-
PDF
- Split View
-
Views
-
Cite
Cite
Nalini Ranjit, Elizabeth A Young, George A Kaplan, Material hardship alters the diurnal rhythm of salivary cortisol, International Journal of Epidemiology, Volume 34, Issue 5, October 2005, Pages 1138–1143, https://doi.org/10.1093/ije/dyi120
Close - Share Icon Share
Abstract
Background In the quest for biological mechanisms underlying socioeconomic differences in health outcomes, attention has turned to the role of the hypothalamic-pituitary-adrenocortical axis. As there is some evidence that both acute and chronic stress raise cortisol levels, and material hardship is a stressor, we examined the relationship of chronic material hardship with salivary cortisol levels over the day.
Methods The data are from a survey of a sample of poor women aged 18–54. Up to four repeated measures of salivary cortisol levels were obtained from 188 women in this sample and modelled as a diurnal profile. Self-reports of a variety of sources of material hardship over the preceding year were combined into a single scale. Specific dimensions of the subjects' cortisol profiles were compared across levels of material hardship.
Results Salivary cortisol varied over the day, and by level of reported material hardship. Upon awakening, salivary cortisol levels were comparable across hardship levels. But soon after waking, women at low levels of hardship experienced both a significantly sharper morning surge and subsequently a sharper decline in salivary cortisol (16.0 and 29.5 nmol/l/h) than women with high hardship levels (5.9 and 24.3 nmol/l/h). These differences in cortisol diurnal pattern tended to be related in a dose-response way to levels of material hardship.
Conclusions Material hardship among poor women is associated with changes in the diurnal rhythms of cortisol, particularly in the waking response, which is blunted in women with high levels of hardship.
Discussions of associations between adverse material conditions and a variety of health outcomes have, in recent years, turned to the biological pathways underlying these associations. This emphasis on biological pathways brings epidemiologists closer to understanding how social structural conditions influence health,1 while at the same time addressing concerns about confounding and reverse causation that may underlie findings of positive associations between material/psychosocial conditions and ill health.2 One widely invoked formalization of a set of biological pathways is the allostatic load hypothesis,3 which draws links between a stressful environment and physical disease via the physiological sequelae of repeated activation of the neuro-endocrine system. Of particular interest in this formulation is the role of stress in modulating the release of cortisol. Cortisol is diverse in its physiologic effects,4 and glucocorticoid receptors are found in the cells of almost all tissues in the body. A wide array of cardiovascular, metabolic, immunologic, and homeostatic functions are regulated by cortisol. Thus, excessive glucocorticoid action is believed to have a role in the development of insulin resistance,5 and is associated with other cardiovascular risk factors such as central obesity6 and hypertension.7 Prolonged exposure to cortisol has been postulated to result in a reduction of cortisol's ability to inhibit the action of pro-inflammatory cytokines.8 Studies have consistently demonstrated enhanced cortisol and corticotropin levels in the peripheral blood of drug-free depressives.9 Alterations of HPA-axis functioning have also been demonstrated in persons with post-traumatic stress disorder.10 In view of this wide array of effects, it is possible that cortisol is part of the pathway between social conditions and health outcomes.
In this study, we explore the consequences of chronic economic stress, operationalized as material hardship, for HPA activity, measured by levels of salivary cortisol over the course of a day. While it has been consistently demonstrated that acute stress raises the level of cortisol,11,12 findings on the effect of chronic stress on cortisol profiles are mixed, as are findings related to chronic economic stress. Lower cortisol concentrations have been observed among women of lower-grade socioeconomic status (SES),13 but among low SES women, high job-demands are associated with higher cortisol levels.14 Another study has shown a positive correlation of cortisol levels with perceived stress, but lowered cortisol secretion among subjects with high levels of burnout.15 A recent review summarizes a series of related findings suggesting that a variety of chronic stress-related bodily disorders, including burnout, chronic fatigue syndrome, and chronic pelvic pain are associated with lowered, rather than elevated cortisol.16 Another review, focusing on children experiencing early adversity, suggests that such hypocortisolism may be limited to awakening levels of cortisol.17
The current analysis extends this literature in two ways. First, we use a comprehensive measure of economic stress, material hardship, that allows us to examine gradations in the cortisol response to stress. Second, we use new approaches to summarize the diurnal cortisol profile that allow us to compare specific features of the cortisol response within women and across levels of stress.
Materials and methods
The participants in this study are drawn from the longitudinal Women's Employment study (WES), based on a random sample of single mothers, about 50% black and 50% white, aged 18–54, with children receiving cash benefits as of February 1997, in an urban county in Michigan in February 1997. In June 2000, a special health supplement was administered to a random sub-sample (n = 298) of participants in the third wave of the WES survey.
Cortisol measures
Up to four salivary cortisol samples were obtained from each respondent, using Salivettes®™. The first measure was obtained during a clinic or home visit, where the respondent was provided with three labelled collection tubes and directions for obtaining subsequent salivary samples at home. Respondents were instructed to collect all three samples on one day, with the first sample collected upon awakening, the second 30 min later and before eating or brushing their teeth, and the third at bedtime. They were instructed to record the time of sample collection for each sample and to delay breakfast until after the second sample was collected. The collection protocol was designed to capture, with as few measures as possible, critical aspects of the diurnal variation in cortisol, including the morning awakening increase and sharp decline, as well as the more gradual decline to the evening nadir. Samples were returned by mail and frozen at −208°C until assay, at which time they were thawed and spun prior to assay. They were assayed using DPC Coat-a-Count tubes using 200 ml of saliva per tube following manufacturer's directions. Inter-assay variability was 10%.
Material hardship measures
Our measure of chronic economic stress in this population was operationalized as material hardship. The material hardship scale comprised a series of eight questions asking whether the participant had each experienced a number of economic hardships in the 12 months preceding the survey, covering a range of life activities. Items were binary responses to questions on housing, such as evictions and homelessness; questions on daily living, such as gas or electricity shut-offs, availability of phone facilities and sharing of household expenses; and questions on affordability of basic health resources, such as doctor visits, and winter clothing. These items are similar to those used in previous studies of poor populations.18,19 Measures of material hardship are widely used to examine the well-being of low-income families, and are considered preferable to traditional income-based poverty measures, in that they employ direct indicators of consumption and physical living conditions.20 A composite score, ranging from 0 to 8, and comprising the sum of the eight items, all equally weighted, was used to characterize the overall level of material hardship.
Statistical analysis
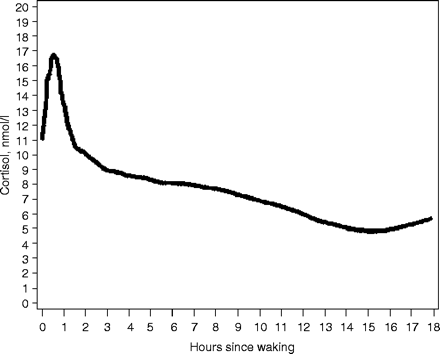
Analyses presented in this paper are restricted to the 188 respondents (62.9% of the original sample) for whom at least one valid cortisol measure was obtained, where a valid measure is defined as one that includes both a value for cortisol as well as the time of collection. Graphical visualizations of the cortisol profile were done using loess-based smoothers to minimize noise (Figure 1). The graphs provided a basis for fixing the inflection points as knots for regression spline models. Random effects models with linear splines were used to estimate and profile the entire pattern of cortisol variation over the day. The advantages of using these methods for modelling cortisol rhythms have been described elsewhere.21 Such models account for the correlation of measures within respondents, can accommodate varying numbers of measures per respondent, and exploit the considerable variability in collection times observed in such data. Respondents' diurnal profiles are characterized by stylized profiles of cortisol employing four distinct 'phases' of change over the day, modelled as regression splines. Thus, we are able to examine the slope of each of the four segments and describe these separately.
Plot of salivary cortisol measures obtained over the course of the day from 188 respondents. Loess-smoothed plot based on 631 salivary cortisol measures, obtained from 188 respondents in sample
Interactions of material hardship and the regression splines were used to model cortisol profiles over the day as a function of chronic stress. All models were adjusted for possible confounders, including age, waking time of the respondent, smoking status (current smoker vs not), body mass index (BMI), and race. Confounders were included in the models on the basis of significant bivariate associations. Measures of whether or not the respondent had smoked, or eaten, immediately prior to collection of the salivary sample, and pregnancy status of the respondent were tested and not found to significantly alter the level or shape of the cortisol profile; accordingly, they were not included in the final models. Random effects were assumed for the intercept (awakening value of cortisol), and for up to three of the slopes parameters depicting phases of the profile during the day. Variants of the material hardship measure were tested to demonstrate robustness as well to identify the best specification of the effect.
Results
Women who provided cortisol measures were similar to non-respondents on a variety of measures, including age and race composition, physical health barriers, and level of material hardship. While the range of the hardship measure as constructed is from 0–8, the measure is heavily skewed towards the right, with ∼45% of the cortisol sample having a value of 0, and 95% of respondents scoring 3 or less on this scale. For analyses utilizing the hardship scale as an ordinal categorical measure, cutpoints were set at 0, 1, and 2 or more, corresponding to 45, 26, and 29% of the respondents respectively.
Of the 188 women with at least one valid cortisol measure, 61% had four measures, while 83% had at least three measures, and 8.5% (16) women provided only one measurement. The small group of women who had only one measurement had a higher mean level of chronic stress than women with two or more cortisol measurements (1.5 vs 0.96), but this difference was not statistically significant (P > 0.18). Table 1 presents cortisol collection details by level of hardship. There is little evidence that the number, sequence or timing of measurements was systematically related to level of hardship. Waking time appears to be higher among women with high levels of hardship, but the difference is small and not statistically significant (P > 0.21). Higher levels of hardship are also associated with slight delays in the timing of the first morning measurements; again, these differences are not significant. No differences by hardship are apparent for the other collection times. The observed differences in waking time and time of collection of first sample was taken into account in specification and testing of the spline models. As per protocol, collection times were clustered in the first 1.5 h after awakening (49% of all samples), and in the period after 12 h (23% of all samples). In contrast, a large majority of the clinic samples were taken between 8 and 11 h after waking. Thus, the inclusion of the clinic sample allowed for a reasonably uniform distribution of samples over the rest of the day, but did not alter inferences in the critical hour after waking. The range of cortisol in the final sample was 0.012–109.3 nmol/l, with a mean of 10.8 and a SD of 10.1.
Cortisol sample collection details by level of hardship
. | Low hardship (n = 85) . | Moderate hardship (n = 49) . | High hardship (n = 54) . |
|---|---|---|---|
| Mean number of measures (95% CI) | 3.3 (3.1–3.5) | 3.7 (3.4–3.9) | 3.1 (2.9–3.4) |
| Proportion with four measures | 57.7 | 73.4 | 53.7 |
| Mean waking time | 7:36 am | 7:30 am | 7:03 am |
| Median time difference between waking and 1st measure (minutes) | 5 | 10 | 10 |
| Median time difference between 1st and 2st measure (minutes) | 31 | 34 | 31 |
| Median time difference between 2nd morning measure and bedtime measure (hours:minutes) | 13:45 | 13:12 | 13:23 |
| Median hours between waking and clinic sample | 10:49 | 10:25 | 10:40 |
. | Low hardship (n = 85) . | Moderate hardship (n = 49) . | High hardship (n = 54) . |
|---|---|---|---|
| Mean number of measures (95% CI) | 3.3 (3.1–3.5) | 3.7 (3.4–3.9) | 3.1 (2.9–3.4) |
| Proportion with four measures | 57.7 | 73.4 | 53.7 |
| Mean waking time | 7:36 am | 7:30 am | 7:03 am |
| Median time difference between waking and 1st measure (minutes) | 5 | 10 | 10 |
| Median time difference between 1st and 2st measure (minutes) | 31 | 34 | 31 |
| Median time difference between 2nd morning measure and bedtime measure (hours:minutes) | 13:45 | 13:12 | 13:23 |
| Median hours between waking and clinic sample | 10:49 | 10:25 | 10:40 |
Four cases and 22 controls were analysed from Rome and Naples, whereas 18 cases and 93 controls were analysed from Sicily.
Cortisol sample collection details by level of hardship
. | Low hardship (n = 85) . | Moderate hardship (n = 49) . | High hardship (n = 54) . |
|---|---|---|---|
| Mean number of measures (95% CI) | 3.3 (3.1–3.5) | 3.7 (3.4–3.9) | 3.1 (2.9–3.4) |
| Proportion with four measures | 57.7 | 73.4 | 53.7 |
| Mean waking time | 7:36 am | 7:30 am | 7:03 am |
| Median time difference between waking and 1st measure (minutes) | 5 | 10 | 10 |
| Median time difference between 1st and 2st measure (minutes) | 31 | 34 | 31 |
| Median time difference between 2nd morning measure and bedtime measure (hours:minutes) | 13:45 | 13:12 | 13:23 |
| Median hours between waking and clinic sample | 10:49 | 10:25 | 10:40 |
. | Low hardship (n = 85) . | Moderate hardship (n = 49) . | High hardship (n = 54) . |
|---|---|---|---|
| Mean number of measures (95% CI) | 3.3 (3.1–3.5) | 3.7 (3.4–3.9) | 3.1 (2.9–3.4) |
| Proportion with four measures | 57.7 | 73.4 | 53.7 |
| Mean waking time | 7:36 am | 7:30 am | 7:03 am |
| Median time difference between waking and 1st measure (minutes) | 5 | 10 | 10 |
| Median time difference between 1st and 2st measure (minutes) | 31 | 34 | 31 |
| Median time difference between 2nd morning measure and bedtime measure (hours:minutes) | 13:45 | 13:12 | 13:23 |
| Median hours between waking and clinic sample | 10:49 | 10:25 | 10:40 |
Four cases and 22 controls were analysed from Rome and Naples, whereas 18 cases and 93 controls were analysed from Sicily.
By visual inspection, cortisol levels rise sharply from an awakening level of about 11 nmol/l for ∼30–45 min after awakening (Phase 1), followed by a rapid decline for approximately the next half hour (Phase 2), and a slower decline over the rest of the day (Phase 3). Visual inspection suggests another inflection point at ∼14 h after awakening, at which a very slight increase in cortisol levels ensues; accordingly, this point was set to mark the beginning of a final phase (Phase 4). A regression-spline model confirmed that at each of these points, there was a significant change in slope from the previous phase. Thus, the knots (or inflection points) for the stress model were fixed at 30 min past awakening, 75 min past awakening, and 14 h past awakening. The fit of this regression model was robust to small changes (within 10 min before or after) in the selected inflection points.
Table 2 presents model-based estimates of the waking level of cortisol (intercept), and slopes of cortisol change for hardship levels of 0 and (on a scale of 1 to 8) for a unit difference on the hardship scale. The underlying model regresses diurnal profiles of salivary cortisol against material hardship levels, controlling for age, race, BMI, smoking status, and time of waking. The intercepts are calculated for a hypothetical black woman aged 35 with BMI of 32, and waking time set at 7:30. The slope (phase) values (in nmol/l/h) describe rate of change in cortisol levels over the course of the day. Waking levels of cortisol, represented by the intercept, do not differ by level of hardship. The small decline in cortisol level (−0.26 nmol/l) associated with a unit increase in hardship level is not significantly different from zero. The slope parameter of Phase 1 implies that cortisol levels rise at the rate of 16.12 nmol/l/h, in the first 30 min after awakening, for a person with a hardship level of 0. An increase in hardship level by one unit attenuates the steepness of this morning rise to 12.97 nmol/l/h. The difference in slopes during this rising phase, −3.19 nmol/l/h, is marginally significant (P = 0.0642). The slope parameters of Phase 2 imply a decline in cortisol, at the rate of −9.88 nmol/l/h for women with a hardship level of 0, and a slower decline, at −7.95 nmol/l/h for women with a hardship level of 1. The difference in slopes for a unit increase in stress in Phase 2 is significant (1.93 nmol/l/h, P = 0.04). There is no evidence that level of hardship moderates the period of slow decline described by Phases 3 and 4. Of the confounders examined, only age, BMI, and waking time were seen to be associated with the waking level of cortisol in these multivariate models. In addition to the fixed effects shown in the table, the intercept and the slopes for Phases 1–3 were allowed to vary randomly; the estimates obtained for these effects confirm that there is significant variability between individuals in each of these parameters.
Model-based estimates of salivary cortisol levels on waking and over the course of day, for a one unit contrast in hardship level
| . | . | . | Estimated effect of a unit increase in stress . | . | |
|---|---|---|---|---|---|
. | Estimated parameters for hardship level = 0 Estimate (SE) . | Estimated parameters for hardship level = 1 Estimate (SE) . | Difference in estimates for one unit (SE) . | P-value for unit Difference . | |
| Intercept (waking level) | 11.33 (1.14) | 11.07 (0.98) | −0.26 (0.73) | 0.7209 | |
| Phase 1 (0–30min): period of morning rise | 16.16 (2.20) | 12.97 (1.81) | −3.19 (1.71) | 0.0642 | |
| Phase 2 (30–75min): period of morning sharp decline | −9.88 (1.36) | −7.95 (1.07) | 1.93 (0.94) | 0.0399 | |
| Phase 3 (75min–14h): period of slow decline over the rest of waking day | −0.59(0.10) | −0.55 (0.07) | 0.04 (0.06) | 0.5609 | |
| Phase 4 (beyond 14h): period of change in last few hours before bedtime | 0.35 (0.47) | 0.58(0.33) | 0.24 (0.32) | 0.4660 | |
| Age (centred at 35 years) | −1.18 (0.46) | −1.18 (0.46) | |||
| . | . | . | Estimated effect of a unit increase in stress . | . | |
|---|---|---|---|---|---|
. | Estimated parameters for hardship level = 0 Estimate (SE) . | Estimated parameters for hardship level = 1 Estimate (SE) . | Difference in estimates for one unit (SE) . | P-value for unit Difference . | |
| Intercept (waking level) | 11.33 (1.14) | 11.07 (0.98) | −0.26 (0.73) | 0.7209 | |
| Phase 1 (0–30min): period of morning rise | 16.16 (2.20) | 12.97 (1.81) | −3.19 (1.71) | 0.0642 | |
| Phase 2 (30–75min): period of morning sharp decline | −9.88 (1.36) | −7.95 (1.07) | 1.93 (0.94) | 0.0399 | |
| Phase 3 (75min–14h): period of slow decline over the rest of waking day | −0.59(0.10) | −0.55 (0.07) | 0.04 (0.06) | 0.5609 | |
| Phase 4 (beyond 14h): period of change in last few hours before bedtime | 0.35 (0.47) | 0.58(0.33) | 0.24 (0.32) | 0.4660 | |
| Age (centred at 35 years) | −1.18 (0.46) | −1.18 (0.46) | |||
Model-based estimates of salivary cortisol levels on waking and over the course of day, for a one unit contrast in hardship level
| . | . | . | Estimated effect of a unit increase in stress . | . | |
|---|---|---|---|---|---|
. | Estimated parameters for hardship level = 0 Estimate (SE) . | Estimated parameters for hardship level = 1 Estimate (SE) . | Difference in estimates for one unit (SE) . | P-value for unit Difference . | |
| Intercept (waking level) | 11.33 (1.14) | 11.07 (0.98) | −0.26 (0.73) | 0.7209 | |
| Phase 1 (0–30min): period of morning rise | 16.16 (2.20) | 12.97 (1.81) | −3.19 (1.71) | 0.0642 | |
| Phase 2 (30–75min): period of morning sharp decline | −9.88 (1.36) | −7.95 (1.07) | 1.93 (0.94) | 0.0399 | |
| Phase 3 (75min–14h): period of slow decline over the rest of waking day | −0.59(0.10) | −0.55 (0.07) | 0.04 (0.06) | 0.5609 | |
| Phase 4 (beyond 14h): period of change in last few hours before bedtime | 0.35 (0.47) | 0.58(0.33) | 0.24 (0.32) | 0.4660 | |
| Age (centred at 35 years) | −1.18 (0.46) | −1.18 (0.46) | |||
| . | . | . | Estimated effect of a unit increase in stress . | . | |
|---|---|---|---|---|---|
. | Estimated parameters for hardship level = 0 Estimate (SE) . | Estimated parameters for hardship level = 1 Estimate (SE) . | Difference in estimates for one unit (SE) . | P-value for unit Difference . | |
| Intercept (waking level) | 11.33 (1.14) | 11.07 (0.98) | −0.26 (0.73) | 0.7209 | |
| Phase 1 (0–30min): period of morning rise | 16.16 (2.20) | 12.97 (1.81) | −3.19 (1.71) | 0.0642 | |
| Phase 2 (30–75min): period of morning sharp decline | −9.88 (1.36) | −7.95 (1.07) | 1.93 (0.94) | 0.0399 | |
| Phase 3 (75min–14h): period of slow decline over the rest of waking day | −0.59(0.10) | −0.55 (0.07) | 0.04 (0.06) | 0.5609 | |
| Phase 4 (beyond 14h): period of change in last few hours before bedtime | 0.35 (0.47) | 0.58(0.33) | 0.24 (0.32) | 0.4660 | |
| Age (centred at 35 years) | −1.18 (0.46) | −1.18 (0.46) | |||
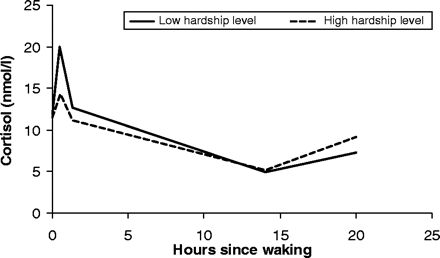
In an alternative specification, the material hardship variable was employed as an ordinal categorical variable, with three categories corresponding to approximate tertiles, as shown in Table 1. The predicted cortisol rhythms for women at the lowest and highest tertiles of stress are presented in Figure 2. There is no difference in the waking level of cortisol between women at the highest and lowest level of hardship (10.6 nmol/l as compared to 10.9 nmol/l). However, differences in cortisol profiles by hardship levels appear soon after waking. Women at low levels of hardship experience a sharp rise in the first half-hour after waking [16.0 nmol/l/h (CI: 11.3–20.7)], while this rise is blunted among women with high hardship levels [5.9 nmol/l/h (CI: 0–13.4; P-value for difference = 0.03)]. The blunted morning rise associated with hardship is associated with a significantly blunted morning decline as well. Under low hardship conditions, the rate of decline is −9.5 nmol/l/h (CI: −12.5 to 26.6); with high levels of hardship, the decline is slowed to −4.3 nmol/l/h (CI: −8.4 to 20.1), for a significant difference of −5.3 nmol/l/h (P = 0.02). This slower morning increase and decline in cortisol levels among highly stressed women results in their maintaining slightly (but not significantly) lower levels of cortisol over most of the rest of the day, despite the fact that waking levels of cortisol do not vary by level of hardship. There is some suggestion that high levels of stress are associated with elevations in evening cortisol, but the data are too sparse in this region to support a statistically significant conclusion.
Fitted cortisol profiles for low and high levels of material hardship. Estimated values of cortisol for women at high and low levels of hardship obtained from a model regressing cortisol level against a 3-category ordinal variable describing material hardship, and controlling for age, race, BMI, smoking status, and time of waking
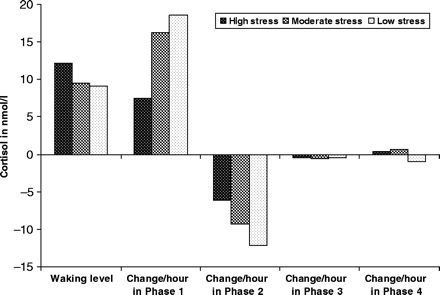
To test that these effects were robust, the model was re-estimated with more and less inclusive definitions of the basic hardship variable, as well as multiple specifications of the hardship variable used above, including collapsing the range at the upper end, and specifying it as an ordinal variable with two, three or four categories. The direction of association of the spline slope parameters with hardship level persisted across the models, although the statistical significance of the association (in terms of P-value) varied with specification. Figure 3 presents parameter estimates from one of these alternative specifications, which employed a more inclusive measure of hardship, ranging from 0 to 13, to permit finer gradations. The graded response of estimated parameters to hardship level evident in Figure 3 indicates that material hardship has a dose-response effect on the slopes of the morning rise and fall in cortisol levels, with the degree of attenuation of parameters dependent on the degree of hardship.
Effects of differing levels of hardship on modelled estimates of awakening level and slopes of change in cortisol level during each phase
Thus, while level of hardship is not associated with awakening levels of cortisol, it is evident that elevated hardship level is associated with blunting of both the morning Phase 1 (0–30 min after awakening) rise in cortisol as well as the subsequent Phase 2 (30–75 min after awakening) decline, with the degree of blunting related to the degree of stress. Over the rest of the day (after 75 min past awakening), the rate of decline in cortisol levels does not vary by hardship level. Gradation in cortisol response by level of hardship was evident in some specifications, where the lowest and highest levels of hardship were clearly separated; however, P-values did not attain significance in those models because of small sample sizes in the extreme hardship category.
Discussion
Examining the effects of chronic stress on cortisol is an exercise fraught with difficulties. The particular measure of stress employed may or may not impact on salivary cortisol levels, as the earlier review of the literature suggested. The process of obtaining repeated and carefully timed measures of cortisol within a laboratory setting may itself have consequences for the stress level of participants. The circadian rhythms of cortisol, the high degree of inter-individual variation, and the marked differences by age and sex in cortisol production all make for considerable difficulty in arriving at useful summary measures or credible conclusions regarding the sensitivity of cortisol secretion to chronic or acute exposures.
The results of the analysis show differences in cortisol profiles by level of chronic economic stress, operationalized here by measures of material hardship. The difference in profile is most marked immediately after awakening (morning challenge), when both the cortisol rise at awakening and the decline subsequent to awakening are sluggish among women who are economically stressed, resulting in lower cortisol levels for these women in the morning. The lowered cortisol levels seen among highly stressed women over the rest of the day are a consequence of the blunting of the morning rise and decline. The results support the suggestion that the HPA axis adapts to chronic stress by downregulation, but it is in the waking response that this downregulation is most evident. A recent review22 concludes that it is indeed the awakening response of cortisol that may have the most significance in terms of a link between psychosocial factors and physiological functioning.
The study has some limitations. Since this is already a chronically stressed sample, the range of exposure observed here is limited, and the findings may not be generalizable outside this range, or may underestimate the magnitude of the differences in cortisol response seen in the population. The findings are limited to women; other studies14 have shown male–female differences in cortisol responses to chronic stress. Finally, on the basis of visual inspection of the data, the model assumed equal timing of phases across levels of stress. With larger samples, and similar statistical models, it should be possible to test variants of this assumption. The increasing number of population-based studies obtaining cortisol data provides an opportunity to apply these methods and test these findings more generally, and to re-evaluate their implications for the assumed physiological sequelae of stress associated with chronic socioeconomic disadvantage.
Data collection and analysis were supported by an award from the National Institute for Child Health and Development (HD P50 HD38986) and by funds from the Michigan Initiative on Inequalities in Health to George A Kaplan. Major funding of the WES study was provided by the Charles Stewart Mott Foundation and the Joyce Foundation. Elizabeth A Young is funded by an award from the National Institute of Mental Health (MH01931). We thank the participants in the WES who so generously participated in the study.
References
Brunner EJ. Towards a new social biology. In: Berkman L, Kawachi I (eds). Social Epidemiology. New York: Oxford University Press,
Macleod J, Davey Smith G. Psychosocial factors and public health: a suitable case for treatment?
Reynolds RM, Walker BR. Human insulin resistance: the role of glucocorticoids.
Whitworth JA, Brown MA, Kelly JJ, Williamson PM. Mechanisms of cortisol-induced hypertension in humans.
Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model.
Bonne O, Brandes D, Segman R, Pitman RK, Yehuda R, Shalev AY. Prospective evaluation of plasma cortisol in recent trauma survivors with posttraumatic stress disorder.
Dugue B, Leppanen E, Grasbeck R. The driving license examination as a stress model: effects on blood picture, serum cortisol and the production of interleukins in man.
Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity.
Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot M. Socioeconomic status and stress-related biological responses over the working day.
Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day.
Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening.
Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders.
Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development.
Mayer SE, Jencks C. Poverty and the distribution of material hardship.
Edin K, Lein L. Work, welfare, and single mother's economic survival strategies.
Ouellette T, Burstein N, Long D, Beecroft E. Measures of material hardship: Final Report. Prepared for: U.S. Department of Health and Human Services. http://aspe.hhs.gov/hsp/material-hardship04/ (June 6, 2005 date last accessed).
Ranjit N, Young EA, Raghunathan TE, Kaplan G. Modeling cortisol rhythms in a population-based study.