-
PDF
- Split View
-
Views
-
Cite
Cite
Vittorio Sambri, Antonella Marangoni, Lorenzo Giacani, Renato Gennaro, Rossella Murgia, Roberto Cevenini, Marina Cinco, Comparative in vitro activity of five cathelicidin-derived synthetic peptides against Leptospira, Borrelia and Treponema pallidum, Journal of Antimicrobial Chemotherapy, Volume 50, Issue 6, December 2002, Pages 895–902, https://doi.org/10.1093/jac/dkf220
Close - Share Icon Share
Abstract
Objective: The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of five different cathelicidin-derived synthetic peptides (SMAP-29, LL-37, PG-1, CRAMP and BMAP-28) for Leptospira interrogans, Borrelia spp. and Treponema pallidum subsp. pallidum were investigated in vitro.
Methods: The MIC of individual peptides was defined as the lowest concentration able to inhibit the motility of spirochaetes after 2 h of incubation, as detected by dark-field microscopy. The MBC of individual peptides was defined as the lowest concentration at which no spirochaetes were subcultured either in cathelicidin-free medium (leptospires and borreliae) or in hamsters (T. pallidum).
Results: The MIC values of peptides for leptospires were highly variable, depending on the compound and the strain used. Of the five cathelicidin-derived peptides, SMAP-29 from sheep and BMAP-28 from cattle were the most active against L. interrogans serovars, with MIC values varying between 3 and 51 mg/L, depending on the strains. The MICs of the remaining synthetic peptides ranged between 4.3 and 224 mg/L. The MIC values of synthetic peptides for T. pallidum ranged between 32.3 mg/L for PG-1 and 449.4 mg/L for LL-37. The MICs of all cathelicidin-derived peptides tested for Borrelia strains ranged between 307 and 449.4 mg/L. The activity of the peptides on the motility of spirochaetes was both dose- and time-dependent. The MBC values of the peptides were the same as the MIC values.
Conclusion: The results of this study demonstrate that the activity of cathelicidin-derived peptides against spirochaetes is fast and highly variable, depending on the species and the strain.
Received 15 March 2002; returned 19 June 2002; revised 5 August 2002; accepted 23 August 2002
Introduction
Cathelicidin-derived antimicrobial peptides are a family of antimicrobial peptides identified in mammalian species.1,2 Despite notable structural diversity, their evolutionary relationship can be inferred from the highly conserved proregion that they share in the intracellular storage form. This N-terminal propiece of ∼100 residues was named cathelin after a polypeptide isolated from pig leucocytes prior to the identification of cathelicidins,3 but it was later seen to correspond to the proregion of a porcine antimicrobial peptide. Cathelicidins are stored as inactive propeptides in the secretory granules of neutrophils,4 and the active peptides are liberated by proteolytic processing upon cellular stimulation and may be released into the phagosome or extracellularly.4–6 In addition to myeloid cells, cathelicidins are expressed in other tissues and blood cells. For instance, the human CAP-18/ LL-37 has been identified in testis,7 in squamous epithelia,8,9 and in specific lymphocyte and monocyte populations.10 The number of different cathelicidins varies between species, with a single congener found in humans1 and up to 10 found in a member of the mammalian order Artiodactyla,1 which consists of the even-toed ungulates.1 The C-terminal antimicrobial domains of different members of this family vary considerably in size and structure and include α-helical, Pro- and Arg-rich, Trp-rich and Cys-containing peptides.1,11,12 In general, cathelicidin-derived peptides exert a potent and broad-spectrum activity in vitro against bacteria and fungi, including clinical isolates multi-resistant to conventional antibiotics, and they have a protective effect in animal models of infection.1,13,14 In addition, some of these peptides bind to lipopolysaccharide (LPS) and neutralize its effects in in vivo models of endotoxaemia.15 These features generated considerable interest in using these peptides as basic compounds for the development of novel anti-infective agents. Although their bactericidal activity has been tested extensively on representative Gram-positive and -negative bacteria and fungi,2,11,12,16 the pathogenic spirochaetes have not been systematically tested.17 The oxygen-dependent mechanisms of neutrophils have a scanty killing effect on Borrelia burgdorferi18 and leptospires (M. Cinco, unpublished data). Therefore, study was made of the potential spirochaeticidal activity of the cationic peptides of neutrophils, which account for an important part of their oxygen-independent killing mechanisms.17,19 In this investigation, the in vitro activity of five synthetic cathelicidin-derived peptides was determined against different pathogenic spirochaetes. Four of the peptides had an α-helical conformation and one a β-sheet hairpin structure.
Materials and methods
Bacterial strains and growing conditions
The following species of spirochaetes were used: Leptospira interrogans serovars copenhageni (Wijnberg strain), hardjo (Hardioprajitno strain), pomona (Mezzano strain), icterohaemorrhagiae (Bianchi strain), Borrelia garinii (P/Bi and BITS strains), Borrelia burgdorferisensu stricto (IRS and Alcaide strains), Borrelia afzelii (BL3 strain), Borrelia anserina (NiNL strain) and the Nichols strain of Treponema pallidum subsp. pallidum. L. interrogans strains were grown at 30°C in Ellinghausen–McCullough–Johnson–Harris (EMJH) medium,20,21 whereas the strains of B. burgdorferisensu lato (s.l.) were maintained at 34°C in Barbour–Stoenner–Kelly (BSK) II medium, as previously reported.22B. anserina NiNL, an avian pathogenic strain, was obtained from the blood of infected chicks and purified in BSK-II medium to produce a blood-cell-free suspension as already described.23T. pallidum subsp. pallidum, Nichols strain, was originally obtained from the Statens Serum Institute (Copenhagen, Denmark) and maintained by passages in testicles of adult male New Zealand White rabbits every 10–14 days.24 Animals were given antibiotic-free food and water ad libitum. Treponemes were extracted from infected testicles with 0.15 M phosphate-buffered saline (PBS) containing 2% (v/v) heat-inactivated rabbit serum (HIRS) as reported elsewhere.24
Peptides
Five synthetic cathelicidin-derived peptides from diverse mammalian species were used: SMAP-29 from sheep,25,26 CRAMP from mice,27 LL-37 from humans,7 BMAP-28 from cattle28 and PG-1 from pigs.29 Each peptide was synthesized as reported previously by solid phase Fmoc chemistry.24 PG-1 was air-oxidized at a concentration of 0.1 g/L under gentle stirring in 0.1 M Tris–HCl buffer, pH 7.5, for 24–36 h at room temperature. The peptides were purified by reversed-phase high-pressure liquid chromatography on a preparative C18 Delta-Pak column with appropriate water–acetonitrile gradients, in the presence of 0.1% trifluoroacetic acid, as described previously.28 Purity of the peptides was assessed by analytical reversed-phase chromatography with a C18 X-Terra column (Waters, Milford, MA, USA) and their molecular mass was determined with an API I electrospray mass spectrometer (SCIEX; Perkin Elmer, Norwalk, CT, USA). After purification, the peptides were lyophilized in 10 mM HCl and stored at 4°C. Stock solutions of each peptide at 1 g/L concentration were prepared in PBS for the evaluation of antimicrobial activity and stored frozen at –80°C in 0.02 mL aliquots until used.
Evaluation of the antibacterial activity of cathelicidin-derived peptides
Stock solutions of individual peptides were two-fold diluted in test tubes containing a total volume of 0.12 mL of spirochaete suspension in PBS–HIRS. The number of spirochaetes was then adjusted in each individual test tube to 1 × 108/mL. Each series of tubes contained duplicates of individual peptide concentrations, plus two control tubes where the peptide was omitted. The tubes were then incubated under appropriate conditions: 95% N2/5% CO2 for T. pallidum experiments and atmospheric air for Leptospira spp. and Borrelia spp. The results reported are the mean of three different experiments.
Determination of MIC and MBC
A uniform and standardized method to test antimicrobials against human pathogenic spirochaetes has not been established. For borreliae and leptospires, the MIC is generally considered the drug concentration at which no motile organisms are observed after 48–72 h of incubation.30,31
In this study, due to the rapid action exerted by the cathelicidin-derived peptides against the microbial cells, preliminary experiments carried out with different concentrations of peptides over a time up to 72 h confirmed that the action of peptides on the motility of borreliae and leptospires was rapid, since the bacteria lost their motility within 2 h of the addition of the various concentrations of the drug. Therefore, the MIC of cathelicidin-derived peptides for spirochaetes was defined as the lowest concentration at which no motile spirochaetes were observed by dark-field microscopy (DF) after 2 h of incubation. T. pallidum cannot be cultivated in vitro; however, this microorganism remains viable for at least 8–16 h in a suitable environment.24,32 Therefore, in this study, due to the rapid action exerted by the cathelicidin-derived peptides, it proved possible to detect the MIC for T. pallidum as it has been obtained for leptospires and borreliae.
The MBCs for borreliae and leptospires are determined by the absence of spirochaetes in subcultures on microscopic examination after various incubation periods.30 The MBC determinations made by these methods require virtually 100% killing of the final inoculum, which is a strict requirement for any antimicrobial drug.33 In this study, the MBC of cathelicidin-derived peptides for borreliae and leptospires was defined as the lowest concentration of the peptides at which no spirochaetes were subcultured (see below). The T. pallidum MBC determination, following Norris et al.,34 was defined as the lowest concentration that abrogated the ability of treated treponemes to cause infection in animals (see below). To determine the MBCs for leptospires and borreliae, after 2 h of incubation in the presence of cathelicidin-derived peptides (each dilution in triplicate) the bacteria were collected by centrifugation and added to vials containing EMJH or BSK-II medium, respectively, up to a final volume of 5 mL. Cultures were then incubated as above over a 2 week period, with daily DF observation.
The MBC for T. pallidum was evaluated in hamsters (authorization no. 16745 by the Ethical-Scientific Committee of the University of Bologna, 07/23/1999). After 2 h of incubation in the presence of peptides, treponemes were collected by centrifugation and inoculated intradermally (1 × 107 organisms in 0.1 mL volume: this is the dose that ensures 100% infection of the animals injected with live organisms; V. Sambri, unpublished data) in an abdominal shaved area of male, 8-week-old, Syrian hamster (LSH strain; Morini, S. Polo d’Enza, Italy). Five hamsters were used for each peptide dilution corresponding to the MIC and the half-MIC, respectively. Each experiment was performed in triplicate. The animals were then caged individually and given antibiotic-free food and water ad libitum. Each hamster was checked weekly over 7 weeks for the appearance of typical skin lesions at the inoculum site.
Detection of T. pallidum infection in hamsters
In the case of an evident chancre, the presence of T. pallidum in the exudate was inferred by DF observation and direct immunofluorescence assay (DFA) with rabbit anti-T. pallidum serum35 and by the evaluation of animal immune response to T. pallidum by western blotting. At the end of the 7 week observation, the hamsters were euthanized and the presence of treponemes was evaluated by DF and DFA on homogenates obtained from spleen and abdominal lymph nodes, as follows: inguinal lymph nodes and the spleen were excised, and teased apart in 0.06 and 0.5 mL of sterile PBS, respectively. Two series of five slides each were prepared: one series prepared with smears obtained from the lymph nodes and the other from the spleen. Two slides of each series were immediately read by DF observation, under the conditions reported above; the remaining slides were air dried, fixed in cold acetone for 10 min and processed by DFA, as indicated below.
As a source of anti-T. pallidum-specific antibodies for the DFA, a serum sample of an experimentally infected rabbit was used. As fluorescein conjugate, swine anti-rabbit immunoglobulins (Dako, Copenhagen, Denmark) diluted 1:30 in PBS were used.
The antibody response to T. pallidum in the animals was evaluated by western blotting performed with peroxidase-conjugated anti-hamster G immunoglobulins (Organon Teknika, Turnhout, Belgium), as described previously.36 A test was considered positive when at least three of the following four bands were present: TpN15, TpN17, TmpA and TpN47, with apparent molecular masses of 15.5, 17, 44.5 and 47 kDa, respectively.37 A test was considered negative when no band or fewer than three of the above-listed T. pallidum-specific bands were present;36 the reader was blinded to the identity of the serum samples when the western blotting strips were examined.
Results
The MIC values of the five peptides for Leptospira, Borrelia and T. pallidum, reported in Table 1, are the means of three different experiments. In particular, the MIC of SMAP-29 for leptospires was 12.8 mg/L when tested against serovars hardjo, pomona and icterohaemorrhagiae, and 51.2 mg/L when tested with serovar copenhageni. The remaining peptides showed variable activity against leptospires: in particular, the second most active peptide was BMAP-28, with MIC values ranging from 3 mg/L (serovar copenhageni) to 36.9 mg/L (serovar icterohaemorrhagiae). CRAMP and LL-37 were the least active: the MIC values ranging between 33.8 mg/L (the MIC of CRAMP for serovars copenhageni and pomona) and 224.7 mg/L (the MIC of LL-37 for serovar icterohaemorrhagiae). All the peptides tested against B. burgdorferi s.l. strains and the strain Ni-NL of B. anserina showed MICs ≥ 204 mg/L. As for T. pallidum, PG-1 was the most active (MIC 32.3 mg/L), followed by SMAP-29 (MIC 38.4 mg/L). In contrast, LL-37, CRAMP and BMAP-28 were much less active.
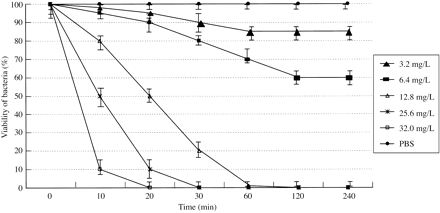
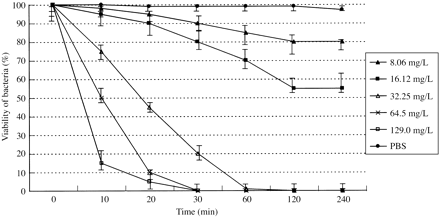
The activity of peptides on the motility of spirochaetes was both dose- and time-dependent. The results are summarized in Figures 1 and 2, which show typical curves for the two most active peptides used in this study, SMAP-29 and PG-1, when tested against leptospires and treponemes, respectively. The higher the concentration used, the faster the activity exerted by synthetic peptides against spirochaetes. In addition, the peptides, when used at a concentration higher than their MIC, were able to immobilize from 50% to 85% of bacteria in 10 min, whereas lower concentrations were unable to immobilize bacteria over a period of 4 h. No immobilization was detected for leptospires when observed for as long as 72 h (data not reported). Control preparations of spirochaetes containing no peptides were seen to maintain their motility at ratios higher than 95% over the total incubation of 4 h.
Determination of the MBC
The MBC values for leptospires and borreliae were determined by recultivating spirochaetes in cathelicidin-free medium, whereas the MBCs for T. pallidum were determined by recultivating treponemes in animals. The MBCs were the same as the MICs for all the spirochaetes tested.
Some details of the bactericidal activity of peptides against treponemes are reported in Table 2. All the animals injected with treponemes incubated in the presence of peptides at a concentration equal to the MIC did not develop any chancre and did not show the presence of spirochaetes in the spleen and lymph nodes by DF and/or DFA. In contrast, several animals injected with T. pallidum treated with concentrations of cathelicidin-derived peptides lower than the MIC developed a skin lesion 19–40 days after injection. Similarly, two of five control animals, injected with treponemes, not treated with any peptide, developed skin lesions after 25 and 37 days, respectively. In addition, the presence of treponemes in biopsies was found by DF and DFA in all the hamsters inoculated with T. pallidum treated with cathelicidin-derived peptides at concentrations lower than the MIC and in the controls, irrespective of the presence of the typical chancre. Animal infection was also confirmed by serological data obtained by western blot analysis; all the sera obtained from hamsters positive by DF and/or by DFA clearly recognized several T. pallidum antigens, including the four specific bands TpN47, TmpA, TpN17 and TpN15. In contrast, sera obtained from animals inoculated with treponemes treated with peptides at concentrations equal to the MIC, and negative by DF and/or DFA examinations, were also negative by western blot.
Discussion
Effective host defence against microbial invasion requires the innate immune system, whose response is both rapid and independent of prior exposure. Neutrophil leucocytes are central cellular effectors of the innate defence system and are expected to play a key role in the clearance of Leptospira spp., Borrelia spp. and T. pallidum during the early phases of infection. After phagocytosis, the clearance of these spirochaetes may proceed through the bactericidal oxygen-dependent and -independent machineries of the neutrophil leucocytes. The oxygen-dependent mechanisms have a scanty killing effect on B. burgdorferi18 and leptospires (M. Cinco, unpublished data). Although the bactericidal activity of neutrophil cationic peptides has been extensively tested on a number of Gram-positive and -negative bacteria,1 only sparse data have been produced on their antispirochaetal activity. Some representatives of defensins found in testicular lesions of syphilitic rabbits have been found to be active in vitro against T. pallidum, suggesting that they play a role in the control of local T. pallidum infection.38,39 Scocchi and co-workers40 reported that the bactenecins Bac5 and Bac7 were bactericidal against L. interrogans and Leptospira biflexa, whereas they were inactive against B. burgdorferi.
The results obtained in this study showed that of five cathelicidin-derived peptides, SMAP-29 from sheep and BMAP-28 from cattle were the most active against L. interrogans serovars, with some differences depending on strains. This observation supports previous data obtained with the bovine bactenecins Bac5 and Bac7,40 and with other cationic peptides of different animal origin (M. Cinco, unpublished data). The PG-1 and SMAP-29 peptides were the only peptides showing intermediate activity against T. pallidum. Finally, all five peptides were much less active (the MICs ranged between 204 and 449 mg/L) against Borrelia strains. It is interesting to note that the killing activity of LL-37 peptide against B. burgdorferi found in this study (449 mg/L) using a high ionic strength buffer (PBS) is comparable to the results obtained by Lusitani et al.17 (>200 mg/L) using the same buffer.
Previous in vitro studies showed that SMAP-29 is the most active of the cathelicidin peptides tested so far for several bacteria, including Pseudomonasaeruginosa, Escherichia coli and Staphylococcus aureus, with MICs ranging between 0.10 and 1.24 mg/L.11,13 The MIC of this drug for P. aeruginosa strains isolated from patients with cystic fibrosis ranged from 0.06 to 8 mg/L.16 The MIC of the same drug for various ovine respiratory pathogens ranged between 0.06 and >20 mg/L.13 In this study, SMAP-29 was the most active drug against leptospires (MIC ranging between 4 and 16 mg/L), whereas the MIC for T. pallidum was 12 mg/L. These data show that leptospires and T. pallidum are clearly less sensitive than other Gram-positive and -negative bacteria to SMAP-29, whereas borreliae were sensitive only at a very high concentration of the drug.
The LL-37 cathelicidin peptide, the only peptide of human origin tested in this study, was much less active, confirming the results of previous studies with other bacteria.11,16
The cationic nature of antimicrobial peptides is important for their initial interaction with negatively charged components of bacterial outer and inner membranes. Antimicrobial peptides adopt an α-helical structure, which seems to be essential for their antibacterial activity, only after binding to negatively charged bacterial cell wall components such as LPS or lipoteichoic acid.11,16,26,41 Unlike leptospires, neither T. pallidum nor B. burgdorferi contains LPS.42,43 This might partly explain the results obtained in the present study, where the overall activity of cationic peptides against leptospires was clearly higher than that observed against Borrelia strains and T. pallidum. Since B. burgdorferi and T. pallidum do not present LPS but express several surface lipoproteins, it is possible that the action of the cathelicidin peptides against these bacteria is due to the ability of peptides to bind lipoproteins.
Previous studies and the present one suggest that cathelicidin peptides have substantial in vitro activity against several bacteria. Much remains to be done to evaluate their interactions with constituents of biological fluids and their in vivo efficacy and safety. Therefore, studies in animal models are needed to understand whether the in vivo studies will confirm in vitro results.
Acknowledgements
This work was supported in part by grants from MURST, ex quota 40%—2000, No. MM06038195 and No. MM05265243, and from the Italian CNR Target Project on Biotechnology.
Corresponding author. Tel: +39-051-429-0913; Fax: +39-051-341632; E-mail: cevenini@almadns.unibo.it
Figure 1. Time curves of motility inhibition of L. interrogans serovar icterohaemorrhagiae by SMAP-29. The activity of different concentrations of SMAP-29 was compared. A suspension of spirochaetes in PBS without any peptide added was used as a negative control. The percentage of motile organisms was detected under DF observation of triplicate 0.01 mL samples of the bacterial suspensions obtained every 10 min during the first half hour and every 30 min for 4 h.
Figure 2. Time curves of motility inhibition of T. pallidum subsp. pallidum by PG-1. The activity of different concentrations of PG-1 was compared. A suspension of spirochaetes in PBS without any peptide added was used as a negative control. The percentage of motile organisms was detected under DF observation of triplicate 0.01 mL samples of bacterial suspensions obtained every 10 min during the first half hour of incubation with peptides and every 30 min for 4 h.
In vitro MBCs of five different cathelicidin-derived peptides for L. interrogans, Borrelia spp. and T. pallidum subsp. pallidum
| L. interrogans (mg/L) | ||||||
| Peptide | serovar copenhageni | serovar hardjo | serovar pomona | serovar icterohae-morrhagiae | Borrelia spp.a (mg/L) | T. pallidum (mg/L) |
| SMAP-29 | 51.2 | 12.8 | 12.8 | 12.8 | 204 | 38.4 |
| LL-37 | 143.8 | 143.8 | 143.8 | 224.7 | 449.4 | 449.4 |
| PG-1 | 69 | 4.3 | 4.3 | 215.6 | 215.6 | 32.3 |
| CRAMP | 67.5 | 33.8 | 33.8 | 131.3 | 375 | 375 |
| BMAP-28 | 3 | 24 | 24 | 36.9 | 307 | 246.2 |
| L. interrogans (mg/L) | ||||||
| Peptide | serovar copenhageni | serovar hardjo | serovar pomona | serovar icterohae-morrhagiae | Borrelia spp.a (mg/L) | T. pallidum (mg/L) |
| SMAP-29 | 51.2 | 12.8 | 12.8 | 12.8 | 204 | 38.4 |
| LL-37 | 143.8 | 143.8 | 143.8 | 224.7 | 449.4 | 449.4 |
| PG-1 | 69 | 4.3 | 4.3 | 215.6 | 215.6 | 32.3 |
| CRAMP | 67.5 | 33.8 | 33.8 | 131.3 | 375 | 375 |
| BMAP-28 | 3 | 24 | 24 | 36.9 | 307 | 246.2 |
aBorrelia species used were: B. garinii, B. burgdorferi s.s., B. afzelii and B. anserina.
In vitro MBCs of five different cathelicidin-derived peptides for L. interrogans, Borrelia spp. and T. pallidum subsp. pallidum
| L. interrogans (mg/L) | ||||||
| Peptide | serovar copenhageni | serovar hardjo | serovar pomona | serovar icterohae-morrhagiae | Borrelia spp.a (mg/L) | T. pallidum (mg/L) |
| SMAP-29 | 51.2 | 12.8 | 12.8 | 12.8 | 204 | 38.4 |
| LL-37 | 143.8 | 143.8 | 143.8 | 224.7 | 449.4 | 449.4 |
| PG-1 | 69 | 4.3 | 4.3 | 215.6 | 215.6 | 32.3 |
| CRAMP | 67.5 | 33.8 | 33.8 | 131.3 | 375 | 375 |
| BMAP-28 | 3 | 24 | 24 | 36.9 | 307 | 246.2 |
| L. interrogans (mg/L) | ||||||
| Peptide | serovar copenhageni | serovar hardjo | serovar pomona | serovar icterohae-morrhagiae | Borrelia spp.a (mg/L) | T. pallidum (mg/L) |
| SMAP-29 | 51.2 | 12.8 | 12.8 | 12.8 | 204 | 38.4 |
| LL-37 | 143.8 | 143.8 | 143.8 | 224.7 | 449.4 | 449.4 |
| PG-1 | 69 | 4.3 | 4.3 | 215.6 | 215.6 | 32.3 |
| CRAMP | 67.5 | 33.8 | 33.8 | 131.3 | 375 | 375 |
| BMAP-28 | 3 | 24 | 24 | 36.9 | 307 | 246.2 |
aBorrelia species used were: B. garinii, B. burgdorferi s.s., B. afzelii and B. anserina.
Activity of cathelicidin-derived peptides against T. pallidum subsp. pallidum, determined by hamster intradermal inoculation after 2 h in vitro incubation
| Cathelicidin-derived peptides (mg/L) | Animals showing cutaneous lesions | Animals positive by DFA and/or DF on lymph nodes and spleen homogenates | Animal sera positive by western blotting |
| SMAP-29 (38.4a) | 0/5 | 0/5 | 0/5 |
| SMAP-29 (19.2) | 2/5 | 5/5 | 5/5 |
| LL-37 (449.4a) | 0/5 | 0/5 | 0/5 |
| LL-37 (224.7) | 1/5 | 5/5 | 5/5 |
| PG-1 (32.3a) | 0/5 | 0/5 | 0/5 |
| PG-1 (16.1) | 3/5 | 5/5 | 5/5 |
| CRAMP (375a) | 0/5 | 0/5 | 0/5 |
| CRAMP (187.5) | 3/5 | 5/5 | 5/5 |
| BMAP-28 (246.2a) | 0/5 | 0/5 | 0/5 |
| BMAP-28 (123.1) | 2/5 | 5/5 | 5/5 |
| Controlb | 2/5 | 5/5 | 5/5 |
| Cathelicidin-derived peptides (mg/L) | Animals showing cutaneous lesions | Animals positive by DFA and/or DF on lymph nodes and spleen homogenates | Animal sera positive by western blotting |
| SMAP-29 (38.4a) | 0/5 | 0/5 | 0/5 |
| SMAP-29 (19.2) | 2/5 | 5/5 | 5/5 |
| LL-37 (449.4a) | 0/5 | 0/5 | 0/5 |
| LL-37 (224.7) | 1/5 | 5/5 | 5/5 |
| PG-1 (32.3a) | 0/5 | 0/5 | 0/5 |
| PG-1 (16.1) | 3/5 | 5/5 | 5/5 |
| CRAMP (375a) | 0/5 | 0/5 | 0/5 |
| CRAMP (187.5) | 3/5 | 5/5 | 5/5 |
| BMAP-28 (246.2a) | 0/5 | 0/5 | 0/5 |
| BMAP-28 (123.1) | 2/5 | 5/5 | 5/5 |
| Controlb | 2/5 | 5/5 | 5/5 |
aMBC.
bNo cathelicidin-derived peptides added to the suspension of treponemes before injection into the hamsters.
Activity of cathelicidin-derived peptides against T. pallidum subsp. pallidum, determined by hamster intradermal inoculation after 2 h in vitro incubation
| Cathelicidin-derived peptides (mg/L) | Animals showing cutaneous lesions | Animals positive by DFA and/or DF on lymph nodes and spleen homogenates | Animal sera positive by western blotting |
| SMAP-29 (38.4a) | 0/5 | 0/5 | 0/5 |
| SMAP-29 (19.2) | 2/5 | 5/5 | 5/5 |
| LL-37 (449.4a) | 0/5 | 0/5 | 0/5 |
| LL-37 (224.7) | 1/5 | 5/5 | 5/5 |
| PG-1 (32.3a) | 0/5 | 0/5 | 0/5 |
| PG-1 (16.1) | 3/5 | 5/5 | 5/5 |
| CRAMP (375a) | 0/5 | 0/5 | 0/5 |
| CRAMP (187.5) | 3/5 | 5/5 | 5/5 |
| BMAP-28 (246.2a) | 0/5 | 0/5 | 0/5 |
| BMAP-28 (123.1) | 2/5 | 5/5 | 5/5 |
| Controlb | 2/5 | 5/5 | 5/5 |
| Cathelicidin-derived peptides (mg/L) | Animals showing cutaneous lesions | Animals positive by DFA and/or DF on lymph nodes and spleen homogenates | Animal sera positive by western blotting |
| SMAP-29 (38.4a) | 0/5 | 0/5 | 0/5 |
| SMAP-29 (19.2) | 2/5 | 5/5 | 5/5 |
| LL-37 (449.4a) | 0/5 | 0/5 | 0/5 |
| LL-37 (224.7) | 1/5 | 5/5 | 5/5 |
| PG-1 (32.3a) | 0/5 | 0/5 | 0/5 |
| PG-1 (16.1) | 3/5 | 5/5 | 5/5 |
| CRAMP (375a) | 0/5 | 0/5 | 0/5 |
| CRAMP (187.5) | 3/5 | 5/5 | 5/5 |
| BMAP-28 (246.2a) | 0/5 | 0/5 | 0/5 |
| BMAP-28 (123.1) | 2/5 | 5/5 | 5/5 |
| Controlb | 2/5 | 5/5 | 5/5 |
aMBC.
bNo cathelicidin-derived peptides added to the suspension of treponemes before injection into the hamsters.
References
Gennaro, R. & Zanetti, M. (
Turner, J., Cho, Y., Dinh, N., Waring, A. J. & Lehrer, R. I. (
Ritonja, A., Kopitar, M., Jerala, R. & Turk, V. (
Zanetti, M., Litteri, L., Gennaro, R., Horstmann, H. & Romeo, D. (
Scocchi, M., Skerlavaj, B., Romeo, D. & Gennaro, R. (
Sorensen, O. E., Follin, P., Johnsen, A. H., Calafat, J., Tjabringa, G. S., Hiemstra, P. S. et al. (
Agerberth, B., Gunne, H., Odeberg, J., Kogner, P., Boman, H. G. & Gudmundsson, G. H. (
Frohm Nilsson, M., Sandstedt, B., Sorensen, O., Weber, G., Borregaard, N. & Ståhle-Bäckdahl, M. (
Frohm, M., Agerberth, B., Ahangari, G., Ståhle-Bäckdahl, M., Lidén, S., Wigzell, H. et al. (
Agerberth, B., Charo, J., Werr, J., Olsson, B., Idali, F., Lindbom, L. et al. (
Travis, S. M., Anderson, N. N., Forsyth, W. B., Espiritu, C., Conway, B. D., Greenberg, E. B. et al. (
Zanetti, M., Gennaro, R. & Romeo, D. (
Brogden, K. A., Kalfa, V. C., Ackermann, M. R., Palmquist, D. E., McCray, P. B., Jr & Tack, B. F. (
Gennaro, R., Scocchi, M., Benincasa, M., Skerlavaj, B., Risso, A., Miani M. et al. (
Larrick, J. W., Hirata, M., Zheng, H., Zhong, J., Bolin, D., Cavaillon, J. M. et al. (
Saiman, L., Tabibi, S., Starner, T. D., San Gabriel, P., Winokur, P. L., Jia, H. P. et al. (
Lusitani, D., Malawista, S. E. & Montgomery, R. R. (
Garcia, R., Gusmani, L., Murgia, R., Guarnaccia, C., Cinco, M. & Rottini, G. (
Elsbach, P., Weiss, J. & Levy, O. (
Ellinghausen, H. C. & McCullough, W. G. (
Marangoni, A., Aldini, R., Sambri, V., Montagnani, M., Ballardini, G., Storni, E. et al. (2000). Uptake and killing of Leptospira interrogans and Borrelia burgdorferi, spirochetes pathogenic to humans, by reticuloendothelial cells in perfused rat liver.
Sambri, V., Marangoni, A., Massaria, F., Farencena, A., La Placa, M., Jr & Cevenini, R. (
Sambri, V., Marangoni, A., Olmo, A., Storni, E., Montagnani, M., Fabbi, M. et al. (
Marangoni, A., Sambri, V., Storni, E., D’Antuono, A., Negosanti, M. & Cevenini, R. (
Bagella, L., Scocchi, M. & Zanetti, M. (
Skerlavaj, B., Benincasa, M., Risso, A., Zanetti, M. & Gennaro, R. (
Gallo, R. L., Kim, K. J., Bernfield, M., Kozak, C. A., Zanetti, M., Merluzzi, L. et al. (
Skerlavaj, B., Gennaro, R., Bagella, L., Merluzzi, L., Risso, A. & Zanetti, M. (
Kokryakov, V. N., Harwig, S. S. L., Panyutich, E. A., Shevchenko, A. A., Aleshina, G. M., Shamova, O. V. et al. (
Levin, J. M., Nelson, J. A., Segreti, J., Harrison, B., Benson, C. A. & Strle, F. (
Agger, W. A., Callister, S. M. & Jobe, D. A. (
Nelson, R. A. & Mayer, M. M. (
Dever, L. L., Jorgensen, J. H. & Barbour, A. G. (
Norris, S. J. & Edmondson, D. J. (
Norris, S. J. & Larsen, S. A. (
Sambri, V., Marangoni, A., Eyer, C., Reichhuber, C., Soutschek, E., Negosanti, M. et al. (
Byrne, R. E., Laska, S., Bell, M., Larson, D., Phillis, J. & Todd, J. (
Borenstein, L. A., Selsted, M. E., Lehrer, R. I. & Miller, J. N. (
Borenstein, L. A., Ganz, T., Sell, S., Lehrer, R. I. & Miller, J. N. (
Scocchi, M., Romeo, D. & Cinco, M. (
Lehrer, R. I., Barton, A. & Ganz, T. (
Hardy, P. H., Jr & Levin, J. (
Author notes
1Sezione di Microbiologia, DMCSS, Ospedale Policlinico S.Orsola, University of Bologna, Via Massarenti 9, 40138 Bologna; 2Dipartimento di Biochimica, Biofisica e Chimica delle Macromolecole; 3Dipartimento di Scienze Biomediche, Sezione di Microbiologia, University of Trieste, Trieste, Italy