-
PDF
- Split View
-
Views
-
Cite
Cite
Jian Chen, Houmin Li, Ruoyu Li, Dingfang Bu, Zhe Wan, Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma, Journal of Antimicrobial Chemotherapy, Volume 55, Issue 1, January 2005, Pages 31–37, https://doi.org/10.1093/jac/dkh507
Close - Share Icon Share
Abstract
Objectives: To monitor changes in itraconazole susceptibility of isolates from a patient undergoing treatment for pulmonary Aspergillus infection and relate these changes to genotypic/phenotypic alterations.
Methods: Six Aspergillus fumigatus isolates were serially recovered from the patient. Itraconazole MICs were determined by Etest and NCCLS methodology. Growth characteristics and phenotype were monitored. Molecular analysis included random amplified polymorphic DNA (RAPD) assay and sequencing of the cyp51A gene.
Results: The MIC of itraconazole against the first isolate before treatment was 0.25 mg/L; the MIC against the second isolate, recovered after 6 months of itraconazole therapy, was >16 mg/L; and that against the third isolate, obtained 2 months after discontinuation of the therapy, was 0.5 mg/L. The MIC against the last three isolates, acquired after restoration of itraconazole therapy for 4–7 months, was >16 mg/L. The six isolates shared identical band patterns of RAPD assay using four primers and the same sequence in intertranscribed spacers (ITS). Therefore, the six isolates were likely to be the same strain of A. fumigatus, and mutations involving itraconazole resistance possibly occurred in these isolates after prolonged itraconazole therapy. Sequencing of the cyp51A gene in the coding region revealed a mutation of M220I in cytochrome P450 sterol 14-α-demethylase in the second resistant isolate and a mutation of G54R in the last three resistant isolates. Expression changes of some pump genes, such as MDR3, may also, in part, be related to the resistance to itraconazole.
Conclusions: We conclude that resistance of A. fumigatus to itraconazole occurred in a patient treated with the drug, and the resistance may result from mutations in the cyp51A gene—the gene encoding the target enzyme for itraconazole.
Introduction
Aspergillus fumigatus, originally viewed as a weak pathogen, is now becoming a major cause of death in immunocompromised patients, with the mortality rate in leukaemic patients approaching 90%, even when treated with antifungal therapy.1 For invasive Aspergillus infections, the treatment options are largely limited to therapy with the polyene drug amphotericin B, broad-spectrum triazoles such as itraconazole and voriconazole and the echinocandin caspofungin. However, amphotericin B therapy can be highly toxic and may result in nephrotoxicity,2 whereas triazoles are fungistatic and subject to drug-resistance development. The safety profile and high therapeutic index afforded by triazole drugs make them particularly suitable for prophylactic, empirical and pre-emptive therapies in many patients with severe immunosuppression. Among triazoles, itraconazole is a commonly used antifungal drug against Aspergillus species,3 is suitable for long-term treatment with intravenous or oral administration and has been widely used in invasive aspergillosis.4
However, repeated exposure to triazole drugs is a major risk factor for drug resistance,5 and in vitro resistance to itraconazole has been documented in clinical isolates and in spontaneous mutants of A. fumigatus.3,6–8 Acquired itraconazole resistance of A. fumigatus isolated from an aspergilloma patient has also been reported.9 The resistance detected in vitro was further confirmed in vivo by animal models,7,9,10 suggesting clinical significance for this resistance. Reduced intracellular accumulation of itraconazole was observed in some resistant strains, possibly due to reduced penetration of the drug and the overexpression of efflux pump genes.7,8 In other strains, the mechanism of itraconazole resistance was related to a mutation in the target enzyme, cytochrome P450 sterol 14-α-demethylase (cyp51A).7,11,12
In this study, we report the acquisition of resistance to itraconazole of A. fumigatus in clinical serial isolates from a patient following prolonged itraconazole therapy. Two missense point mutations in the cyp51A gene may account for the acquired itraconazole resistance.
Materials and methods
Case report
A 46-year-old man with a history of pulmonary tuberculosis, as well as 5 years of lung cavities and fibrosis in the lesion, was admitted in December 2001 with expectoration, haemoptysis and dyspnoea. Acid-fast staining of his sputum for Mycobacterium tuberculosis was negative, but a large number of hyaline and septate hyphae were detected in his sputum smear treated with potassium hydroxide. A large cavity and an aspergilloma were found in his left lung by chest CT scanning, and he was diagnosed with an aspergilloma. Surgical treatment was not performed due to insufficiency of his respiratory function. A. fumigatus was isolated from his sputum, and was named AF1 in this study. Itraconazole 200 mg daily, combined with anti-tuberculosis agents protionamide, rifapentine and sodium para-aminosalicylate, were then started and continued for 6 months. In July 2002, hyphae were still found in sputum by microscopic examination after 6 months of itraconazole treatment. A. fumigatus was isolated from his sputum at that time, and this strain was named AF2 in this study. In August 2002, about 2 months after discontinuation of itraconazole by the patient, he was hospitalized again. Hyphae were positive in sputum and a strain of A. fumigatus, labelled as AF3, was recovered from his sputum. He was then treated with itraconazole 200 mg twice daily intravenously for 2 weeks, followed by itraconazole 200 mg twice daily orally. His general condition improved remarkably, but hyphae were still found in sputum by microscopic examination. During December 2002–February 2003, 4–7 months after reconstitution of the itraconazole therapy, three isolates named AF4, AF5 and AF6 were recovered from his sputum.
A. fumigatus strains
Isolates AF1 and AF2 of A. fumigatus were obtained from the patient before and after 6 months of itraconazole therapy, respectively. Isolate AF3 was also obtained from this patient after 2 months discontinuation of itraconazole therapy. Isolates AF4, AF5 and AF6 were obtained after his itraconazole therapy had been restored for about 4–7 months. These clinical isolates were purified to single spore, from which 10 colonies were then randomly selected for successive studies.
Phenotypic characterization and growth study
To evaluate the phenotypic characterization of the six clinical isolates, they were first tested for the identification of A. fumigatus by microscopic examination and thermotolerance studies. An assessment of growth differences in liquid medium was performed by the MTT assay, a reliable indicator of fungal biomass.13 In brief, 200 μL of 105 conidia/mL in RPMI 1640 was incubated at 35°C for 0, 4, 8, 12, 16, 20, 24 and 40 h. After each of the respective incubation times, 25 μL of RPMI 1640 containing 5 mg/mL MTT and 1 mM menadione was added, and the mixture incubated for 3 h at 37°C. The supernatant was carefully aspirated. Acidic isopropanol (95 mL of isopropanol, 5 mL of 1 M HCl) 0.1 mL per well was added. The plate was shaken on a shaker for 5 min to dissolve the formazan crystals, and measured with a spectrophotometer at 550 nm. Assays in triplicate were performed for each sample. To test whether the hyphal growth rate of these clinical strains was similar, radial growth on solid medium was observed for the six isolates. In brief, a conidia suspension (10 μL, 105 conidia/mL) was inoculated on the centre of an agar plate, incubated at 35°C and the diameter of the growing colony measured every 24 h up to 96 h. Comparison of conidiation on solid medium was also performed using previously described methods.14
Assay of susceptibility to itraconazole and voriconazole
A susceptibility assay was performed with two methods at least twice, and the yielded MICs were usually reproducible on re-testing. Itraconazole susceptibility for the six clinical isolates was first determined by the Etest method according to the manufacturer's instructions (AB Biodisk). A. fumigatus conidia (106) were plated onto RPMI 1640 agar supplemented with 2% glucose, and the plate was allowed to dry. Etest strips containing itraconazole were applied, and the MIC was determined after 48 h of incubation at 35°C. The Etest MIC was considered to be the drug concentration at which dense colony growth intersected the strip, but sparse subsurface hyphal growth at the margins was ignored. The six clinical isolates and the 60 monosporal isolates derived from them were also evaluated for susceptibility to itraconazole and voriconazole, using the broth microdilution method M-38A from the National Committee for Clinical Laboratory Standards (NCCLS).15 RPMI 1640 medium containing l-glutamine and buffered to pH 7.0 with 0.165 M MOPS was used as the culture medium. Itraconazole (Janssen Pharmaceutica, Xian, China) and voriconazole (Pfizer, Hong Kong, China) were dissolved in dimethylsulphoxide (DMSO) to 1600 mg/L, and then diluted to the final concentration of 0.03–16 mg/L with the medium, according to the standard additive two-fold drug dilution scheme described in the NCCLS reference method. After growing on PDA agar (Difco, Kansas, MO, USA) slant at 35°C for 7 days, the inoculum was washed from the slant with 1 mL of 0.85% NaCl containing 0.05% Tween 20. The conidia were re-suspended in culture medium, adjusted to a turbidity equivalent to that of a 0.5 McFarland standard, further diluted in culture medium with various concentrations of itraconazole, inoculated into a 96-well plate at a concentration of ∼0.4–5 × 104 cells/mL and incubated at 35°C for 48 h. As recommended by the NCCLS M-38A document,15 MIC was defined as the lowest drug concentration at which the drug prevented the fungi from any discernible growth. The results of MICs were in close concordance with those from the Etest (data not shown).
Molecular typing of A. fumigatus isolates from the patient
Conidia of the isolates were cultured in liquid YPG medium (yeast extract 10 g/L, peptone 20 g/L and glucose 20 g/L) with shaking at 37°C for 24 h. Genomic DNA was extracted from mycelia by pulverization in liquid nitrogen16 and repeated phenol–chloroform extractions.17,18
A segment of ribosomal RNA genes with intertranscribed spacers (ITS) was amplified from genomic DNA by PCR using primers ITS1 (5′-TCCGTAGGTGAACCTGCGG) and ITS4 (5′-TCCTCCGCTTATTGATATGC). Amplification was performed in 25 μL containing 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 2.0 mM MgCl2, 100 μM each dNTP, 0.4 μM each primer, 50 ng of genomic DNA and 1 U of Taq DNA polymerase. The amplification was carried out at 95°C for 5 min followed by 30 cycles of 95°C for 30 s, 58°C for 30 s and 72°C for 1 min and then one cycle of 72°C for 10 min. Direct sequencing of the PCR product was performed on a sequencer (ABI 3700). To rule out the errors introduced by the PCR amplification, PCR and sequencing were carried out in duplicate for the samples tested.
Random amplified polymorphic DNA (RAPD) assay was performed by using four primers: primer 2 (5′-GCTGGTGG), primer 5 (5′-GCGCACGG),19 primer R108 (5′-GTATTGCCCT) and primer R151 (5′-GCTGTAGTGT).20 PCR was carried out in 50 μL containing 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 0.001% gelatin, 2.5 mM MgCl2, 200 μM each dNTP, 1.0 μM primer, 50 ng of genomic DNA and 2.5 U of Taq DNA polymerase. After denaturing at 94°C for 5 min, the thermal cycle conditions were 94°C for 1 min, 35°C for 1 min and 72°C for 2 min for 45 cycles, followed by final extension at 72°C for 10 min. PCR product was separated in a 1.5% agarose gel, stained with ethidium bromide and photographed. RAPD assay for each primer was repeated at least twice.
Sequencing of the cyp51A gene
The entire coding region of the cyp51A gene (GenBank AF222068), the gene of the enzyme for ergosterol biosynthesis and the target enzyme for azole antifungals, was amplified from genomic DNA of A. fumigatus by PCR using three pairs of primers (Table 1). PCR conditions were similar to those for the amplification of the ribosomal RNA gene. PCR product was purified and sequenced (ABI 3700) from both directions using one of the PCR primers as the sequencing primer. The six isolates and their monosporal strains were amplified and sequenced twice to exclude PCR-introduced errors.
Measurement of mRNAs potentially involved in resistance to itraconazole by quantitative reverse transcription-PCR
Expression changes of genes possibly involved in the resistance to itraconazole were evaluated for A. fumigatus isolates by quantitative reverse transcription-PCR. The mRNAs measured included those coding for the target enzyme of azole antifungals, CYP51A transcribed from the cyp51A gene, ATP-binding cassette transporter transcribed from the atrF gene, and multidrug-resistant proteins 1, 2, 3 and 4 transcribed from MDR1, 2, 3 and 4, respectively. For calibration of cDNA samples loaded in PCR mixtures, β-tublin mRNA transcribed from the TUB1 gene, one of the housekeeping genes constitutively expressed at a high level under various conditions, was also measured as an internal standard. Conidia of the six isolates were cultured in liquid YPG medium with shaking at 37°C for 24 h. Total RNA was extracted from mycelia by using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and treated with RNase-free DNase I (Takara, China) to eliminate DNA contamination. First-strand cDNA was synthesized from 1 μg of total RNA by using random primers and M-MuLV reverse transcriptase. The PCR mixture contained 10 pmol of each primer (Table 2), 2.0 mM MgCl2, 100 μM each dNTP, 0.5 U of AmpliTaq Gold DNA polymerase, 2 μL of 10 × SYBR Green buffer (Applied Biosystems, Sparks, MD, USA) and 1 μL of 1:5 diluted cDNA sample in a total volume of 20 μL. Real-time quantitative PCR was run in a spectrofluorometric thermal cycler (Applied Biosystems 5700 Prism), and each sample was measured three times. Specificity of PCR product was examined by the melting curve of amplicons and the single band after agarose electrophoresis. The relative amount of the cDNA was calibrated by the amount of β-tublin cDNA in the sample. The calibrated amount of cDNA was used for further analysis.
Results
Phenotypic characterization of the isolates
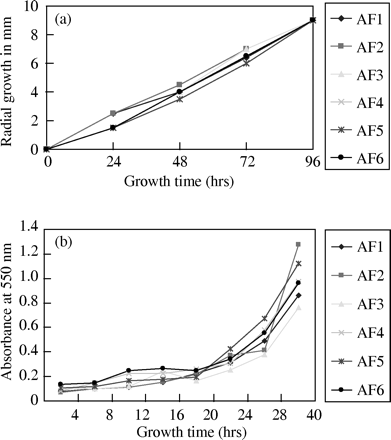
The six clinical isolates were identified to be A. fumigatus by microscopic examination and thermotolerance studies. Similarity in growth rate was confirmed by the MTT assay (Figure 1b). No obvious differences in the radii of growth (Figure 1a) and the conidial production per unit area (data not shown) were found among the six isolates.
In vitro susceptibility to itraconazole and voriconazole
Results of susceptibility tests are shown in Table 3. AF1, AF3 and their monosporal clones were susceptible to itraconazole with MICs of 0.25–0.5 mg/L. In contrast, AF2, AF4, AF5, AF6 and their monosporal isolates were resistant to itraconazole with MICs > 16 mg/L. For voriconazole, all of the six isolates and their monosporal clones were highly to moderately susceptible to the drug with an MIC range of 0.25–1 mg/L.
Molecular typing of the isolates
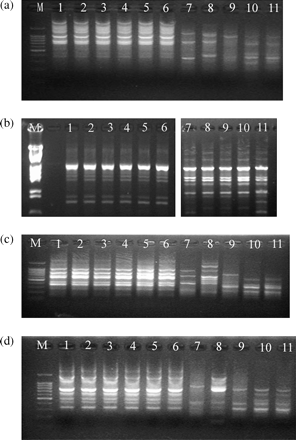
RAPD was performed to detect any gross difference in genomes among the isolates. If RAPD showed the same or very similar patterns among the six isolates, the patient was probably infected by one strain of A. fumigatus, and changes in susceptibility to itraconazole would therefore result from mutations somewhere in its genome. RAPD patterns using the four primers are shown in Figure 2. Identical band patterns were obtained from all of the six isolates when tested with the four primers. However, the control A. fumigatus strains and other species strains performed along with the six isolates exhibited apparent band differences, and they can be distinguished from each other by the assay used for each primer.
The PCR product amplified from A. fumigatus using primers ITS1 and ITS4 was ∼600 bp in size, containing a segment of the 18S ribosomal RNA gene, internal transcribed spacer 1, the 5.8S ribosomal RNA gene, internal transcribed spacer 2 and a segment of the 28S ribosomal RNA gene. Sequencing data of the PCR products were 100% identical for the six isolates, but showed 1–3 base differences among control A. fumigatus strains (data not shown).
Mutations in the cyp51A gene
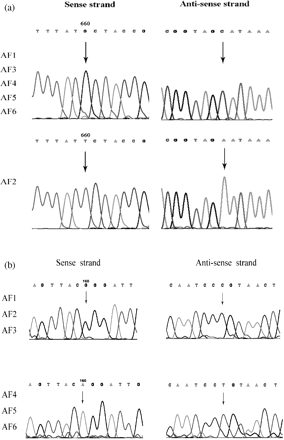
The entire coding region of the cyp51A gene from the six isolates and their 60 clones from single spores was amplified by PCR and sequenced. From isolate AF2 (the isolate resistant to itraconazole) and its 10 monosporal clones, a point transversion mutation of G660T, which converts methionine into isoleucine at residue 220 (M220I) in CYP51A, was found (Figure 3a). In AF4, AF5, AF6 and their monosporal clones, a point transition mutation of G160A (Figure 3b) was found, which results in the amino acid change of glycine to arginine at codon 54 (G54R). AF1, AF3 and the control A. fumigatus strain had the same normal sequence as that of GenBank accession number AF222068.21
Expression of genes potentially involved in resistance to itraconazole
Expression of the genes CYP51A, atrF and MDR1, 2, 3 and 4 in the six isolates was estimated by quantitative reverse transcription-PCR. Table 4 shows that the expression of cyp51A, MDR2, MDR3 and MDR4 genes was elevated in some of the resistant strains. Expression of the cyp51A gene was increased 1.4-, 1.24- and 1.5-fold in resistant strains AF2, AF4 and AF5, respectively, but not in the resistant strain AF6. Expression of the MDR2 gene was increased 1.68-fold only in the resistant strain AF5. Expression of MDR3 was increased 6.2-, 2.08-, 1.05- and 1.35-fold in resistant strains AF2, AF4, AF5 and AF6, respectively, but was also increased 2.98-fold in the susceptible strain AF3, as compared with that of the susceptible strain AF1. Expression of MDR4 was slightly increased by 1.28-, 1.25-, 2.17- and 1.36-fold in resistant strains AF2, AF4, AF5 and AF6.
Discussion
In this study, the six A. fumigatus isolates were sequentially isolated from the sputum of a patient with aspergilloma over a period of 24 months. Isolate AF1 was obtained before itraconazole treatment, isolate AF2 was obtained after 6 months of itraconazole treatment and isolate AF3 was acquired after discontinuation of itraconazole therapy for 2 months. AF4, AF5 and AF6 were recovered after reconstitution of itraconazole therapy for 4–7 months. In vitro susceptibility tests for itraconazole showed that isolates AF1 and AF3 were susceptible to itraconazole, and isolates AF2, AF4, AF5 and AF6 were highly resistant to the drug. If the patient was infected by only one strain of the fungus, some alterations must happen in the fungi in the disease course, and characterization of these alterations will be valuable for understanding the mechanism of resistance to itraconazole in A. fumigatus.
RAPD was used for typing fungal strains1,16 and RAPD using primers R108, 2, 5 and R151 was found to provide helpful information for the discrimination of various A. fumigatus strains.19,20,22 From the RAPD using the four primers, the six isolates were found to have identical band patterns, but the control strains of A. fumigatus showed remarkable differences in band pattern under the same assay conditions (Figure 2). The sequence of ITS, a hypervariable region in the fungus genome, was also useful for the identification of fungal strain. The six isolates had the same ITS sequence, but other control strains contained variations of 1–3 bases in ITS. In addition, we randomly picked 10 colonies from each of the six isolates, and they manifested the same response pattern to itraconazole as that of their parent strain. It is therefore suggested that the six isolates recovered from the patient at different disease stages originated from one strain of A. fumigatus. In another words, the patient was infected by one strain of A. fumigatus, and mutations resulting in changes of susceptibility to itraconazole developed in the A. fumigatus in the patient's lung during and after itraconazole treatment. Previous fingerprint studies on A. fumigatus isolated from patients with aspergilloma and invasive aspergillosis also showed that most of the patients were infected by one strain.23–26 In this study, we report for the first time that a strain of A. fumigatus acquired resistance to itraconazole after 6 months of itraconazole treatment. This resistance disappeared after discontinuation of the treatment, and was regained following reconstitution of itraconazole therapy. Using the six isolates, we further studied the mechanism underlying the induction and reversion of the resistance to itraconazole.
Itraconazole inhibits fungal ergosterol biosynthesis through the inhibition of the cytochrome P450 enzyme, CPY51A. Alterations in this target enzyme have been related to itraconazole resistance in some A. fumigatus strains.7,11,12 Several studies reported that a point mutation that replaced the glycine at codon 54 of CYP51A was found in A. fumigatus resistant to itraconazole.11,12,27 Recently, a study of five clinical A. fumigatus isolates exhibiting reduced susceptibility to itraconazole and other triazole drugs revealed that all of the five strains harboured mutations in cyp51A, resulting in the replacement of methionine at residue 220 by valine, lysine or threonine.28 We sequenced the coding region of cyp51A and found two mutations that were completely in accordance with itraconazole resistance but not with resistance to voriconazole (Table 3). The mutation M220I was found in resistant isolate AF2, and the mutation G54R was found in resistant isolates AF4, AF5 and AF6. Each monosporal clone had the same mutation as that of its respective parent isolate. Isolates AF1 and AF3 (susceptible to the drug) showed the normal sequence of the enzyme gene. Consequently, mutations in the cyp51A gene may be responsible for the resistance to itraconazole.
Superficially, reversible resistance to itraconazole, as well as changeable mutations in cyp51A, occurred in A. fumigatus in this patient. Although A. fumigatus resistant to itraconazole in vitro and in an animal aspergillosis model was reported previously,6–10 and A. fumigatus with acquired resistance to itraconazole from an aspergilloma patient was documented,9 reversible resistance to itraconazole has not been reported clinically. In A. fumigatusin vitro exposed to itraconazole, the incidence of resistance to the drug was rare, and was estimated to be 1 × 10–8.8,12 Alternatively, in this patient, a small portion of A. fumigatus acquired mutations in the cyp51A gene and resistance to itraconazole after itraconazole treatment. The mutant clone grew predominantly under the pressure of itraconazole, and AF2 or AF4, AF5 and AF6 were isolated. After discontinuation of the drug, the parent clone susceptible to itraconazole may be more suited to grow, and AF3 was found. More in vitro and animal experiments are needed to understand susceptibility changes to itraconazole and variable mutations in cyp51A in A. fumigatus from this patient.
Alignment of the amino acid sequences of cytochrome P450 sterol 14-α-demethylases from A. fumigatus and M. tuberculosis (MT-CYP51) locates the M220I substitution found in this study in the loop between helices F and G. According to the crystal structure of cytochrome P450 14-α-sterol demethylase from other species, there is a pathway consisting of the F/G loop, the β1 sheet and either the B helix or the B/B’ loop in the model.29 Enzyme substrate and azole antifungals enter into the active centre of the enzyme through the pathway.30,31 The M220I substitution may change the conformation of the F/G loop and the pathway, and, as a result, reduce the affinity for azoles. The G54R mutation is located in a very conserved region, possibly involved in inhibitor- or substrate-induced structure changes in the enzyme molecule.
In this study, no cross-resistance to itraconazole and voriconazole was found in the resistant isolates AF2, AF4, AF5 and AF6. Recently, a homology model of CYP51 from A. fumigatus, constructed on the basis of the X-ray structure of CYP51 from M. tuberculosis,32 suggested that some of the residues in the F/G loop (T215 to P230) in CYP51 were predicted to contact with itraconazole and posaconazole, but not directly with voriconazole.32 Mutation at G54 in CYP51A was associated with cross-resistance to itraconazole and posaconazole.12 However, the exact approach by which substitutions at M220 and G54 of CYP51A interfere with the binding of azole antifungals is uncertain.
Reduced susceptibility to itraconazole may also be related to decreased accumulation of the drug in cells due to overexpression of efflux pump genes and/or decreased penetration of the drug into the cells.7,8MDR1, 2, 3 and 4 and atrF genes, encoding multiple drug resistance-related transporters, may be involved in itraconazole resistance.7,27,33 In this study, expression of MDR3 and 4 genes was increased in the itraconazole-resistant isolates of AF2, AF4, AF5 and AF6, but the expression of MDR3 was also increased in AF3 susceptible to itraconazole. Therefore, the efflux pump genes may be, in part, related to the resistance to itraconazole. We also evaluated the expression changes of these genes in the six isolates in the absence and presence of itraconazole at the concentration of 0.25 × MIC (0.06 mg/L for AF1; 0.12 mg/L for AF3; 4 mg/L for AF2, AF4, AF5 and AF6). Expression of MDR1, 2, 3 and 4 remained unchanged in the six isolates, but that of atrF was slightly reduced in AF2 after exposure to itraconazole (data not shown). Failure to up-regulate the expression of these genes in the presence of itraconazole implies that these pump genes may not be the major factors in the resistance to itraconazole in the six isolates.
These authors contributed equally.
(a) Comparison of the radial growth of six isolates growing on PDA at 35°C. Radial growth was measured as the radius of the expanding colony in millimetres (y-axis) relative to hours of incubation (x-axis). (b) The MTT assay. Growth curve was measured using the production of formazan by the six isolates over time.
RAPD assay with the four primers: (a) primer R108; (b) primer 2; (c) primer 5; (d) primer R151. Samples were separated in 1.5% agarose gels and run in 1 × TAE at 3.0 V/cm. M, DNA molecular standards. Samples in lanes 1, 2, 3, 4, 5 and 6 were from isolates AF1, 2, 3, 4, 5 and 6, respectively. Samples in lanes 7, 8, 9,10 and 11 were from control strains.
(a) A point transversion mutation of G660T, which converts methionine into isoleucine at residue 220 (M220I) in cytochrome P450 sterol 14-α-demethylase, was found in AF2. No changes were found in the enzyme from the other five isolates. (b) Another point transition mutation of G160A, which converts glycine into arginine at residue 54 (G54R) in cytochrome P450 sterol 14-α-demethylase, was found in AF4, 5 and 6. No changes were found in the enzyme from isolates AF1, AF2 and AF3.
Primers used for amplification of the cyp51A gene
| Primer . | Sequence . |
|---|---|
| CYP1-L | 5′-CACCCTCCCTGTGTCTCCT |
| CYP1-R | 5′-AGCCTTGAAAGTTCGGTGAA |
| CYP2-L | 5′-CATGTGCCACTTATTGAGAAGG |
| CYP2-R | 5′-CCTTGCGCATGATAGAGTGA |
| CYP3-L | 5′-TTCCTCCGCTCCAGTACAAG |
| CYP3-R | 5′-CCTTTGAAGTCCTCGATGGT |
| Primer . | Sequence . |
|---|---|
| CYP1-L | 5′-CACCCTCCCTGTGTCTCCT |
| CYP1-R | 5′-AGCCTTGAAAGTTCGGTGAA |
| CYP2-L | 5′-CATGTGCCACTTATTGAGAAGG |
| CYP2-R | 5′-CCTTGCGCATGATAGAGTGA |
| CYP3-L | 5′-TTCCTCCGCTCCAGTACAAG |
| CYP3-R | 5′-CCTTTGAAGTCCTCGATGGT |
Primers used for amplification of the cyp51A gene
| Primer . | Sequence . |
|---|---|
| CYP1-L | 5′-CACCCTCCCTGTGTCTCCT |
| CYP1-R | 5′-AGCCTTGAAAGTTCGGTGAA |
| CYP2-L | 5′-CATGTGCCACTTATTGAGAAGG |
| CYP2-R | 5′-CCTTGCGCATGATAGAGTGA |
| CYP3-L | 5′-TTCCTCCGCTCCAGTACAAG |
| CYP3-R | 5′-CCTTTGAAGTCCTCGATGGT |
| Primer . | Sequence . |
|---|---|
| CYP1-L | 5′-CACCCTCCCTGTGTCTCCT |
| CYP1-R | 5′-AGCCTTGAAAGTTCGGTGAA |
| CYP2-L | 5′-CATGTGCCACTTATTGAGAAGG |
| CYP2-R | 5′-CCTTGCGCATGATAGAGTGA |
| CYP3-L | 5′-TTCCTCCGCTCCAGTACAAG |
| CYP3-R | 5′-CCTTTGAAGTCCTCGATGGT |
Primers used for quantitative RT-PCR
| Primer . | Sequence . | Reference sequence in GenBank . |
|---|---|---|
| tub1 L | 5′-CTG TAT CGA CAA CGA GGC TCT | AY048754 |
| tub1 R | 5′-AGT TGA GCT GAC CAG GGA AA | |
| CYP51 L | 5′-TTG CGT GCA GAG AAA AGT ATG | AF222068 |
| CYP51 R | 5′-GAC CTC TTC CGC ATT GAC AT | |
| atrF L | 5′-TGC CCA GAG AAA TCG ACA AC | AJ311940 |
| atrF R | 5′-CCA CCT CGT CGC AGA TAG TC | |
| MDR1 L | 5′-GCT CTT CCC TTG TTC ACA ATT C | U62933 |
| MDR1 R | 5′-CGG CAA TAC CGA GAT ACA CA | |
| MDR2 L | 5′-TGC CAC ATT CTT TAG CTC CAC | U62935 |
| MDR2 R | 5′-AAG ACC GAA CAT GCT TGA CC | |
| MDR3 L | 5′-GAT GCA TCC TGC AAA GTA CG | AF503774 |
| MDR3 R | 5′-AGG CTC CTT GGT GCT TGA C | |
| MDR4 L | 5′-CAC TGA ACG CAA CTC CTG AA | AF503773 |
| MDR4 R | 5′-TCT TTC TGG CTT CCT CCT CA |
| Primer . | Sequence . | Reference sequence in GenBank . |
|---|---|---|
| tub1 L | 5′-CTG TAT CGA CAA CGA GGC TCT | AY048754 |
| tub1 R | 5′-AGT TGA GCT GAC CAG GGA AA | |
| CYP51 L | 5′-TTG CGT GCA GAG AAA AGT ATG | AF222068 |
| CYP51 R | 5′-GAC CTC TTC CGC ATT GAC AT | |
| atrF L | 5′-TGC CCA GAG AAA TCG ACA AC | AJ311940 |
| atrF R | 5′-CCA CCT CGT CGC AGA TAG TC | |
| MDR1 L | 5′-GCT CTT CCC TTG TTC ACA ATT C | U62933 |
| MDR1 R | 5′-CGG CAA TAC CGA GAT ACA CA | |
| MDR2 L | 5′-TGC CAC ATT CTT TAG CTC CAC | U62935 |
| MDR2 R | 5′-AAG ACC GAA CAT GCT TGA CC | |
| MDR3 L | 5′-GAT GCA TCC TGC AAA GTA CG | AF503774 |
| MDR3 R | 5′-AGG CTC CTT GGT GCT TGA C | |
| MDR4 L | 5′-CAC TGA ACG CAA CTC CTG AA | AF503773 |
| MDR4 R | 5′-TCT TTC TGG CTT CCT CCT CA |
Primers used for quantitative RT-PCR
| Primer . | Sequence . | Reference sequence in GenBank . |
|---|---|---|
| tub1 L | 5′-CTG TAT CGA CAA CGA GGC TCT | AY048754 |
| tub1 R | 5′-AGT TGA GCT GAC CAG GGA AA | |
| CYP51 L | 5′-TTG CGT GCA GAG AAA AGT ATG | AF222068 |
| CYP51 R | 5′-GAC CTC TTC CGC ATT GAC AT | |
| atrF L | 5′-TGC CCA GAG AAA TCG ACA AC | AJ311940 |
| atrF R | 5′-CCA CCT CGT CGC AGA TAG TC | |
| MDR1 L | 5′-GCT CTT CCC TTG TTC ACA ATT C | U62933 |
| MDR1 R | 5′-CGG CAA TAC CGA GAT ACA CA | |
| MDR2 L | 5′-TGC CAC ATT CTT TAG CTC CAC | U62935 |
| MDR2 R | 5′-AAG ACC GAA CAT GCT TGA CC | |
| MDR3 L | 5′-GAT GCA TCC TGC AAA GTA CG | AF503774 |
| MDR3 R | 5′-AGG CTC CTT GGT GCT TGA C | |
| MDR4 L | 5′-CAC TGA ACG CAA CTC CTG AA | AF503773 |
| MDR4 R | 5′-TCT TTC TGG CTT CCT CCT CA |
| Primer . | Sequence . | Reference sequence in GenBank . |
|---|---|---|
| tub1 L | 5′-CTG TAT CGA CAA CGA GGC TCT | AY048754 |
| tub1 R | 5′-AGT TGA GCT GAC CAG GGA AA | |
| CYP51 L | 5′-TTG CGT GCA GAG AAA AGT ATG | AF222068 |
| CYP51 R | 5′-GAC CTC TTC CGC ATT GAC AT | |
| atrF L | 5′-TGC CCA GAG AAA TCG ACA AC | AJ311940 |
| atrF R | 5′-CCA CCT CGT CGC AGA TAG TC | |
| MDR1 L | 5′-GCT CTT CCC TTG TTC ACA ATT C | U62933 |
| MDR1 R | 5′-CGG CAA TAC CGA GAT ACA CA | |
| MDR2 L | 5′-TGC CAC ATT CTT TAG CTC CAC | U62935 |
| MDR2 R | 5′-AAG ACC GAA CAT GCT TGA CC | |
| MDR3 L | 5′-GAT GCA TCC TGC AAA GTA CG | AF503774 |
| MDR3 R | 5′-AGG CTC CTT GGT GCT TGA C | |
| MDR4 L | 5′-CAC TGA ACG CAA CTC CTG AA | AF503773 |
| MDR4 R | 5′-TCT TTC TGG CTT CCT CCT CA |
Mutations in the cyp51A gene and MICs of azole antifungals for the six clinical isolates
. | . | MIC (mg/L) . | . | |
|---|---|---|---|---|
| Isolate . | Mutation in cyp51A . | itraconazole . | voriconazole . | |
| AF1 | – | 0.25 | 0.25 | |
| AF2 | M220I | >16 | 1 | |
| AF3 | – | 0.5 | 0.5 | |
| AF4 | G54R | >16 | 0.5 | |
| AF5 | G54R | >16 | 0.5 | |
| AF6 | G54R | >16 | 0.5 | |
. | . | MIC (mg/L) . | . | |
|---|---|---|---|---|
| Isolate . | Mutation in cyp51A . | itraconazole . | voriconazole . | |
| AF1 | – | 0.25 | 0.25 | |
| AF2 | M220I | >16 | 1 | |
| AF3 | – | 0.5 | 0.5 | |
| AF4 | G54R | >16 | 0.5 | |
| AF5 | G54R | >16 | 0.5 | |
| AF6 | G54R | >16 | 0.5 | |
Mutations in the cyp51A gene and MICs of azole antifungals for the six clinical isolates
. | . | MIC (mg/L) . | . | |
|---|---|---|---|---|
| Isolate . | Mutation in cyp51A . | itraconazole . | voriconazole . | |
| AF1 | – | 0.25 | 0.25 | |
| AF2 | M220I | >16 | 1 | |
| AF3 | – | 0.5 | 0.5 | |
| AF4 | G54R | >16 | 0.5 | |
| AF5 | G54R | >16 | 0.5 | |
| AF6 | G54R | >16 | 0.5 | |
. | . | MIC (mg/L) . | . | |
|---|---|---|---|---|
| Isolate . | Mutation in cyp51A . | itraconazole . | voriconazole . | |
| AF1 | – | 0.25 | 0.25 | |
| AF2 | M220I | >16 | 1 | |
| AF3 | – | 0.5 | 0.5 | |
| AF4 | G54R | >16 | 0.5 | |
| AF5 | G54R | >16 | 0.5 | |
| AF6 | G54R | >16 | 0.5 | |
Relative expression levels of cyp51A, atrF, MDR1, MDR2, MDR3 and MDR4 in isolates AF1, 2, 3, 4, 5 and 6
| Gene . | AF1 . | AF2 . | AF3 . | AF4 . | AF5 . | AF6 . |
|---|---|---|---|---|---|---|
| cyp51A | 1.0 (0.93–1.07) | 1.40 (1.04–1.80) | 0.25 (0.22–0.35) | 1.24 (1.05–1.32) | 1.5 (1.48–1.64) | 0.98 (0.85–1.06) |
| atrF | 1.0 (0.92–1.09) | 0.80 (0.77–0.83) | 0.40 (0.36–0.43) | 0.58 (0.55–0.62) | 0.86 (0.79–0.89) | 0.75 (0.45–1.05) |
| MDR1 | 1.0 (0.86–1.24) | 0.35 (0.34–0.37) | 0.15 (0.14–0.16) | 0.20 (0.17–0.23) | 0.48 (0.46–0.50) | 0.56 (0.45–0.60) |
| MDR2 | 1.0 (0.96–1.06) | 0.58 (0.48–0.69) | 0.13 (0.12–0.15) | 0.69 (0.45–0.78) | 1.68 (1.47–1.91) | 1.02 (0.85–1.21) |
| MDR3 | 1.0 (0.96–1.06) | 6.20 (5.13–7.27) | 3.98 (3.26–4.70) | 2.08 (1.92–2.24) | 1.05 (0.85–1.23) | 1.35 (1.14–1.42) |
| MDR4 | 1.0 (0.85–1.08) | 1.28 (0.91–1.65) | 0.84 (0.63–1.04) | 1.25 (1.08–1.34) | 2.17 (2.07–2.33) | 1.36 (1.22–1.45) |
| Gene . | AF1 . | AF2 . | AF3 . | AF4 . | AF5 . | AF6 . |
|---|---|---|---|---|---|---|
| cyp51A | 1.0 (0.93–1.07) | 1.40 (1.04–1.80) | 0.25 (0.22–0.35) | 1.24 (1.05–1.32) | 1.5 (1.48–1.64) | 0.98 (0.85–1.06) |
| atrF | 1.0 (0.92–1.09) | 0.80 (0.77–0.83) | 0.40 (0.36–0.43) | 0.58 (0.55–0.62) | 0.86 (0.79–0.89) | 0.75 (0.45–1.05) |
| MDR1 | 1.0 (0.86–1.24) | 0.35 (0.34–0.37) | 0.15 (0.14–0.16) | 0.20 (0.17–0.23) | 0.48 (0.46–0.50) | 0.56 (0.45–0.60) |
| MDR2 | 1.0 (0.96–1.06) | 0.58 (0.48–0.69) | 0.13 (0.12–0.15) | 0.69 (0.45–0.78) | 1.68 (1.47–1.91) | 1.02 (0.85–1.21) |
| MDR3 | 1.0 (0.96–1.06) | 6.20 (5.13–7.27) | 3.98 (3.26–4.70) | 2.08 (1.92–2.24) | 1.05 (0.85–1.23) | 1.35 (1.14–1.42) |
| MDR4 | 1.0 (0.85–1.08) | 1.28 (0.91–1.65) | 0.84 (0.63–1.04) | 1.25 (1.08–1.34) | 2.17 (2.07–2.33) | 1.36 (1.22–1.45) |
Relative expression levels of cyp51A, atrF, MDR1, MDR2, MDR3 and MDR4 in isolates AF1, 2, 3, 4, 5 and 6
| Gene . | AF1 . | AF2 . | AF3 . | AF4 . | AF5 . | AF6 . |
|---|---|---|---|---|---|---|
| cyp51A | 1.0 (0.93–1.07) | 1.40 (1.04–1.80) | 0.25 (0.22–0.35) | 1.24 (1.05–1.32) | 1.5 (1.48–1.64) | 0.98 (0.85–1.06) |
| atrF | 1.0 (0.92–1.09) | 0.80 (0.77–0.83) | 0.40 (0.36–0.43) | 0.58 (0.55–0.62) | 0.86 (0.79–0.89) | 0.75 (0.45–1.05) |
| MDR1 | 1.0 (0.86–1.24) | 0.35 (0.34–0.37) | 0.15 (0.14–0.16) | 0.20 (0.17–0.23) | 0.48 (0.46–0.50) | 0.56 (0.45–0.60) |
| MDR2 | 1.0 (0.96–1.06) | 0.58 (0.48–0.69) | 0.13 (0.12–0.15) | 0.69 (0.45–0.78) | 1.68 (1.47–1.91) | 1.02 (0.85–1.21) |
| MDR3 | 1.0 (0.96–1.06) | 6.20 (5.13–7.27) | 3.98 (3.26–4.70) | 2.08 (1.92–2.24) | 1.05 (0.85–1.23) | 1.35 (1.14–1.42) |
| MDR4 | 1.0 (0.85–1.08) | 1.28 (0.91–1.65) | 0.84 (0.63–1.04) | 1.25 (1.08–1.34) | 2.17 (2.07–2.33) | 1.36 (1.22–1.45) |
| Gene . | AF1 . | AF2 . | AF3 . | AF4 . | AF5 . | AF6 . |
|---|---|---|---|---|---|---|
| cyp51A | 1.0 (0.93–1.07) | 1.40 (1.04–1.80) | 0.25 (0.22–0.35) | 1.24 (1.05–1.32) | 1.5 (1.48–1.64) | 0.98 (0.85–1.06) |
| atrF | 1.0 (0.92–1.09) | 0.80 (0.77–0.83) | 0.40 (0.36–0.43) | 0.58 (0.55–0.62) | 0.86 (0.79–0.89) | 0.75 (0.45–1.05) |
| MDR1 | 1.0 (0.86–1.24) | 0.35 (0.34–0.37) | 0.15 (0.14–0.16) | 0.20 (0.17–0.23) | 0.48 (0.46–0.50) | 0.56 (0.45–0.60) |
| MDR2 | 1.0 (0.96–1.06) | 0.58 (0.48–0.69) | 0.13 (0.12–0.15) | 0.69 (0.45–0.78) | 1.68 (1.47–1.91) | 1.02 (0.85–1.21) |
| MDR3 | 1.0 (0.96–1.06) | 6.20 (5.13–7.27) | 3.98 (3.26–4.70) | 2.08 (1.92–2.24) | 1.05 (0.85–1.23) | 1.35 (1.14–1.42) |
| MDR4 | 1.0 (0.85–1.08) | 1.28 (0.91–1.65) | 0.84 (0.63–1.04) | 1.25 (1.08–1.34) | 2.17 (2.07–2.33) | 1.36 (1.22–1.45) |
This project was supported by the National Natural Science Foundation Committee (no. 30170054).
References
Latge, J.-P. (
Georgopapadakou, N. H. & Walsh, T. J. (
Dannaoui, E., Persat, F., Monier, M. F. et al. (
Denning, D. W. (
Johnson, E. M. & Warnock, D. W. (
Chryssanthou, E. (
Denning, D. W., Venkateswarlu, K., Oakley, K. L. et al. (
Manavathu, E. K., Vazquez, J. A. & Chandrasekar, P. H. (
Dannaoui, E., Borel, E., Monier, M. F. et al. (
Dannaoui, E., Borel, E., Persat, F. et al. (
Diaz-Guerra, T. M., Mellado, E., Cuenca-Estrella, M. et al. (
Mann, P. A., Parmegiani, R. M., Wei, S. Q. et al. (
Meletiadis, J., Mouton, J. W., Meis, J. F. G. M. et al. (
Shimizu, K. & Keller, N. P. (
National Committee for Clinical Laboratory Standards. (
Lin, D., Lehmann, P. F., Hamory, B. H. et al. (
James, M. J., Lasker, B. A., McNeil, M. M. et al. (
Lasker, B. A., Elie, C. M., Lott, T. J. et al. (
Loudon, K. W., Burnie, J. P., Coke, A. P. et al. (
Aufauvre-Brown, A., Cohen, J. & Holden, D. W. (
Edlind, T. D., Henry, K. W., Metera, K. A. et al. (
Anderson, M. J., Gull, K. & Denning, D. W. (
Chazalet, V., Debeaupuis, J. P., Sarfati, J. et al. (
Debeaupuis, J. P., Sarfati, J., Chazalet, V. et al. (
Girardin, H., Sarfati, J., Kobayashi, H. et al. (
Girardin, H., Sarfati, J., Traore, F. et al. (
Nascimento, A. M., Goldman, G. H., Park, S. et al. (
Mellado, E., Garcia-Effron, G., Alcazar-Fuoli, L. et al. (
Hasemann, C. A., Kurumbail, R. G., Boddupalli, S. S. et al. (
Podust, L. M., Poulos, T. L. & Waterman, M. R. (
Winn, P. J., Ludemann, S. K., Gauges, R. et al. (
Xiao, L., Madison, V., Chau, A. S. et al. (