-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Glimåker, Jan Sjölin, Styrbjörn Åkesson, Pontus Naucler, Lumbar Puncture Performed Promptly or After Neuroimaging in Acute Bacterial Meningitis in Adults: A Prospective National Cohort Study Evaluating Different Guidelines, Clinical Infectious Diseases, Volume 66, Issue 3, 1 February 2018, Pages 321–328, https://doi.org/10.1093/cid/cix806
Close - Share Icon Share
Abstract
Early treatment is pivotal for favorable outcome in acute bacterial meningitis (ABM). Lumbar puncture (LP) is the diagnostic key. The aim was to evaluate the effect on outcome of adherence to European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), and Swedish guidelines regarding neuroimaging before LP.
The cohort comprised 815 adult ABM patients in Sweden registered prospectively between 2008 and 2015. Primary endpoint was in-hospital mortality and secondary endpoint was favorable outcome at 2–6 months of follow-up.
Indications for neuroimaging before LP existed in 7%, 32%, and 65% according to Swedish, ESCMID, and IDSA guidelines, respectively. The adjusted odds ratio (aOR) was 0.48 (95% confidence interval [CI], .26–.89) for mortality and 1.52 (95% CI, 1.08–2.12) for favorable outcome if Swedish guidelines were followed. ESCMID guideline adherence resulted in aOR of 0.68 (95% CI, .38–1.23) for mortality and 1.05 (95% CI, .75–1.47) for favorable outcome. Following IDSA recommendations resulted in aOR of 1.09 (95% CI, .61–1.95) for mortality and 0.59 (95% CI, .42–.82) for favorable outcome. Performing prompt vs neuroimaging-preceded LP was associated with aOR of 0.38 (95% CI, .18–.77) for mortality and 2.11 (95% CI, 1.47–3.00) for favorable outcome. The beneficial effect of prompt LP was observed regardless of mental status and immunosuppression.
Adherence to Swedish guidelines in ABM is associated with decreased mortality and increased favorable outcome in contrast to adherence to ESCMID or IDSA recommendations. Our findings support that impaired mental status and immunocompromised state should not be considered indications for neuroimaging before LP in patients with suspected ABM.
Acute bacterial meningitis (ABM) is a life-threatening disease. Despite modern treatment and intensive care, the mortality is still 10%–30%, and the risk of persistent neurologic or hearing deficit is high [1–3]. Early adequate treatment is pivotal to achieve a favorable outcome [1, 4]. Lumbar puncture (LP) is the basis of ABM diagnosis and, in clinical practice, LP and cerebrospinal fluid (CSF) analyses are often decisive to start correct antibiotics and corticosteroids in meningitis dosages [4, 5].
It is recommended that, in certain situations with suspected increased intracranial pressure (ICP) and cerebral mass lesion, the clinician should refrain from prompt LP and instead first perform computed tomography (CT) of the brain, as it is argued that LP may increase the risk of brain herniation [6–8]. However, firm evidence that LP may cause herniation is lacking, and the natural course of ABM or brain abscess may itself result in herniation [6, 9, 10]. Furthermore, it is shown that cerebral CT is poor at predicting the risk of herniation in ABM [11–13]. Guidelines differ as to when to perform neuroimaging before LP in patients with suspected bacterial meningitis. Table 1 shows the recommendations in the current Swedish, European Society for Clinical Microbiology and Infectious Diseases (ESCMID), and Infectious Diseases Society of America (IDSA) guidelines. The Swedish guidelines are the most liberal regarding prompt LP since impaired mental status and new-onset seizures were omitted as indications for CT before LP after the revision in 2009 [14]. The ESCMID guidelines were revised in 2016 when the level of consciousness indicating CT before LP was lowered from Glasgow Coma Scale score (GCS) <12 to <10 [8]. IDSA guidelines from 2004 are the most conservative regarding prompt LP by recommending CT before LP if abnormal mental status (GCS <15) is observed [7]. In contrast to the Swedish recommendations, both the ESCMID and IDSA guidelines recommend CT before LP in cases with severely immunocompromised state or new-onset seizures.
Recommendations for Neuroimaging of the Brain Before Lumbar Puncture According to Different Guidelines in 815 Patients With Acute Bacterial Meningitis
| . | Swedish Guidelines . | ESCMID Guidelines . | IDSA Guidelines . | |||
|---|---|---|---|---|---|---|
| . | No. (%) . | . | No. (%) . | . | No. (%) . | |
| Impaired mental status | Cerebral herniationa | 10 (1.2) | GCS <10 (RLS >3) | 124 (15.2) | GCS <15 (RLS >1) | 480 (58.9) |
| Neurologic deficit (suspected mass lesion) | Arm or leg drift>4 d of neurological symptoms or ABM-atypical symptoms | 45 (5.5) | Arm or leg drift | 45 (5.5) | Arm or leg drift | 45 (5.5) |
| NAb | Abnormal ocular motility, visual field, dilated pupil | 9 (1.1) | ||||
| New-onset seizures | No recommendation | Within 1 week of presentation | 59 (7.2) | Within 1 week of presentation | 59 (7.2) | |
| Severe immuno-compromised state | No recommendation | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | |
| History of CNS disease | No recommendation | No recommendation | Mass lesion, stroke, focal infection | NAb | ||
| Papilledema | No recommendation | No recommendation | Increased intracranial pressure | NAc | ||
| Totald | 53 (6.5) | 250 (30.7) | 522 (64.0) | |||
| . | Swedish Guidelines . | ESCMID Guidelines . | IDSA Guidelines . | |||
|---|---|---|---|---|---|---|
| . | No. (%) . | . | No. (%) . | . | No. (%) . | |
| Impaired mental status | Cerebral herniationa | 10 (1.2) | GCS <10 (RLS >3) | 124 (15.2) | GCS <15 (RLS >1) | 480 (58.9) |
| Neurologic deficit (suspected mass lesion) | Arm or leg drift>4 d of neurological symptoms or ABM-atypical symptoms | 45 (5.5) | Arm or leg drift | 45 (5.5) | Arm or leg drift | 45 (5.5) |
| NAb | Abnormal ocular motility, visual field, dilated pupil | 9 (1.1) | ||||
| New-onset seizures | No recommendation | Within 1 week of presentation | 59 (7.2) | Within 1 week of presentation | 59 (7.2) | |
| Severe immuno-compromised state | No recommendation | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | |
| History of CNS disease | No recommendation | No recommendation | Mass lesion, stroke, focal infection | NAb | ||
| Papilledema | No recommendation | No recommendation | Increased intracranial pressure | NAc | ||
| Totald | 53 (6.5) | 250 (30.7) | 522 (64.0) | |||
Abbreviations: ABM, acute bacterial meningitis; CNS, central nervous system; ESCMID, European Society for Clinical Microbiology and Infectious Diseases; GCS, Glasgow Coma Scale score; HIV, human immunodeficiency virus; IDSA, Infectious Diseases Society of America; NA, not applicable; RLS, Reaction Level Scale.
aDeep coma with loss of all reactions or GCS <6 or RLS >5 combined with nonreactive dilated pupils, opisthotonus, abnormal breathing pattern, or raised blood pressure combined with bradycardia.
bDuration of cerebral symptoms before admission or history of CNS disease is not registered in the Swedish quality register.
cFunduscopy is not recommended before lumbar puncture in Sweden.
dMore than 1 indication present in some patients.
Recommendations for Neuroimaging of the Brain Before Lumbar Puncture According to Different Guidelines in 815 Patients With Acute Bacterial Meningitis
| . | Swedish Guidelines . | ESCMID Guidelines . | IDSA Guidelines . | |||
|---|---|---|---|---|---|---|
| . | No. (%) . | . | No. (%) . | . | No. (%) . | |
| Impaired mental status | Cerebral herniationa | 10 (1.2) | GCS <10 (RLS >3) | 124 (15.2) | GCS <15 (RLS >1) | 480 (58.9) |
| Neurologic deficit (suspected mass lesion) | Arm or leg drift>4 d of neurological symptoms or ABM-atypical symptoms | 45 (5.5) | Arm or leg drift | 45 (5.5) | Arm or leg drift | 45 (5.5) |
| NAb | Abnormal ocular motility, visual field, dilated pupil | 9 (1.1) | ||||
| New-onset seizures | No recommendation | Within 1 week of presentation | 59 (7.2) | Within 1 week of presentation | 59 (7.2) | |
| Severe immuno-compromised state | No recommendation | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | |
| History of CNS disease | No recommendation | No recommendation | Mass lesion, stroke, focal infection | NAb | ||
| Papilledema | No recommendation | No recommendation | Increased intracranial pressure | NAc | ||
| Totald | 53 (6.5) | 250 (30.7) | 522 (64.0) | |||
| . | Swedish Guidelines . | ESCMID Guidelines . | IDSA Guidelines . | |||
|---|---|---|---|---|---|---|
| . | No. (%) . | . | No. (%) . | . | No. (%) . | |
| Impaired mental status | Cerebral herniationa | 10 (1.2) | GCS <10 (RLS >3) | 124 (15.2) | GCS <15 (RLS >1) | 480 (58.9) |
| Neurologic deficit (suspected mass lesion) | Arm or leg drift>4 d of neurological symptoms or ABM-atypical symptoms | 45 (5.5) | Arm or leg drift | 45 (5.5) | Arm or leg drift | 45 (5.5) |
| NAb | Abnormal ocular motility, visual field, dilated pupil | 9 (1.1) | ||||
| New-onset seizures | No recommendation | Within 1 week of presentation | 59 (7.2) | Within 1 week of presentation | 59 (7.2) | |
| Severe immuno-compromised state | No recommendation | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | Transplant recipients, HIV infection, or severe immunosuppressive treatment | 89 (10.9) | |
| History of CNS disease | No recommendation | No recommendation | Mass lesion, stroke, focal infection | NAb | ||
| Papilledema | No recommendation | No recommendation | Increased intracranial pressure | NAc | ||
| Totald | 53 (6.5) | 250 (30.7) | 522 (64.0) | |||
Abbreviations: ABM, acute bacterial meningitis; CNS, central nervous system; ESCMID, European Society for Clinical Microbiology and Infectious Diseases; GCS, Glasgow Coma Scale score; HIV, human immunodeficiency virus; IDSA, Infectious Diseases Society of America; NA, not applicable; RLS, Reaction Level Scale.
aDeep coma with loss of all reactions or GCS <6 or RLS >5 combined with nonreactive dilated pupils, opisthotonus, abnormal breathing pattern, or raised blood pressure combined with bradycardia.
bDuration of cerebral symptoms before admission or history of CNS disease is not registered in the Swedish quality register.
cFunduscopy is not recommended before lumbar puncture in Sweden.
dMore than 1 indication present in some patients.
The aim of the present study was to evaluate the effect on mortality and favorable outcome of adherence to the Swedish, ESCMID, and IDSA guidelines, respectively, regarding indications for neuroimaging before LP. Furthermore, we evaluated the effect, in itself, of performing prompt vs CT-preceded LP.
METHODS
Study Population
The study comprised adult ABM patients (age >16 years) who were prospectively registered by Web forms in the national Swedish quality register for ABM during the period January 2008 to December 2015. Using conventional diagnostic criteria, ABM diagnoses were defined by specialists in infectious diseases at each of the 29 Swedish infectious disease clinics, where the vast majority of all patients with ABM in Sweden are treated. The diagnoses were based on clinical criteria, CSF analysis, and microbiological tests on blood and CSF as previously described [4]. ABM was defined as community acquired if the patients had not been hospitalized or if they had undergone surgery in the central nervous system within the last 30 days.
In the register sex, age, immunocompromised state, clinical findings and symptoms, mental status, neurological deficit, septic shock, antibiotic and corticosteroid treatment, time from admission to start of antibiotic treatment, intensive care, etiology, in-hospital mortality, neurological sequelae, and hearing deficits are routinely recorded (Table 2). Mental status on admission is recorded as the Reaction Level Scale (RLS) [15], the GCS, or both. In cases where the GCS was noted but not the RLS, the GCS was converted to the RLS for standardized comparison [16]. Adequate antibiotic treatment was defined as intravenous β-lactam antibiotics for which the isolated bacteria were sensitive and administered in meningitis dosages. In patients with unknown etiology, third-generation cephalosporin plus ampicillin or monotherapy with meropenem was considered adequate. Adequate corticosteroid treatment was defined as betamethasone 8 mg every 6 hours intravenously, initiated within 1 hour from the start of antibiotics [17]. Immunocompromised state, new-onset seizures, neurological deficits, typical symptoms (fever, headache, and neck stiffness), septic shock, primary focus of infection, and intensive care were defined as present if recorded as yes in the register and not present if recorded as no, or not recorded at all. Data for all other parameters were defined according to data entry in the register.
Baseline Characteristics, Clinical Presentations, Etiologies, and Time to Adequate Treatment in 815 Adults With Bacterial Meningitis Stratified Into Groups Where Lumbar Puncture Was Performed Without Prior, or After, Cerebral Computerized Tomography
| Characteristic . | Total Patients (n = 815) . | LP Done Without Prior CT (n = 323) . | LP Done After CT (n = 378) . | P Value . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 62 | 61 | 64 | .03 |
| Range | 17–95 | 17–95 | 18–92 | |
| Interquartile range | 48–70 | 47–70 | 49–72 | |
| Male/female sex | 417/398 | 156/167 | 204/174 | .13 |
| Immunocompromised state | ||||
| Severea | 87 (10.7) | 30 (9.3) | 44 (11.6) | .51 |
| Moderateb | 225 (27.6) | 94 (29.1) | 100 (26.5) | .43 |
| Total | 312 (38.3) | 124 (38.4) | 144 (38.1) | .94 |
| Primary focus of infection | ||||
| Ear, sinus, or lungs | 397 (48.7) | 140 (43.3) | 174 (46.0) | .48 |
| Pharynx | 54 (6.6) | 23 (7.1) | 21 (5.6) | .39 |
| Other/unknown | 364 (44.7) | 160 (49.5) | 183 (48.4) | .77 |
| Triad of fever, headache, and neck stiffness | 232 (28.5) | 86 (26.6) | 109 (28.8) | .52 |
| Mental status | (n = 780) | (n = 310) | (n = 362) | |
| RLS 1 | 300 (38.5) | 136 (43.9) | 117 (32.3) | .002 |
| RLS 2–3 | 356 (45.6) | 139 (44.8) | 171 (47.2) | .53 |
| RLS 4–8 | 124 (15.9) | 35 (11.3) | 74 (20.4) | .001 |
| New-onset seizures | 59 (7.2) | 24 (7.4) | 31 (8.2) | .29 |
| Neurological deficit | ||||
| Arm/leg drift | 45 (5.5) | 16 (5.0) | 26 (6.9) | .28 |
| Cranial nerve palsy | 37 (4.5) | 10 (3.1) | 22 (5.8) | .09 |
| Septic shock | 64 (7.9) | 26 (8.1) | 28 (7.4) | .75 |
| Etiology | ||||
| Streptococcus pneumoniae | 420 (51.5) | 144 (44.6) | 216 (57.1) | <.001 |
| Neisseria meningitidis | 87 (10.7) | 52 (16.1) | 24 (6.4) | <.001 |
| Other bacteria | 232 (28.5) | 93 (28.8) | 113 (29.9) | .75 |
| Unidentified etiology | 76 (9.3) | 34 (10.5) | 25 (6.6) | .06 |
| Time from admission to adequate antibiotic and corticosteroid treatment | (n = 700) | (n = 277) | (n = 328) | |
| <1 h | 168 (24.0) | 80 (28.9) | 60 (18.3) | .002 |
| <2 h | 252 (36.0) | 113 (40.8) | 98 (29.9) | .005 |
| Characteristic . | Total Patients (n = 815) . | LP Done Without Prior CT (n = 323) . | LP Done After CT (n = 378) . | P Value . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 62 | 61 | 64 | .03 |
| Range | 17–95 | 17–95 | 18–92 | |
| Interquartile range | 48–70 | 47–70 | 49–72 | |
| Male/female sex | 417/398 | 156/167 | 204/174 | .13 |
| Immunocompromised state | ||||
| Severea | 87 (10.7) | 30 (9.3) | 44 (11.6) | .51 |
| Moderateb | 225 (27.6) | 94 (29.1) | 100 (26.5) | .43 |
| Total | 312 (38.3) | 124 (38.4) | 144 (38.1) | .94 |
| Primary focus of infection | ||||
| Ear, sinus, or lungs | 397 (48.7) | 140 (43.3) | 174 (46.0) | .48 |
| Pharynx | 54 (6.6) | 23 (7.1) | 21 (5.6) | .39 |
| Other/unknown | 364 (44.7) | 160 (49.5) | 183 (48.4) | .77 |
| Triad of fever, headache, and neck stiffness | 232 (28.5) | 86 (26.6) | 109 (28.8) | .52 |
| Mental status | (n = 780) | (n = 310) | (n = 362) | |
| RLS 1 | 300 (38.5) | 136 (43.9) | 117 (32.3) | .002 |
| RLS 2–3 | 356 (45.6) | 139 (44.8) | 171 (47.2) | .53 |
| RLS 4–8 | 124 (15.9) | 35 (11.3) | 74 (20.4) | .001 |
| New-onset seizures | 59 (7.2) | 24 (7.4) | 31 (8.2) | .29 |
| Neurological deficit | ||||
| Arm/leg drift | 45 (5.5) | 16 (5.0) | 26 (6.9) | .28 |
| Cranial nerve palsy | 37 (4.5) | 10 (3.1) | 22 (5.8) | .09 |
| Septic shock | 64 (7.9) | 26 (8.1) | 28 (7.4) | .75 |
| Etiology | ||||
| Streptococcus pneumoniae | 420 (51.5) | 144 (44.6) | 216 (57.1) | <.001 |
| Neisseria meningitidis | 87 (10.7) | 52 (16.1) | 24 (6.4) | <.001 |
| Other bacteria | 232 (28.5) | 93 (28.8) | 113 (29.9) | .75 |
| Unidentified etiology | 76 (9.3) | 34 (10.5) | 25 (6.6) | .06 |
| Time from admission to adequate antibiotic and corticosteroid treatment | (n = 700) | (n = 277) | (n = 328) | |
| <1 h | 168 (24.0) | 80 (28.9) | 60 (18.3) | .002 |
| <2 h | 252 (36.0) | 113 (40.8) | 98 (29.9) | .005 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CT, computed tomography; LP, lumbar puncture; RLS, Reaction Level Scale.
aHuman immunodeficiency virus infection, transplant recipient, malignancy with chemotherapy, or severe immunosuppressive treatment such as tumor necrosis factor–α inhibitors or corresponding for autoimmune disease.
bCortisone treatment irrespective of dose, diabetes, intravenous drug abuse, alcoholism, asplenia, cerebrospinal fluid leakage, or severe liver or kidney failure.
Baseline Characteristics, Clinical Presentations, Etiologies, and Time to Adequate Treatment in 815 Adults With Bacterial Meningitis Stratified Into Groups Where Lumbar Puncture Was Performed Without Prior, or After, Cerebral Computerized Tomography
| Characteristic . | Total Patients (n = 815) . | LP Done Without Prior CT (n = 323) . | LP Done After CT (n = 378) . | P Value . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 62 | 61 | 64 | .03 |
| Range | 17–95 | 17–95 | 18–92 | |
| Interquartile range | 48–70 | 47–70 | 49–72 | |
| Male/female sex | 417/398 | 156/167 | 204/174 | .13 |
| Immunocompromised state | ||||
| Severea | 87 (10.7) | 30 (9.3) | 44 (11.6) | .51 |
| Moderateb | 225 (27.6) | 94 (29.1) | 100 (26.5) | .43 |
| Total | 312 (38.3) | 124 (38.4) | 144 (38.1) | .94 |
| Primary focus of infection | ||||
| Ear, sinus, or lungs | 397 (48.7) | 140 (43.3) | 174 (46.0) | .48 |
| Pharynx | 54 (6.6) | 23 (7.1) | 21 (5.6) | .39 |
| Other/unknown | 364 (44.7) | 160 (49.5) | 183 (48.4) | .77 |
| Triad of fever, headache, and neck stiffness | 232 (28.5) | 86 (26.6) | 109 (28.8) | .52 |
| Mental status | (n = 780) | (n = 310) | (n = 362) | |
| RLS 1 | 300 (38.5) | 136 (43.9) | 117 (32.3) | .002 |
| RLS 2–3 | 356 (45.6) | 139 (44.8) | 171 (47.2) | .53 |
| RLS 4–8 | 124 (15.9) | 35 (11.3) | 74 (20.4) | .001 |
| New-onset seizures | 59 (7.2) | 24 (7.4) | 31 (8.2) | .29 |
| Neurological deficit | ||||
| Arm/leg drift | 45 (5.5) | 16 (5.0) | 26 (6.9) | .28 |
| Cranial nerve palsy | 37 (4.5) | 10 (3.1) | 22 (5.8) | .09 |
| Septic shock | 64 (7.9) | 26 (8.1) | 28 (7.4) | .75 |
| Etiology | ||||
| Streptococcus pneumoniae | 420 (51.5) | 144 (44.6) | 216 (57.1) | <.001 |
| Neisseria meningitidis | 87 (10.7) | 52 (16.1) | 24 (6.4) | <.001 |
| Other bacteria | 232 (28.5) | 93 (28.8) | 113 (29.9) | .75 |
| Unidentified etiology | 76 (9.3) | 34 (10.5) | 25 (6.6) | .06 |
| Time from admission to adequate antibiotic and corticosteroid treatment | (n = 700) | (n = 277) | (n = 328) | |
| <1 h | 168 (24.0) | 80 (28.9) | 60 (18.3) | .002 |
| <2 h | 252 (36.0) | 113 (40.8) | 98 (29.9) | .005 |
| Characteristic . | Total Patients (n = 815) . | LP Done Without Prior CT (n = 323) . | LP Done After CT (n = 378) . | P Value . |
|---|---|---|---|---|
| Age, y | ||||
| Median | 62 | 61 | 64 | .03 |
| Range | 17–95 | 17–95 | 18–92 | |
| Interquartile range | 48–70 | 47–70 | 49–72 | |
| Male/female sex | 417/398 | 156/167 | 204/174 | .13 |
| Immunocompromised state | ||||
| Severea | 87 (10.7) | 30 (9.3) | 44 (11.6) | .51 |
| Moderateb | 225 (27.6) | 94 (29.1) | 100 (26.5) | .43 |
| Total | 312 (38.3) | 124 (38.4) | 144 (38.1) | .94 |
| Primary focus of infection | ||||
| Ear, sinus, or lungs | 397 (48.7) | 140 (43.3) | 174 (46.0) | .48 |
| Pharynx | 54 (6.6) | 23 (7.1) | 21 (5.6) | .39 |
| Other/unknown | 364 (44.7) | 160 (49.5) | 183 (48.4) | .77 |
| Triad of fever, headache, and neck stiffness | 232 (28.5) | 86 (26.6) | 109 (28.8) | .52 |
| Mental status | (n = 780) | (n = 310) | (n = 362) | |
| RLS 1 | 300 (38.5) | 136 (43.9) | 117 (32.3) | .002 |
| RLS 2–3 | 356 (45.6) | 139 (44.8) | 171 (47.2) | .53 |
| RLS 4–8 | 124 (15.9) | 35 (11.3) | 74 (20.4) | .001 |
| New-onset seizures | 59 (7.2) | 24 (7.4) | 31 (8.2) | .29 |
| Neurological deficit | ||||
| Arm/leg drift | 45 (5.5) | 16 (5.0) | 26 (6.9) | .28 |
| Cranial nerve palsy | 37 (4.5) | 10 (3.1) | 22 (5.8) | .09 |
| Septic shock | 64 (7.9) | 26 (8.1) | 28 (7.4) | .75 |
| Etiology | ||||
| Streptococcus pneumoniae | 420 (51.5) | 144 (44.6) | 216 (57.1) | <.001 |
| Neisseria meningitidis | 87 (10.7) | 52 (16.1) | 24 (6.4) | <.001 |
| Other bacteria | 232 (28.5) | 93 (28.8) | 113 (29.9) | .75 |
| Unidentified etiology | 76 (9.3) | 34 (10.5) | 25 (6.6) | .06 |
| Time from admission to adequate antibiotic and corticosteroid treatment | (n = 700) | (n = 277) | (n = 328) | |
| <1 h | 168 (24.0) | 80 (28.9) | 60 (18.3) | .002 |
| <2 h | 252 (36.0) | 113 (40.8) | 98 (29.9) | .005 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: CT, computed tomography; LP, lumbar puncture; RLS, Reaction Level Scale.
aHuman immunodeficiency virus infection, transplant recipient, malignancy with chemotherapy, or severe immunosuppressive treatment such as tumor necrosis factor–α inhibitors or corresponding for autoimmune disease.
bCortisone treatment irrespective of dose, diabetes, intravenous drug abuse, alcoholism, asplenia, cerebrospinal fluid leakage, or severe liver or kidney failure.
Definitions of Guideline Adherence and Outcomes
Adherence to guidelines was defined as (1) LP performed without previous neuroimaging when the guidelines did not recommend CT before LP, and (2) CT performed before LP when the guidelines recommended this sequence. Table 1 shows conditions where CT before LP is recommended according to the 3 guidelines, respectively. Abnormal level of consciousness, where IDSA guidelines recommend CT before LP, was defined as RLS >1, corresponding to GCS <15. ESCMID guidelines recommend CT before LP if GCS <10, corresponding to RLS >3. Signs of cerebral herniation, where Swedish guidelines recommend CT before LP, were defined as previously described [14]. ESCMID and IDSA recommend CT-preceded LP if severe immunocompromised state, defined as human immunodeficiency virus infection, transplant recipient, or severe immunosuppressive treatment for malignancy or autoimmune disease. Moderate immunocompromised state was defined as cortisone treatment irrespective of dose, diabetes, intravenous drug abuse, alcoholism, asplenia, CSF leakage, or severe liver or kidney failure.
Primary endpoint was mortality during hospital stay and secondary endpoint favorable outcome at follow-up 2–6 months after discharge. Favorable outcome was defined as Glasgow Outcome Score (GOS) 5 and no neurological sequelae or hearing deficits. Neurological sequelae were specified as cognitive dysfunction/dementia, vertigo, epileptic seizures, ataxia, or persistent neurological deficits as defined by the clinician at the follow-up visit. Hearing disability was defined by the patient as new-onset of impairment and audiometry was performed when appropriate.
Statistical Analysis
Categorical variables were analyzed using χ2 or Fisher exact test as appropriate. Mann-Whitney U test was used for continuous data. Logistic regression analyses were performed to assess the effect of adherence to the different guidelines with adjustments for sex, age, typical symptom triad (fever, headache, and neck stiffness), septic shock, and etiology. In logistic regression analyses comparing the outcomes and time to start of adequate antibiotics and corticosteroids related to prompt LP vs CT-preceded LP, adjustments were made for sex, age, immunocompromised state, typical symptom triad, mental status (3 categories; reaction level scale 1, 2–3, and >3), new-onset seizures, cranial nerve palsy, septic shock, and etiology.
Ethical Considerations
The study was approved by the ethics committee at Karolinska Institutet (Dnr 2015/756-32).
RESULTS
Baseline characteristics in the 815 patients are described in Table 2. The overall mortality was 8% (68/815), and favorable outcome was noticed in 50% (336/677) of patients with available follow-up data.
Guideline Adherence of Lumbar Puncture and Neuroimaging
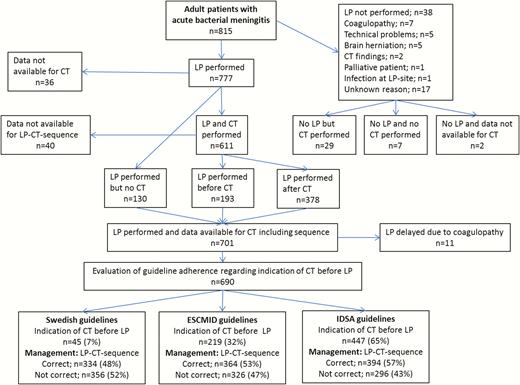
The diagnostic management with LP and neuroimaging, indications for CT before LP, and adherence to the 3 guidelines are presented in Figure 1. Data for prompt LP vs CT-preceded LP were available in 701 patients. Eleven of these were excluded when evaluating guideline adherence regarding the LP-CT sequence because LP was delayed due to coagulopathy and not because of neuroimaging. Hence, guideline adherence was evaluated in 690 patients. Of these, indications for CT before LP were observed in 45 (7%), 219 (32%), and 447 (65%) patients according to Swedish, ESCMID, and IDSA guidelines, respectively. Adherence to the Swedish guidelines was observed in 334 (48%), to ESCMID guidelines in 364 (53%), and to IDSA guidelines in 394 (57%) patients. Adherence to the 3 guidelines was stable during the study period (Supplementary Figure 1).
Management with lumbar puncture (LP) and computed tomography (CT) of the brain, the number of patients with indication for CT before LP according to the different guidelines, and the number of patients with correct and incorrect management according to the guidelines. 1LP was not performed because CT indicated a subdural empyema and increased intracranial pressure in 1 patient and in another because signs of meningitis were observed on CT which, together with the clinical picture, set the diagnosis. Abbreviations: CT, computed tomography; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; LP, lumbar puncture.
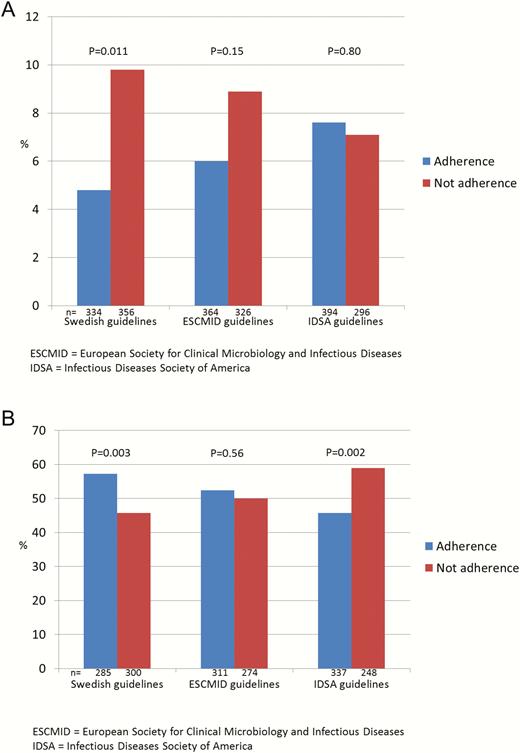
Figure 2 shows that adherence to the Swedish guidelines was associated with significantly lower mortality and increased favorable outcomes. No significant differences were observed by following the ESCMID guidelines. Regarding the IDSA guidelines, similar mortality was found whether there was adherence or not, and significantly fewer favorable outcomes were observed if the guidelines were followed. Adjusted for sex, age, typical symptoms, septic shock, and etiology, the odds ratio (OR) for mortality was 0.48 (95% confidence interval [CI], .26–.89) and the OR for favorable outcome was 1.52 (95% CI, 1.08–2.12) when Swedish guidelines were followed (Supplementary Table 1 and 2). Adherence to ESCMID guidelines resulted in an adjusted OR of 0.68 (95% CI, .38–1.23) for mortality and 1.05 (95% CI, .75–1.47) for favorable outcome. Following the IDSA recommendations resulted in an adjusted OR of 1.09 (95% CI, .61–1.95) for mortality and 0.59 (95% CI, .42–.82) for favorable outcome. The results were similar when restricted to 14-day in-hospital mortality (Supplementary Table 3).
Mortality during hospital stay (A) and favorable outcome at follow-up after 2–6 months (B) related to adherence or not to different guidelines for neuroimaging before lumbar puncture. Abbreviations: ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America.
Prompt or Computed Tomography-Preceded Lumbar Puncture
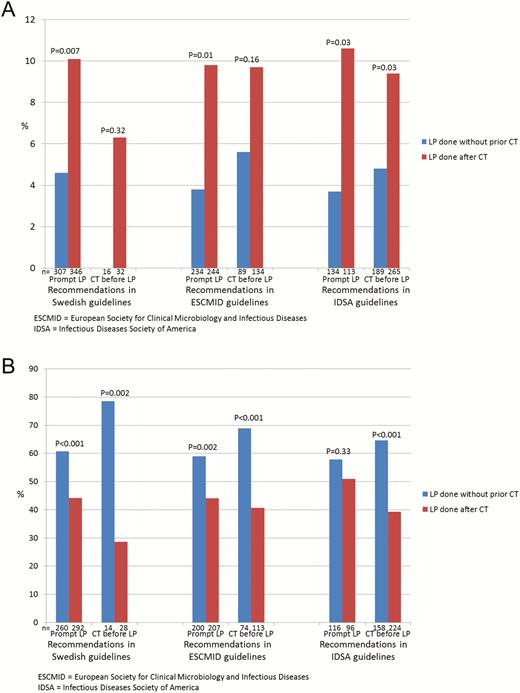
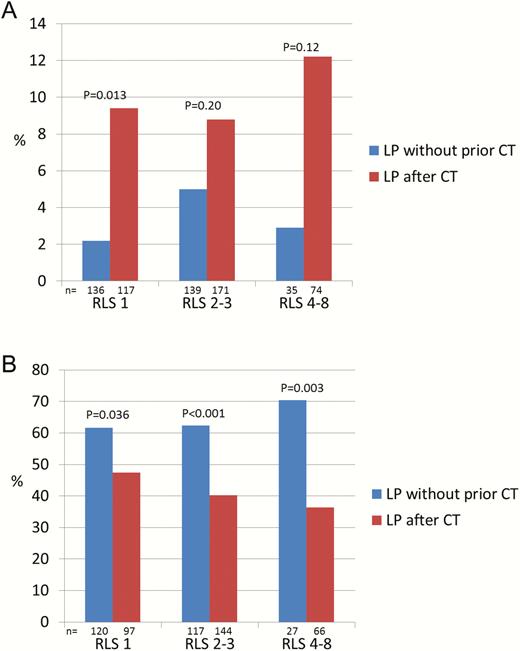
Prompt LP was done in 323 (46%) and CT-preceded LP in 378 (54%) of the 701 cases with available data (Figure 1). Performing LP without previous CT scan was associated with a mortality of 14 of 323 (4%) vs 37 of 378 (10%; P < .001) if CT was done before LP. In patients with available data at follow-up, a favorable outcome was noticed in 169 of 274 (62%) patients with prompt LP vs 137 of 320 (43%; P < .001) if CT preceded LP. The ORs for mortality and favorable outcome, adjusted for sex, age, immunocompromised state, typical symptoms, mental status, new-onset seizures, cranial nerve palsy, septic shock, and etiology, were 0.38 (95% CI, .18–.77) and 2.11 (95% CI, 1.47 to 3.00), respectively. Figure 3 shows mortality and favorable outcomes related to if LP was done promptly or after neuroimaging, stratified into groups where immediate LP and CT-preceded LP, respectively, were recommended in the different guidelines. Lower mortality and increased favorable outcomes were observed in patients in whom LP was done promptly vs after CT scan in all groups, regardless of indications for CT before LP. A reduced mortality and increased favorable outcome in patients who underwent prompt LP was observed also in analyses stratified according to impaired mental status (RLS 2–3 and 4–8) (Figure 4). New-onset seizures occurred in 7% with fatal outcome in 3 of 24 (13%) cases with prompt LP vs 3 of 31 (10%; P = .74) if CT preceded LP, and in patients with available data a favorable outcome was observed in 13 of 22 (59%) vs 13 of 25 (52%), respectively (P = .63). Severe immunocompromised state was noticed in 11%. The mortality if prompt LP was performed was 1 of 30 (3%) vs 5 of 44 (11%) in cases with CT-preceded LP (P = .21). In those with available data, a favorable outcome was noticed in 18 of 25 (72%) if prompt LP was done vs 13 of 36 (36%) if CT was performed before LP (P = .006).
Mortality during hospital stay (A) and favorable outcome at follow-up after 2–6 months (B) related to if lumbar puncture (LP) was performed without prior, or after, computed tomography (CT) of the brain stratified into groups where prompt LP is recommended vs where CT is recommended before LP, according to different guidelines. Abbreviations: CT, computed tomography; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; LP, lumbar puncture.
Mortality during hospital stay (A) and favorable outcome at follow-up after 2–6 months (B), stratified according to mental status on admission, related to if lumbar puncture was performed without prior computed tomography (CT) of the brain vs after CT. Abbreviations: CT, computed tomography; LP, lumbar puncture; RLS, Reaction Level Scale.
Prompt or Computed Tomography-Preceded Lumbar Puncture Related to Time to Treatment
Among 362 patients with CT-preceded LP and available data, antibiotics were started before the CT investigation in 170 (47%). Adequate treatment with antibiotics and corticosteroids <1 hour, and <2 hours, from admission were observed in 80 of 277 (29%) and 113 of 277 (41%), respectively, in patients with prompt LP vs 60 of 328 (18%; P = .002) and 98 of 328 (30%; P = .005), respectively, in cases with CT-preceded LP. The OR for adequate treatment <1 hour from admission if the LP was done promptly vs after CT was 2.46 (95% CI, 1.60–3.79) adjusted for sex, age, immunocompromised state, typical symptoms, mental status, new-onset seizures, cranial nerve palsy, septic shock, and etiology. The corresponding adjusted OR for treatment <2 hours was 2.12 (95% CI, 1.45–3.10).
DISCUSSION
This is, to our knowledge, the first study evaluating different guidelines regarding early management with LP and neuroimaging. The Swedish, ESCMID, and IDSA guidelines differ substantially in their recommendations to perform CT prior to LP, such that 7%, 32%, and 65%, respectively, of the patients in the present study had indications for CT-preceded LP. Adherence to the 3 guidelines was about 50%, which is comparable with earlier findings [18–20]. Importantly, following the Swedish guidelines resulted in 50% lower mortality and increased favorable outcomes at follow-up in contrast to adherence to ESCMID and IDSA guidelines. Following IDSA recommendations resulted in significantly decreased favorable outcomes at follow-up.
LP without previous CT scan was performed in 46% of the patients, which is more frequent than in the Netherlands (26%) and Canada (29%) [5, 19]. Prompt LP resulted in significantly decreased mortality and increased long-term favorable outcomes regardless of guideline indications for CT before LP. These findings are in contrast to a recent Dutch study where CT before LP was not associated with increased unfavorable outcome at discharge (mortality was not assessed) [19]. This discrepancy could possibly be explained by earlier CT management resulting in less delay in the Netherlands compared with Sweden. In line with earlier reports, impaired mental status (RLS 2–3) was noticed in more than half, and coma (RLS 4–8) in 15%, of the patients in the present study [2]. The beneficial effect of prompt LP was observed regardless of mental status, indicating that impaired mental status in the absence of signs of cerebral herniation should not be an indication to perform CT before LP.
New-onset seizures occurred in 7% and severe immunocompromised state was noticed in 11%, which is in line with earlier studies [2, 13]. If prompt LP was performed, similar outcomes were observed in those with new-onset seizures and better outcomes in those with immunocompromised state compared to if CT preceded LP. The obviously positive effect of prompt LP in ABM, shown in the present and an earlier study [5], should be weighed against a potential risk if the patient suffers from a cerebral mass lesion. Clinical findings, other than seizure or immunocompromised state, should decide whether ABM or a mass lesion is primarily suspected. A prompt LP should be done if the clinical picture is more compatible with ABM than with a mass lesion.
Significantly earlier treatment with antibiotics and corticosteroids was achieved by performing prompt LP, which is concordant with earlier findings [3, 5, 21, 22]. The decreased mortality and increased favorable outcomes that were observed by performing prompt LP were most probably associated with this difference in timely management. The importance of early antibiotic treatment is emphasized in all guidelines, and there is a strong recommendation that whenever LP is delayed (eg, due to neuroimaging), empiric antibiotics must be started immediately on clinical suspicion, even if the diagnosis has not been established. Yet in line with earlier findings, antibiotics were started before neuroimaging in only half of the patients where LP was done after the CT scan [5, 19]. Among these patients, starting antibiotics before CT was associated with significantly decreased mortality (5% vs 13%) but similar outcomes at follow-up compared with if antibiotics were started after the CT (data not shown). Thus, in clinical practice many patients with CT-preceded LP are not started on antibiotics before the CT scan despite the well-known recommendations of antibiotics before CT. Furthermore, recent reports indicate that most clinicians in the United States and Netherlands do not adhere to IDSA or ESCMID guidelines, such that CT is performed before LP in the majority of patients regardless of guideline indication [19, 20]. In the present study, similar poor adherence to Swedish guidelines was observed. This is alarming as 61% of the patients were treated at an intensive care unit and 18% were administered ICP-targeted therapy and a highly plausible diagnosis of ABM, accomplished only by LP, is usually required to rapidly reach the decision to administer these advanced treatment modalities.
In aggregate, our findings indicate that LP is safe in ABM patients with impaired mental status or coma, which is in agreement with earlier reports [2, 4, 9]. Furthermore, the importance of prompt LP increases in severely ill ABM cases—that is, in patients for whom a CT scan is often recommended before LP [20]. Thus, adherence to IDSA and, to a lesser degree, ESCMID guidelines may be associated with delayed treatment in many patients with ABM with increased risk of unfavorable outcomes.
The strengths of the study are the large sample size with well-characterized patients enabling identification of indications for CT before LP. Comprehensive clinical and management data enabled evaluation of guideline adherence by multivariate analyses with adjustment for all important risk factors [23] except low cell count in CSF, which is not available in the register. The time from admission to start of adequate treatment was registered, which made it possible to determine delays associated with performing CT scan before LP. Furthermore, because a large number of patients collected from multiple sites from all over Sweden were included, based on clinical as well as laboratory findings, the generalizability from the present study to clinical practice is high.
A limitation is that the present study is based on confirmed ABM, whereas guidelines should be applicable to patients with suspected ABM where other diagnoses such as mass lesions exist. ESCMID and IDSA guidelines are based on the study of Hasbun et al from 2001 of 301 patients with suspected meningitis where certain clinical findings were associated with abnormalities on cerebral CT, but not with risks of performing LP [22]. It is stated that LP can be hazardous if ¨brain shift¨ is present due to space-occupying lesions [7, 8]. However, to our knowledge, evidence from human or animal studies supporting this hypothesis is very limited. It has been stated that LP may cause fatal herniation in cases with brain abscess, although the potential risk has been estimated to be small, about 1% [7, 24]. However, LP is usually not beneficial in the diagnosis of brain abscess or mass lesion. Thus, in patients with suspected brain abscess or other mass lesion, a cerebral CT should be performed initially mainly because this is the best diagnostic tool. A mass lesion should be suspected in patients with a long (>4 days) or ABM-atypical history of cerebral symptoms and if arm or leg drift is noticed [25]. These clinical findings should be indications for initial CT scan. Another limitation is the lack of randomization for the comparison of efficacy of the different guidelines. However, multivariate analyses were performed to compensate for bias. Furthermore, data for LP and CT management were missing in 14% of the patients.
In conclusion, the present study indicates that adherence to Swedish guidelines regarding LP and neuroimaging is associated with decreased mortality and increased favorable outcome in contrast to adherence to ESCMID or IDSA recommendations where no beneficial effect was found. CT performed before LP causes significant delay and is a risk factor for increased mortality and unfavorable outcome irrespective of mental status. In patients with clinical findings indicating a mass lesion or impending herniation, a cerebral CT should be performed initially. Otherwise, guidelines should promote prompt LP liberally and impaired mental status, new-onset seizures, and immunocompromised state should not be considered indications for neuroimaging before LP in patients with suspected ABM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. G., J. S., and P. N. conceived the study. M. G. and S. Å. organized and controlled the data collection. M. G., P. N., and J. S. conducted the analyses and reviewed the results. M. G. made an original draft of the manuscript and all authors contributed to the final version. M. G. and P. N. assume responsibility for the work as a whole.
Financial support. This work was supported by the Stockholm County Council.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments