | 0 | |||
| . | Overall no. of patients (%) . | No. of onsets in each period (months) . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
. | . | I (3–23) . | II (24–59) . | III (60–95) . | IV (96+) . | |||
| Metabolic acidosis | 9 (15.8) | 4 | 4 | 1 | 0 | |||
| Inguinal hernia | 7 (12.3) | 5 | 1 | 1 | 0 | |||
| Need for intermittent catheterization | 5 (8.8) | 4 | 0 | 1 | 0 | |||
| Neobladder calculi | 4 (7.0) | 1 | 3 | 0 | 0 | |||
| Upper urinary tract calculi | 3 (5.3) | 0 | 1 | 2 | 0 | |||
| Urethroileal anastomotic stricture | 2 (3.5) | 2 | 0 | 0 | 0 | |||
| Febrile urinary tract infection | 1 (1.8) | 1 | 0 | 0 | 0 | |||
DISCUSSION
The results of this study indicated that Studer's ileal neobladder maintained favorable voiding and storage functions for many years after the operation. Although the neobladder capacity is insufficient for the first 3 months after operation, it increases to 400–500 ml at 6 months (3,5,6). In this study, the appropriate capacity was maintained even >8 years after the operation. This tendency is comparable with that observed in Studer's series (6,7). However, in our study, two patients developed a capacity of >1000 ml over 5 years after the operation. Periodic assessment with a frequency/volume chart and reinstruction of neobladder management are required to avoid its overextension and too large a storage volume.
Although we did not identify the specific factors, our recommendations for patients to wake up and void at least once in the middle of the night, and to refrain from drinking an excessive amount of water before going to sleep may have contributed to the reduction of incontinence frequency.
Qmax was 10–20 ml/s immediately after the operation, as has been reported by others (8,9), and it was stable in the long term. Urinary retention occurred in one female patient. It was associated with neither anastomotic stricture nor urethral recurrence of carcinoma. Although urinary retention is rare, it occurs more frequently in female patients (10). One of the speculated causes of urinary retention in females is kinking of the urethra (6,7,10), which is probably caused by denervation of the proximal urethra and is considered to be the main cause (10,11). However, neither voiding cystourethrography nor cystourethroscopy revealed such an apparent cause for retention in our patient. Thus the episode was due to other, as yet unknown functional or anatomical causes.
The percentage of our patients who needed intermittent catheterization was 8.8%, which was comparable with that in other reports (9,12). Of those patients, one had a PVR that increased to >150 ml 5 years after the operation. Although a large PVR was reported to be a result of inguinal or incisional hernias (7,10), our patient had neither inguinal nor incisional hernia, and had a functional capacity >800 ml. These findings suggest that overextension is a cause of increased PVR. Mikuma et al. pointed out that in patients with a low Qmax and a high PVR, the anastomosis between the neobladder and membranous urethra was not located at the bottom of the pouch and a cystocele-like change was observed (13). Although that was not confirmed by radiographic examination, in our patient, a cystocele-like change resulting from overextension of the neobladder that occurred several years after the operation might have been involved in the increase of PVR.
The incidence of inguinal hernias was unexpectedly high in this study. Studer et al. reported that the incidence of inguinal or abdominal wall hernias was 7% in their series with a median follow-up period of 30.2 months (5). Our longer follow-up, 57 months, might be related to the difference in the rate from that of others, although we did not find any specific explanations for the incidence. Ichioka et al. reported that 21.3% of patients who underwent radical retropubic prostatectomy developed inguinal hernia. On the other hand, in patients with cystectomy and mainly incontinent urinary diversion in their series, the incidence of inguinal hernias was 5.4% (14). When compared with the rate in radical prostatectomy, the lower rate in their cystectomy series was explained by the increased volume of the abdominal cavity after operation and lesser abdominal pressure provided by the operative procedure so that the peritoneum was left open. However, patients who receive an ileal neobladder need to strain to void. We speculate that this situation has inherent potential to increase to some extent the incidence of inguinal hernias in patients with an ileal neobladder.
CONCLUSIONS
Studer's ileal neobladder had a favorable long-term functional outcome in our study. Although the late complication rate was generally low and all complications were already known to occur, there were several patients who had to undergo CIC for their poor voiding condition resulting in a larger PVR. A unique complication was inguinal hernia, the rate of which was relatively high in our series. This is considered to be one of the definite late complications in patients with an ileal neobladder.
References
Stein JP, Skinner DG. Orthotopic urinary diversion. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell's Urology, 8th edn. Philadelphia: Saunders,
Studer UE, Danuser H, Hochreiter W, Springer JP, Turner WH, Zingg EJ. Summary of 10 years' experience with an ileal low-pressure bladder substitute combined with an afferent tubular isoperistaltic segment.
Yokoo A, Hirose T, Mikuma N, Tsukamoto T. Ileal neobladder for bladder substitution after radical cystectomy.
Gburek BM, Lieber MM, Blure ML. Comparison of Studer ileal neobladder and ileal conduit urinary diversion with respect to perioperative outcome and late complications.
Studer UE, Zingg EJ. Ileal orthotopic bladder substitutes. What we have learned from 12 years' experience with 200 patients.
Madersbacher S, Möhrle K, Burkhard F, Studer UE. Long-term voiding pattern of patients with ileal orthotopic bladder substitutes.
Perimenis P, Burkhard FC, Kessler TM, Gramann T, Studer UE. Ileal orthotopic bladder substitute combined with an afferent tubular segment: long-term upper urinary tract changes and voiding pattern.
Cancrini A, Carli PD, Pompeo V, Fattahi H, Lamanna L, Giuseppe C, et al. Lower urinary tract reconstruction following cystectomy: experience and results in 96 patients using the orthotopic ileal bladder substitution of Studer et al.
Yoneda T, Igawa M, Shiina H, Shigeno K, Urakami S. Postoperative morbidity, functional results and quality of life of patients following orthotopic neobladder reconstruction.
Varol C, Studer UE. Managing patients after an ileal orthotopic bladder substitution.
Benson MC, Seaman EK, Olsson CA. The ileal ureter neobladder is associated with a high success and a low complication rate.
Mikuma N, Hirose T, Yokoo A, Tsukamoto T. Voiding dysfunction in ileal neobladder.