-
PDF
- Split View
-
Views
-
Cite
Cite
Robin Zur Nieden, Joachim W. Deitmer, The Role of Metabotropic Glutamate Receptors for the Generation of Calcium Oscillations in Rat Hippocampal Astrocytes In Situ, Cerebral Cortex, Volume 16, Issue 5, May 2006, Pages 676–687, https://doi.org/10.1093/cercor/bhj013
Close - Share Icon Share
Abstract
Ca2+ oscillations are part of the intra- and intercellular signalling in many cell types. We have studied Ca2+ oscillations in astrocytes in acute brain slices of the hippocampus of juvenile rats (postnatal 8–14 days old), using confocal laser scanning microscopy and bulk-loading of the Ca2+-sensitive dye Fluo-4. Astrocytes were identified morphologically in the stratum radiatum, and by their Ca2+ response in the absence of external K+. Thirty-five per cent of astrocytes (43 slices) showed spontaneous Ca2+ oscillations, with a frequency of 1.26 ± 0.11 transients/min (n = 366). These Ca2+ signals were unaffected by tetrodotoxin (0.5 μM) and Ni2+ (2 mM), but were sensitive to interference with the phospholipase C-mediated Ca2+ release from intracellular stores. Spontaneous Ca2+ oscillations were reduced or suppressed by antagonists of metabotropic glutamate receptors (mGluRs) of groups I and II, but not affected by antagonists of group III. Glutamate (1–100 μM) and specific agonists of mGluR groups I and II evoked concentration-dependent Ca2+ signals, which were oscillatory at intermediate concentrations (e.g. at 10 μM glutamate). Our results indicate that mGluRs of both groups I and II are involved in mediating Ca2+ oscillations in astrocytes, which might be glial responses to micromolar changes of glutamate in the extracellular spaces.
Introduction
Single and repetitive Ca2+ transients, which can occur in various cell types, spontaneously or as evoked signals, can initiate or change cell activity (for review, see Berridge et al., 2003). These may be short-term changes due to the activation of Ca2+/Calmodulin-dependent proteins or long-term effects as a result of gene expression. Our knowledge about the function and mechanisms of Ca2+ oscillations in cells is, however, still rather limited. In electrically non-excitable cells, Ca2+ oscillations may be of particular importance for generating or initiating specific cellular functions (Dolmetsch et al., 1998; Li et al., 1998; Rose and Konnerth, 2001; Morita et al., 2003).
In the nervous system, spontaneous Ca2+ transients have been reported in both neurons (Woodhall et al., 1999) and glial cells, in culture and in situ (Cornell-Bell et al., 1990; Charles, 1998; Strahonja-Packard and Sanderson, 1999; Aguado et al., 2002; Nett et al., 2002). Neurons appear to show spontaneous Ca2+ oscillations primarily at early developmental stages, which might be linked to the formation of neuronal circuits in the developing brain (Garaschuk et al., 2000). In neurons, Ca2+ release is associated with transmitter exocytosis, synaptic plasticity and electrical excitability (Rose and Konnerth, 2001). Astrocytes are electrically non-excitable cells, which often employ Ca2+ signalling in response to chemical or mechanical stimuli (Chen et al., 1997; Kimelberg et al., 1997; Deitmer et al., 1998), but have also been found spontaneously, i.e. in the absence of given stimuli (Parri et al., 2001; Aguado et al., 2002; Beck et al., 2004). Ca2+ signals in astrocytes often occur as oscillations (repetitive transients), and can propagate along cell processes and possibly even beyond cell boundaries to neighbouring cells (Parri et al., 2001; Nett et al., 2002; Schipke et al., 2002). They have also been associated with Ca2+-dependent exocytosis of transmitters (Bezzi et al., 1998; Innocenti et al., 2000; Angulo et al., 2004; Fiacco and McCarthy, 2004; Zhang et al., 2004) and modulation of regional blood flow (Zonta et al., 2003; Mulligan and MacVicar, 2004). The mechanisms, however, that initiate and maintain Ca2+ oscillations in astrocytes in situ are not yet known.
The aim of the present study was to elucidate the role of neurotransmitters, in particular glutamate, in generating Ca2+ oscillations in astrocytes. We have therefore investigated Ca2+ transients and Ca2+ oscillations in rat hippocampal slices using confocal fluorescent Ca2+ imaging. We have recorded spontaneous Ca2+ oscillations in cells identified as astrocytes, and used a number of transmitter receptor ligands to elucidate the mechanism involved in initiating these Ca2+ signals. Our results suggest that metabotropic glutamate receptors (mGluRs) of groups I and II play a major role in mediating Ca2+ oscillations in astrocytes in situ. We discuss whether the initiation of different types of Ca2+ signals in astrocytes might reflect the level of extracellular, non-synaptic glutamate in the hippocampal tissue. Some of the results have been reported in preliminary form (Zur Nieden and Deitmer, 2002, 2004).
Material and Methods
Slice Preparation
Hippocampal slices were prepared from juvenile rats (postnatal 8–14 days old). In brief, rats were decapitated and their brain were quickly transferred into a chilled (4°C) calcium-reduced (0.5 mM) artificial cerebrospinal fluid (aCSF) following the method of Edwards et al. (1989). The saline was continuously gassed with carbogen (95% O2/5% CO2) and buffered to pH 7.4 by bicarbonate throughout the entire preparation. Frontal slices of the forebrain containing the hippocampal formation were cut using a Vibratome (Leica VTS1000, Bensheim, Germany). The brain slices (250–300 μm thick) were immediately transferred into a second chamber, where they were stored for at least 1 h in Ca2+-reduced aCSF (0.5 mM Ca2+) at 30°C before dye-loading.
Identification of Cell Types
Cells types were identified by their change in intracellular Ca2+-concentration ([Ca2+]i) in response to application of K+-free and high-K+ (50 mM) aCSF, and by their stellate morphology. In brief, hippocampal astrocytes in rat and mice show a rise in [Ca2+]i when perfused with nominal K+-free saline for 3–5 min (Dallwig et al., 2000; Dallwig and Deitmer, 2002). More than 80% of the fluo-4-AM-stained cells in the stratum radiatum, as well as those in the stratum lacunosum-moleculare of the CA1 region of the rat hippocampus, responded to K+-free aCSF with an increase in [Ca2+]i. These cells often displayed a stellate morphology and were presumed to be astrocytes, as indicated by immunocytochemical markers (Beck et al., 2004). Cells showing only a weak (<10% change in fluorescence F/F0) or strongly delayed onset ( >1 min) in their response to K+-free saline were not considered to be viable astrocytes. Cells showing a fast onset in their response to high-K+ containing aCSF but poor fluo-4-AM staining with no change in intracellular Ca2+-concentration during the application of K+-free saline were presumed to be neurons.
Solutions
The standard aCSF for acute brain slices contained (in mM): NaCl 125, KCl 2.5, CaCl2 2, MgCl2 1, D-glucose 25, NaHCO3 26, NaH2PO4 1.25, L-lactate 0.5, gassed during the entire experiment by carboxygen to adjust the pH to 7.4. In Ca2+-reduced saline (0.5 mM), 1.5 mM CaCl2 was replaced by 1.5 mM MgCl2. In nominally Ca2+-free aCSF, CaCl2 was substituted by equimolar amounts of MgCl2, and 1 mM EGTA was added to reduce the remaining free Ca2+ to <10 nM. In K+-free saline (0K) or high-K+ solution (50 mM K+, 50K), KCl was exchanged by/for NaCl.
Metabotropic glutamate receptor agonists, (RS)-3,5-dihydroxyphenylglycine (DHPG), (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC), (±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (trans-ACPD), L-(+)-2-amino-4-phosphonobutyric acid (L-AP4) and (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG IV) as well as the group-specific mGluR antagonists (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY 341495), (RS)-1-amino-5-phosphonoindan-1-carboxylic acid (APICA), (RS)-α-ethyl-4-carboxyphenylglycine (MCPG) and the SERCA-pump inhibitor cyclopiazonic acid (CPA) were obtained from Tocris Cookson (Bristol, UK). Tetrodotoxin (TTX) was purchased from Alomone Labs (Jerusalem, Israel). ATP and the purinergic P2 antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) were obtained from Sigma-Aldrich (Taufkirchen, Germany). Bafilomycin A1 (ALEXIS Corp., Lausen, Switzerland), a potent inhibitor of vacuolar-type H+-ATPase, was dissolved (4 mM) in dimethylsulfoxide (DMSO) and added to the dye-loading solution, to make a final concentration of 4 μM and incubated for at least 90 min. The final concentration of DMSO never exceeded 0.1% in the saline. All stock solutions were stored at −20°C. The pH of aCSF was readjusted to pH 7.4 with HCl after adding all drugs. All saline for acute brain slices were gassed throughout the entire experiment with carbogen to maintain pH and oxygen at constant levels.
Dye-loading
Acute brain slices were incubated in the dark at room temperature (21–24°C) in Ca2+-reduced aCSF containing 2 μM fluo-4-AM (Molecular Probes, Eugene, Oregon). The dye was dissolved in DMSO (final concentration <0.1%) to make a 10 mM stock solution. The brain slices were bulk-loaded with the dye for 60 min at room temperature (20–25°C). After dye-loading, slices were transferred onto a nylon mesh and kept dark in aCSF until the beginning of the experiment.
Laser Scanning Microscopy
Measurements of Ca2+ changes in cells of an acute brain slice were performed with a confocal laser scanning microscope (Zeiss LSM 510, Oberkochen, Germany). The Ca2+-sensitive dye fluo-4 was excited using the 488 nm line of an argon laser. To separate the excitation from the emission pathway, a dichroic mirror was used. The emission signal was truncated by a optical bandpass filter at wavelengths of 505 and 550 nm. To measure Ca2+-dynamics, images were acquired in one focal plane with a frequency of 0.5–1.0 Hz. The regions of interest (ROIs) were defined, and fluorescence changes were normalized to the average baseline level (F0) of the first 20 time points. All changes are expressed as the relative change of the fluorescence intensity F normalized to F0 as F/F0. A deflection of the baseline of ≥10% F/F0 (the noise was usually <5% F/F0) could be clearly identified as a Ca2+ signal. Laser-, optical filter- and microscope settings as well as data acquisition were controlled by PC software (AIM 3.0, Zeiss).
Patch-clamp Recording
Hippocampal slices from juvenile rats (10–16 days old) were fixed in a recording chamber with a U-shaped platinum wire and nylon grid on the stage of an upright microscope (Axioscope, Zeiss, Oberkochen, Germany). The chamber was continuously perfused with carbon-gassed aCSF (see above). Pipettes were pulled with a horizontal puller and heat-polished to a tip resistance of 2–3 MΩ, filled with an ‘intracellular solution’ containing (in mM): CsCl, 120; tetraethylammonium chloride, 20; MgCl2, 2; Na-ATP, 2; EGTA, 0.5; HEPES, 10; pH adjusted to 7.4 with NaOH. This pipette solution strongly reduced background K+ currents and allowed a better observation of spontaneous postsynaptic currents (Konnerth et al., 1990). Series resistance was 4–15 MΩ and was compensated. Recordings were controlled with pCLAMP software (Axon Instruments, Forster City, CA) with a digidata 1200 interface and an Axopatch-1D amplifier. For further details, see Brockhaus and Deitmer (2002).
Statistics
Measurements are given as mean values ± SEM, with n as the number of cells or the number of slices as indicated. Significance of statistical differences were calculated under the assumption that the results show a normal distribution using Student's t-test and performed by Origin 7.0 (OriginLab Corp., Northampton, MA). Figures were prepared using Corel Draw (Corel Corp., Toronto, Canada).
Results
Identification of Spontaneous Ca2+ Oscillations
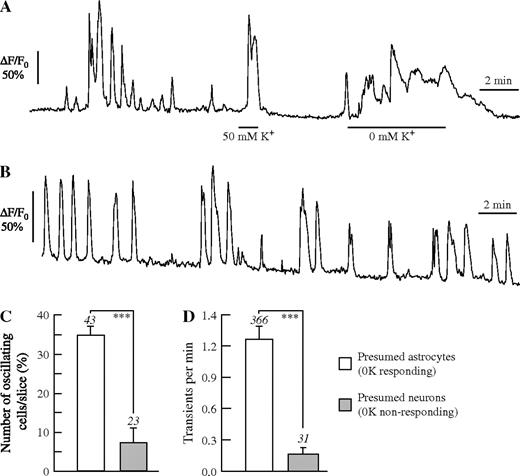
Acutely dissected brain slices were continuously superfused in the experimental chamber with saline for at least 5 min before recording of intracellular Ca2+ were begun. Over the next 10 min, spontaneous Ca2+ signals were recorded in normal saline as control. The experimental protocol included the 10–15 min after different agonists and antagonists were applied. At the end of each experiment, the slices were superfused with saline containing 50 mM K+ (50K) and/or 0 mM K+ (0K), for identification of the cell type (Fig. 1A; see also Dallwig and Deitmer, 2002; Beck et al., 2004). While both neurons and astrocytes responded to 50K (see Fig. 2C for a Ca2+ response in an astrocyte) with a prominent Ca2+ transient, only astrocytes showed Ca2+ rises in 0K. Ca2+ oscillations were defined as two or more repetitive Ca2+ transients, and they occurred spontaneously, i.e. without exogenous stimulus, in presumed astrocytes (Fig. 1B). The amplitude of these Ca2+ oscillations was variable, amounting to a mean increase of the relative fluorescence by 44 ± 12% F/F0 in 156 cells of 17 brain slices (n = 156/17). Often, the Ca2+ transients, especially those evoked by added agonists, declined in amplitude over the course of an experiment, and the amplitude could vary from slice to slice. Therefore, we focused on analysing the number of cells with Ca2+ responses and the frequency of the Ca2+ responses.
Ca2+ oscillations in acute rat hippocampal slices. (A, B) Identification of astrocyte by Ca2+ signals to 50 mM K+ and to 0 mM K+. (B) Continuous recording of spontaneous Ca2+ signals in a presumed astrocyte for 15 min. The percentage of oscillating cells (C) and the frequency of Ca2+ transients (D) in presumed astrocytes and neurons. The numbers above standard error bars indicate the number of slices (C) or the number of cells (D) analysed. Asterisks indicate level of significance (***P < 0.005).
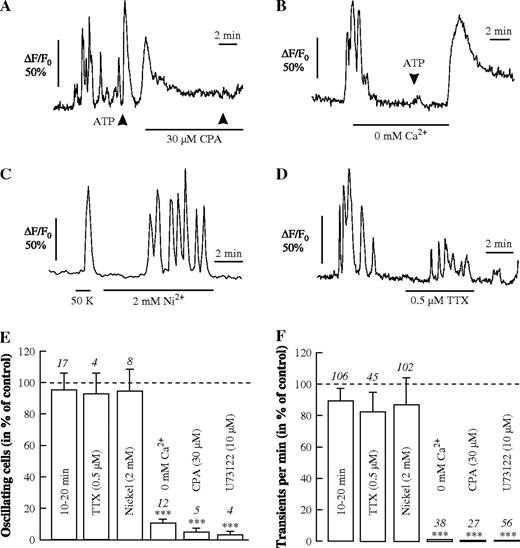
Spontaneous Ca2+ signalling and evoked Ca2+ responses in astrocytes. (A, B) ATP (10 μM for 10 s, arrowheads) evokes a single Ca2+ transient, which is suppressed after depletion of the Ca2+ stores by cyclopiazonic acid (CPA, 30 μM; A) or in the prolonged absence of external Ca2+ (B). The spontaneous Ca2+ oscillations are not blocked either by 2 mM Ni2+ in an astrocyte showing a Ca2+ transient in response to 50 mM K+ (50 K) (C) or by 0.5 μM TTX (D). The relative number of oscillating cells (E) and the frequency of Ca2+ transients (F) during a control period in normal saline during the first 10–20 min, or with substances applied as indicated during the subsequent period of 10 min, except U73122 (10 μM), in which slices were incubated for 30–60 min before recording. The numbers above standard error bars indicate the number of slices (E) or the number of cells (F) analysed.
Spontaneous Ca2+ oscillations could be recorded in 35 ± 2% (n = 43 slices) of the cells identified as astrocytes due to their response to 0K, in a given brain slice over a time period of 10 min, while only 7 ± 3% (n = 23 slices) of cells, identified as neurons by their morphology and due to the lack of a Ca2+ response to 0K, showed Ca2+ oscillations during this time (Fig. 1C). The overall frequency of spontaneous Ca2+ transients averaged over 10 min was 1.26 ± 0.11 transients/min (n = 366 cells) in astrocytes and 0.15 ± 0.06 transients/min in neurons (n = 31; Fig. 1D). Thus, spontaneous Ca2+ oscillations were much more frequent in astrocytes than in neurons in these hippocampal slices.
We also looked at coincident Ca2+ responses in neighbouring astrocytes in a brain slice, or propagation of Ca2+ transients from one astrocyte to another. We found poor correlation between the Ca2+ responses in different cells, however, and rarely observed Ca2+ responses crossing cell borders in form of waves. The lack of coincident and propagated Ca2+ responses might be due to the two-dimensional analysis of the Ca2+ responses, which misses all events that might occur in the z-axis.
Spontaneous Ca2+ Oscillations Dependent on Functional Intracellular Ca2+ Stores and Independent of Neuronal Activity
After addition of cyclopiazonic acid (CPA, 30 μM; Fig. 2A), which depletes Ca2+ stores by inhibiting the Ca2+-ATPase of the endoplasmic reticulum, almost no Ca2+ transients could be observed and the percentage of astrocytes displaying spontaneous Ca2+ oscillations dropped to 5 ± 2% (n = 5 slices). ATP was applied in some of these experiments to check that Ca2+ release from functional Ca2+ stores was still working (Fig. 2A,B). Bath-applied ATP (100 μM, 1 min), which evoked a single Ca2+ transient under control conditions (n = 27/5) but no Ca2+ oscillations, also failed to elicit a Ca2+ response in CPA, suggesting that Ca2+ release from intracellular stores was involved in the generation of spontaneous Ca2+ oscillations. When extracellular Ca2+ was removed (0 mM Ca2+), one or few Ca2+ transients could still be recorded in the initial period of application (1–2 min) before they stopped altogether (Fig. 2B). ATP was applied in some of these experiments to check that Ca2+ release from functional Ca2+ stores was still working (Fig. 2A,B). Also, under these conditions ATP no longer elicited a significant Ca2+ response, indicating that the Ca2+ stores had been emptied. The Ca2+ oscillations were also suppressed after preincubation of the brain slices with the phospholipase inhibitor U73122 (10 μM for 30–60 min) containing saline. This also suppressed all Ca2+ responses to ATP and glutamate in these astrocytes (Fig. 2E,F). The spontaneous Ca2+ oscillations during the first 10 min of recording (Control, 100%) and the second 10 min of recording under the same control conditions were very similar with respect to the number of Ca2+ oscillating cells (95 ± 10%, n = 17; Fig. 2E) and the frequency of Ca2+ transients (99 ± 8%, n = 106; Fig. 2F). These results indicate that the Ca2+ oscillations in astrocytes were likely due to phospholipase C-mediated Ca2+ release from intracellular stores.
In order to suppress the electrical and synaptic activity of neurons, we used 0.5 μM TTX or 2 mM Ni2+ to inhibit the generation of action potentials and calcium-dependent transmitter release, respectively (Fig. 2C,D). Both TTX and Ni2+ had no significant effect on the spontaneous Ca2+ oscillations in astrocytes (Fig. 2E,F). In some experiments, a smaller amplitude of the Ca2+ responses was observed (Fig. 2D). These experiments suggest that the Ca2+ oscillations in astrocytes were not evoked or maintained by spontaneous neuronal activity.
Ca2+ Oscillations after Inhibition of mGluRs
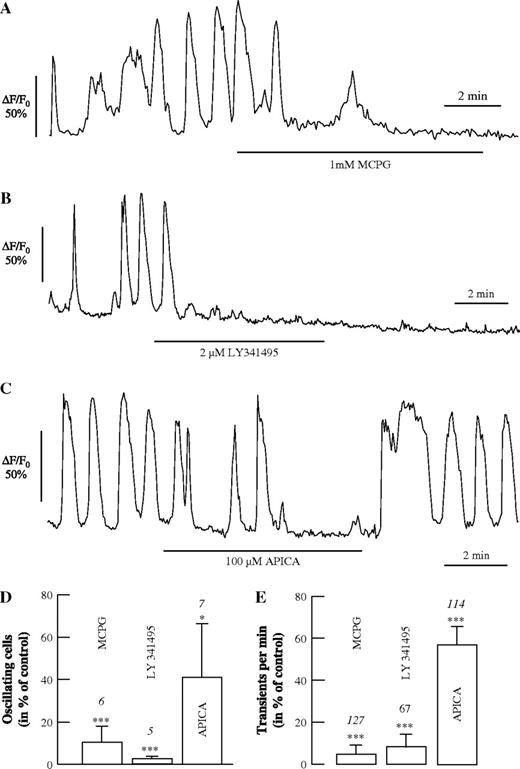
Different antagonists of mGluRs were applied to evaluate the role of these receptors for initiating the spontaneous Ca2+ oscillations in astrocytes (Fig. 3). Both MCPG, an antagonist of group I and possibly group II mGluRs (1 mM; Fig. 3A) and LY341495, a potent antagonist of group II/III mGluRs at the concentration of 2 μM as used here (Fig. 3B), led to a reduction or termination of spontaneous Ca2+ oscillations within the first two min of application. The group II mGluR antagonist APICA also reduced the Ca2+ oscillations, though less so than LY341495 (Fig. 3C). The fraction of oscillating cells were reduced to 10 ± 7% (n = 6 slices) by MCPG, to 3 ± 1% (n = 5) by LY341495 and to 41 ± 25% (n = 7) by APICA (Fig. 3D; Control without antagonist, 100%), while the frequency of remaining Ca2+ transient decreased to 6 ± 4% (n = 127), 9 ± 5% (n = 67) and 61 ± 10% (n = 114) by these antagonists, respectively (Fig. 3E). The effects of MCPG and LY341495 were irreversible over the period of wash-out (<15 min), while Ca2+ oscillations were resumed after the removal of APICA (Fig. 3C). These results strongly suggest that activation of mGluRs are involved in generating the spontaneous Ca2+ oscillations in hippocampal astrocytes.
The effect of antagonists of mGluRs on spontaneous Ca2+ oscillations in astrocytes. (A–C) Effect of MCPG, an antagonist of mGluR group I and II (1 mM; A), LY341495, an antagonist of mGluR group II (2 μM; B), and APICA, an antagonist of mGluR group II (100 μM; C), on spontaneous Ca2+ oscillations. Summary of the effects of the glutamate receptor antagonists on the number of oscillating astrocytes (D) and on the frequency of Ca2+ transients (E).
Ca2+ Oscillations Can Be Evoked by Activating mGluRs
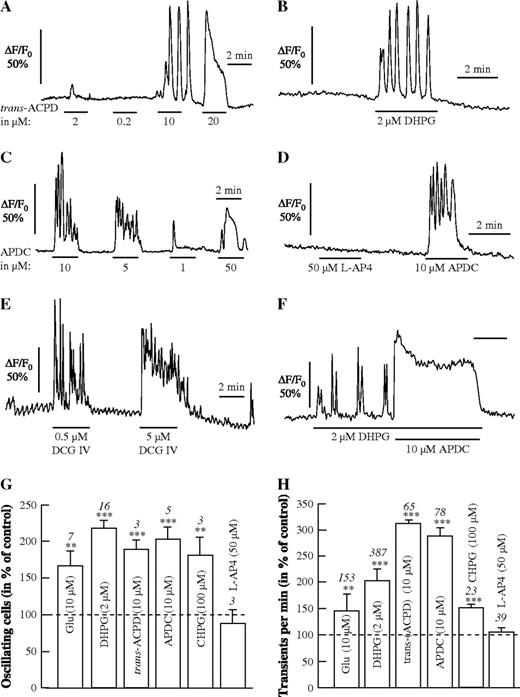
In order to check the involvement of different types of mGluR in mediating Ca2+ oscillations, we applied different agonists of mGluRs to test their ability to initiate Ca2+ oscillations. Interestingly, trans-ACPD, at 2 μM, evoked a single Ca2+ transient, while it elicited Ca2+ oscillations at 10 μM (Fig. 4A). At 20 μM, trans-ACPD evoked a biphasic response consisting of a fast transient response followed by a shoulder. Since trans-ACPD is known to activate group I as well as group II mGluRs, and group II antagonists also inhibited spontaneous Ca2+ oscillations, we tested different concentrations of DHPG, a potent and specific group I mGluR agonist, and APDC, a specific group II mGluR agonist. Both agonists exhibited a concentration-dependent profile similar to trans-ACPD, evoking Ca2+ oscillations at a concentration of 2 μM for DHPG (Fig. 4B) and at concentrations of 5–10 μM for APDC (Fig. 4C), respectively. At concentrations of 1 and 50 μM, APDC elicited different Ca2+ responses, but no oscillations. L-AP4 (50 μM), a group III-specific agonist, failed to elicit Ca2+ changes in the same astrocytes, in which APDC evoked Ca2+ oscillations (Fig. 4D). Another group I mGluR agonist, CHPG (100 μM), also increased highly significantly the number of Ca2+ oscillating cells and increased the rate of transients (Fig. 4G,H).
Ca2+ signals in response to agonists of mGluRs. (A–F) Different concentrations of trans-ACPD (A) and APDC (C) evoke single Ca2+ transiens at low concentrations (1 μM), larger Ca2+ transients with shoulder at high concentrations (20, 50 μM) and Ca2+ oscillations at intermediate concentrations (5–10 μM). The group I mGluR agonist DHPG evokes Ca2+ oscillations at 2 μM (B), while the group III agonist L-AP4 (50 μM) has no effect, in contrast to the group II agonist APDC (10 μM) (D). The group II agonist DCG IV evokes Ca2+ oscillations at 0.5 μM and Ca2+ oscillations superimposed on a tonic response at 5 μM (E). Co-application of the group I agonist DHPG and the group II agonist APDC at intermediate concentrations evoked a large Ca2+ transient with shoulder (F). Summary of the effect of different mGluR ligands on the number of oscillating astrocytes (G) and on the frequency of Ca2+ transients (H).
The highly selective group II mGluR agonist DCG IV elicited Ca2+ oscillations at 0.5 μM, while 5 μM DCG IV evoked a tonic Ca2+ rise onto which Ca2+ oscillations were superimposed (Fig. 4E). When agonists of groups I and II were applied together, e.g. DHPG and APDC, at concentrations at which they alone evoked Ca2+ oscillations, a sustained Ca2+ response was observed, as elicited by one of these agonists at a higher concentration (Fig. 4F).
The influence of different group-specific mGluR agonists is summarized in Figure 4G,H, showing that all agonists of groups I and II mGluRs, but not those of group III, significantly increased the number of oscillating cells and the frequency of Ca2+ transients as compared with the control without agonists (set to 100%).
In order to evaluate the involvement of mGluRs in initiating Ca2+ oscillations, we compared the responses to glutamate with those to other metabotropic transmitter receptor agonists that are known to elicit Ca2+ responses in astrocytes, such as ATP and phenylephrine (Shao and McCarthy, 1995). Whereas application of glutamate at a concentration of 10 μM elicited Ca2+ oscillations (Fig. 5A), higher concentrations (100 μM, Fig. 5B) induced biphasic Ca2+ changes (peak with shoulder) or a sustained Ca2+ rise, and lower concentrations (1 μM, data not shown) elicited only a single Ca2+ transient. Perfusion of the acute brain slices with ATP (5, 10 and 30 μM; Fig. 5C,D) evoked Ca2+ signals, but rarely Ca2+ oscillations, when applied at different concentrations (5–100 μM). At the lowest concentration used (5 μM ATP; 1 μM glutamate) both agonists predominantly evoked a single calcium transient (ATP: 76% in three slices; glutamate: 57% in four slices; Fig. 5E). At a concentration of 10 μM, glutamate evoked Ca2+ oscillations in 63% of all astrocytes (seven slices, Fig. 5E), while application of 10 μM ATP predominantly (69% in seven slices) led to a biphasic Ca2+ response (a peak with shoulder). Only a small portion (4%) of astrocytes exposed to 10 μM ATP displayed Ca2+ oscillations. Application of a higher agonist concentration (e.g. 100 μM) resulted in the case of ATP in 86% (four slices) and in the case of glutamate in 90% (three slices) of all astrocytes in a Ca2+ transient followed by a shoulder or sustained Ca2+ rise (Fig. 5B,D). Since ADP (10–100 μM) could also evoke Ca2+ responses, but not Ca2+ oscillations, the responses to ATP were likely mediated by activation of P2Y receptors in the astrocytes. The Ca2+ responses evoked by ATP were reduced by PPADS (10 μM), a P2 receptor antagonist, but PPADS alone had no effect on either the frequency of spontaneous nor of glutamate-evoked Ca2+ oscillations in these cells.
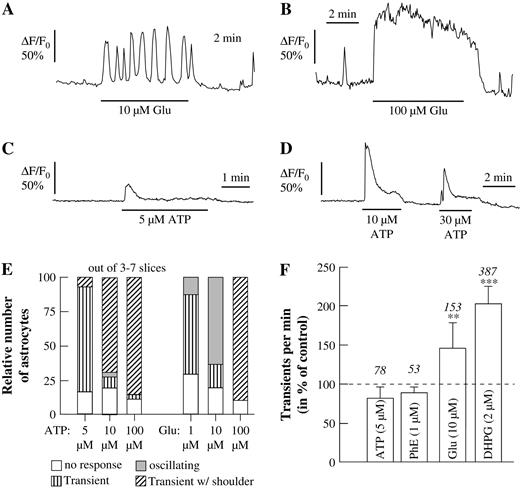
Ca2+ responses to metabotropic receptor agonists in astrocytes. (A–D) Glutamate applied at an intermediate concentration (10 μM; A) evoked Ca2+ oscillations and at higher concentrations (100 μM; B) a sustained Ca2+ response, while ATP evoked single Ca2+ transients without shoulder at lower concentrations (5 μM; C) and with shoulder at higher concentrations (10–30 μM; D). (E) Relative number of cells responding to different concentrations of ATP or glutamate with Ca2+ transients and Ca2+ oscillations. Each column represents the analysis from at least 78 astrocytes responding with a Ca2+ signal to either ATP or glutamate. (F) Frequency of Ca2+ transients as elicited by ATP, phenylephrine (PhE), glutamate (Glu) and DHPG.
Similar to ATP, phenylephrine (5–100 μM) evoked Ca2+ oscillations only in a minority of cells (<10%; data not shown). While ATP and phenylephrine did not increase the frequency of spontaneous Ca2+ transients, glutamate (10 μM) and DHPG (2 μM) increased the frequency by 50 and 100%, respectively (Fig. 5F).
The Effect of Agonists of Group II mGluRs
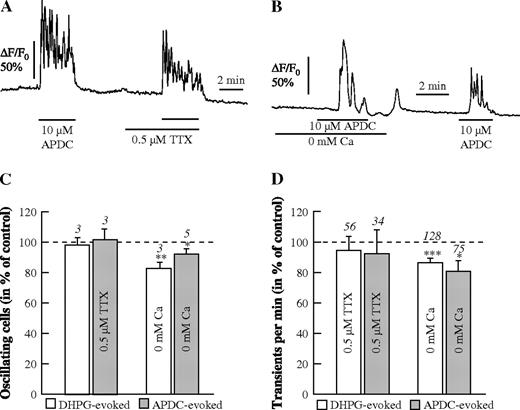
In order to further confirm the role of both group I and group II mGluRs in directly mediating Ca2+ oscillations in astrocytes, we applied different agonists and antagonists of these receptor classes under different experimental conditions. First, we tested the group II-specific mGluR agonist APDC in the presence of TTX (Fig. 6A,C) and in the absence of external Ca2+ (Fig. 6B,D). The results show that the action of APDC is not dependent on either neuronal activity or Ca2+ influx. In the same cells, this was confirmed also for the group I mGluR agonist DHPG (2 μM; Fig. 6C,D). As stated before, in Ca2+-free saline Ca2+ responses could only be evoked once by the mGluR agonists, presumably because this one response depleted the intracellular Ca2+ stores when Ca2+ influx was suppressed due to the absence of extracellular Ca2+. After readdition of Ca2+ to the bathing solution, APDC could again evoke Ca2+ oscillations (Fig. 6B).
APDC- and DHPG-evoked Ca2+ oscillations. (A, B) Ca2+ oscillations evoked by 10 μM APDC in the absence and presence of TTX (0.5 μM; A), and in the absence and presence of external Ca2+ (B). Summary of the DHPG- and the APDC-evoked responses with respect to the number of oscillating astrocytes (C) and to the frequency of Ca2+ transients in the oscillating astrocytes (D).
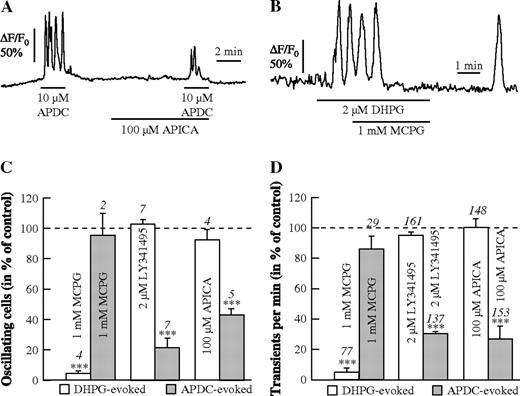
The agonists DHPG and APDC were applied with various antagonists of group I and II mGluRs to the same brain slices to get a differential pharmacological profile of the evoked Ca2+ oscillations in astrocytes (Fig. 7). The group II antagonist APICA significantly reduced the Ca2+ oscillations evoked by APDC (Fig. 7A), and MCPG stopped the Ca2+ oscillations evoked by DHPG (Fig. 7B). While MCPG terminated the response to the group I mGluR agonist DHPG, the group II-specific mGluR antagonists, LY341495 and APICA, had no effect on the DHPG-evoked responses, but reduced the number of cells and Ca2+ transients evoked by APDC to 20–45% (Fig. 7C,D).
Pharmacological profile of APDC- and DHPG-evoked Ca2+ oscillations. (A, B) Reduction of APDC-evoked Ca2+ oscillations by APICA (100 μM; A) and blockade of DHPG-evoked Ca2+ oscillations by MCPG (1 mM; B). Summary of the DHPG- and APDC-evoked responses with respect to the number of oscillating astrocytes (C) and to the frequency of Ca2+ transients in the oscillating astrocytes (D) in the presence of different antagonists of group I and group II mGluRs.
Since activation of group II mGluRs has been linked primarily to the cAMP-mediated pathway (see Discussion), we performed experiments with inhibitors of either the cAMP or the IP3 pathway. Adenylyl cyclase inhibition with SQ22.536 (200 μM) had no effect on APDC-evoked Ca2+ oscillations, while superfusion of the brain slices with the phospholipase C blocker U73122 (10 μM, 60 min) suppressed the APDC-evoked Ca2+ oscillations (data not shown). This indicates that the Ca2+ oscillations are elicited by APDC presumably via the IP3-mediated pathway.
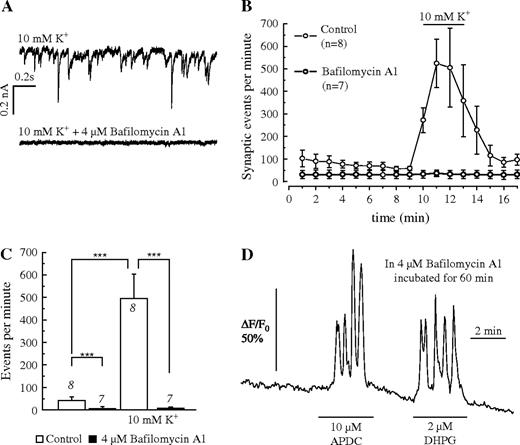
Both APDC and DHPG could elicit Ca2+ oscillations even after preincubating the brain slices in 4 μM bafilomycin A1, which blocks vesicular transmitter release (Fig. 8). The efficacy of bafilomycin A1 was tested using patch-clamp recordings from pyramidal neurons in acute hippocampal brain slices. Spontaneous postsynaptic currents were recorded in aCSF and at elevated extracellular K+ concentration (10 mM), which greatly increased the frequency of the postsynaptic currents. After incubation of the slices in 4 μM bafilomycin A1, the frequency of postsynaptic events was reduced and the frequency increase in 10 mM K+ was abolished (Fig. 8A–C). Brain slices which were treated the same way with bafilomycin A1 still showed Ca2+ oscillations in response to 10 μM APDC and 2 μM DHPG (Fig. 8D). The frequency of spontaneous Ca2+ oscillations was not affected by preincubation with bafilomycin A1. This result was obtained in 102 cells from five brain slices, indicating that Ca2+ oscillations evoked by group I- and II-specific mGluR agonists are not induced by increased vesicular release of neurotransmitters activated by these agonists.
Effect of the inhibitor of neurotransmitter release bafilomycin A1. (A) Suppression of postsynaptic currents in a pyramidal neuron of a hippocampal brain slice at an elevated K+ concentration (10 mM) without (upper trace) incubation and after incubation of the slice in 4 μM bafilomycin A1 for 30 min. (B) Plot of the recorded synaptic events (spontaneous postsynaptic currents) at normal and elevated K+ concentrations without incubation (open circles) and after incubation of the slices in 4 μM bafilomycin A1 (filled circles), indicating the loss of synaptic currents in bafilomycin-treated brain slices (C). (D) Recording of Ca2+ oscillations evoked by APDC and DHPG in a brain slice incubated in 4 μM bafilomycin A1 the same way as the slices from which the membrane currents were recorded (A–C).
Discussion
The present study suggests that activation of mGluRs is needed for the generation of spontaneous Ca2+ transients in rat hippocampal astrocytes. Glial Ca2+ oscillations occur independently of neuronal transmitter release, and are presumably due to tonic activation of group I and group II metabotropic receptors by basal levels of extracellular glutamate. The Ca2+ oscillations could be inhibited by antagonists of mGluR of both group I and group II. Agonists of mGluR groups I and II, but not mGluR group III, elicited dose-dependent Ca2+ oscillations in astrocytes. Like the Ca2+ responses mediated by mGluR group I (Porter and McCarthy, 1995; Nakahara et al., 1997; this study), activation of mGluR group II also appeared to result in Ca2+ release from CPA-sensitive intracellular stores. The dose-dependent activation of mGluR led, at low concentration (e.g. 10 μM for glutamate), to Ca2+ oscillations, while activation with higher agonist concentrations (e.g. 100 μM for glutamate) resulted in responses consisting of a fast transient Ca2+ increase followed by a shoulder, presumably reflecting in part capacitive and non-capacitive calcium influx (Shuttleworth and Thompson, 1999; Jung et al., 2000).
Spontaneous Ca2+ Oscillations in Rat Astrocytes
Spontaneous Ca2+ oscillations in astrocytes have been described for cultured (Fatatis and Russel, 1992; Harris-White et al., 1998) and acutely dissociated cells (Charles, 1994), as well as for acute brain slices (Parri et al., 2001; Nett et al., 2002). Since action potential firing and synaptic transmitter release are not needed to sustain astrocytic Ca2+ oscillations in astrocytes, it was proposed that these Ca2+ oscillations are due to ‘intrinsic factors’ (Nett et al., 2002). We could confirm that the Ca2+ oscillations are not dependent on TTX-sensitive electrical activity and Ni2+-sensitive transmitter release. In contrast to mouse hippocampal slices, where inhibitors of mGluRs did not affect spontaneous Ca2+ oscillations in astrocytes (Nett et al., 2002; Aguado et al., 2002), we show here that Ca2+ oscillations in rat astrocytes were initiated and maintained by activation of mGluR groups I and II, but not group III. On the other hand, blocking Ca2+ channels with Ni2+ had no effect on the Ca2+ oscillations, while spontaneous glial Ca2+ oscillations in mouse preparations were reduced by Co2+ (Aguado et al., 2002).
The frequency of spontaneously active astrocytes over at least 10 min was 35%, with 1.26 Ca2+ transients/min on average in the juvenile rats. Both these parameters may vary with the stage of development and the type of preparation. In mouse hippocampal brain slices we found that a higher percentage of astrocytes were spontaneously active than in rat hippocampal slices under the same conditions (unpublished observations). An in vivo study on rat cortical astrocytes of similar age as in the present study showed that >50% of astrocytes showed a Ca2+ response within 10 min, but with a much lower frequency of occurrence (0.12/min; Hirase et al., 2004). The reasons for these variations are still unclear, but indicate that influences from the whole system, as present under in vivo conditions, may alter the spontaneous activity.
Our results show that antagonists of either group I or group II mGluRs are able to abolish the Ca2+ oscillations in hippocampal astrocytes, but addition of an agonist of either group I or group II mGluRs is sufficient to evoke Ca2+ oscillations. The effect of blocking either group I or group II receptors suggests that mGluRs of both groups need to be activated to allow Ca2+ oscillations, while the initiation of Ca2+ oscillations by the addition of a single, specific agonist for either group I or group II mGluRs suggests that activation of either group I or group II mGluRs is sufficient to evoke Ca2+ oscillations. It must be noted here that the degree of specificity of mGluR agonists and antagonists for either group I and/or group II varies; therefore our results might be explained by the lack of group-specificity of the ligands used here. This may explain the action of MCPG, which is believed to block group I and group II mGluRs but did not inhibit the APDC-evoked Ca2+ oscillations in our experiments. On the other hand, even when adding a group-specific agonist for mGluRs which alone is able to elicit Ca2+ oscillations, both types of mGluR might be activated due to the presence of endogenous, extracellular glutamate in the slice (probably in the low micromolar range; see below). Assuming different affinities and desensitisation properties of the different mGluRs, the background glutamate level in the tissue might be sufficient to activate one, and the added group-specific agonist the other, mGluR, leading to the initiation of Ca2+ oscillations. Experimental evidence, however, is yet needed to prove either of these possibilities.
Biophysical models simulating oscillating cytosolic Ca2+ either employ oscillating protein kinase C activity and IP3 production (Hofer et al., 2004) or are based on oscillating Ca2+ membrane fluxes into and out of the cytosol (Sneyd et al., 2004). Application of U73122, a blocker of phospholipase C, and CPA, a potent inhibitor of sarcoendoplasmatic Ca2+-ATPase, abolished spontaneous Ca2+ oscillations in rat astrocytes. From this, and the result that Ni2+ does not influence Ca2+ oscillations, we conclude that oscillating Ca2+ plasma membrane fluxes are not responsible for the Ca2+ oscillations in rat astrocytes. Furthermore, it is known that evoked neuronal activity elicits Ca2+ rises in astrocytes (Porter and McCarthy, 1996) and, in turn, glial Ca2+ rises are able to release glutamate (Fellin et al., 2004) and increase synaptic activity in hippocampal interneurons through activation of kainate receptors (Liu et al., 2004), or enhance currents through NMDA receptors in interneurons of the thalamus (Parri et al., 2001). The findings that astrocytes are able to respond even to small changes in the extracellular glutamate concentration and release glutamate in response to an increase in intracellular Ca2+ could mean that a positive feedback mechanism operates inducing ‘glutamate-induced glutamate release’ from astrocytes. This would not only be functional during neuronal activation, but might also be caused by tonic activation of glial mGluR.
Group II Metabotropic Glutamate Receptor-mediated Ca2+ Release in Astrocytes
Group II mGluRs have been shown to be located mainly on presynaptic terminals (Petralia et al., 1996). They have been suggested to be involved in the negative feedback on glutamatergic and GABAergic transmitter release (Moldrich et al., 2001; Smolders et al., 2004). However, in situ hybridization and antibody staining revealed that group II mGluRs are found not only in neurons, but also in astrocytes of the hippocampus (Petralia et al., 1996; Schools and Kimelberg, 1999). In addition to the negative coupling of group II mGluRs to the activity of the adenylyl cyclase via Gi/o-proteins (Schoepp et al., 1995; Anwyl, 1999), there is evidence that group II mGluRs might also stimulate phospholipase C activity (Otani et al., 2002). The specific agonist DCG IV induced long-term depression in the prefrontal cortex, which was shown to be sensitive to U73122 and the IP3 receptor antagonist heparin. Furthermore, phospholipase D activation by mGluR group II agonists in hippocampal slices was reported by Klein et al. (1997), and application of DCG IV increased PLD and PLC activity with an EC50 of 22 nM. Our results suggest that activation of glial group II mGluRs leads to a phospholipase C-mediated Ca2+ release from intracellular stores. An indirect effect of mGluR group II via modulation of external glutamate levels is unlikely, since inhibition of synaptic transmitter release by Ni2+ or Ca2+-free saline as well as incubation with V-ATPase inhibitor bafilomycin A1 failed to inhibit mGluR group II-mediated Ca2+ oscillations. In addition, mGluR2/3 (group II) stimulation via DCG IV or APDC can decrease extracellular glutamate levels in the hippocampus in situ (Moldrich et al., 2001). Release of glutamate by the glutamate–cystine exchanger of astrocytes, as described by Tomi et al. (2003), could be a Ca2+-independent source for external glutamate. Since it has been reported for the nucleus accumbens that activation of group II mGluRs results in an inhibition of the glutamate–cystine exchanger (Xi et al., 2002), and therefore in a decrease of glutamate release, an involvment of X(c−) transporters seems unlikely.
Furthermore, the inhibition of the adenylyl cyclase by SQ22.536 did not inhibit DCG IV- or APDC-triggered Ca2+ responses, and supports our notion that the cAMP-pathway is not involved in the mGluR2/3 (group II)-mediated Ca2+ responses. These findings cannot exclude the possibility that NMDA receptors and group II mGluR interact as described by Mistry et al. (1998), who reported that activation of group II mGluRs by DCG IV (<1 μM) leads to an increase in phosphoinositide turnover. This mechanism cannot be excluded here, though NMDA receptors are generally believed to be absent in hippocampal astrocytes (Seifert and Steinhäuser, 2001; Matthias et al., 2003).
The Role of Extracellular Glutamate for Spontaneous Ca2+ Oscillations in Astrocytes
Extracellular glutamate levels are derived from vesicular and non-vesicular sources. While glutamate is released during synaptic transmission, it is taken up by glial cells via the excitatory amino acid transporter. Under pathophysiological conditions like stroke and ischaemia, increased levels of glutamate lead to cell death. Although group II mGluRs are known to act as autoreceptors at presynaptic sites, or as heteroceptors at neuronal cell somata, and are able to tune down synaptic transmitter release, the role of group II mGluRs expressed in astrocytes is not yet clear. Our findings show that astrocytes respond to different concentrations of glutamate with different Ca2+ responses. This may enable astrocytes to sense different levels of extracellular glutamate levels in the micromolar range (normal ∼3 μM). Activation of purinergic, histaminergic and adrenerigic metabotropic receptors failed to show this response profile. Prolonged application of low agonist concentrations (e.g. PhE, 3 μM or ATP, 10 μM) resulted in a single Ca2+ transient, which was followed at a reduced Ca2+ level by a ‘shoulder’ at higher agonist concentrations. While the transient change in intracellular Ca2+ seems to reflect receptor response and IP3 production, the subsequent shoulder is presumably due to receptor inactivation, store depletion and/or store-operated Ca2+ influx (Shuttleworth and Thompson, 1999; Jung et al., 2000). This also infers that glial cells may sense extracellular glutamate, and respond with different types of Ca2+ transients and frequencies of Ca2+ oscillations to micromolar glutamate concentrations or to small step changes of the glutamate concentration in the extracellular spaces.
We thank Dr Christian Lohr for comments on an earlier version of this manuscript. This study was supported by the Deutsche Forschungsgemeinschaft, SFB 530, B1.
References
Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E (
Angulo MC, Kozlov AS, Charpak S, Audinat E (
Anwyl R (
Beck A, Zur Nieden R, Schneider HP, Deitmer JW (
Berridge MJ, Bootman MD, Roderick HL (
Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A (
Brockhaus J, Deitmer JW (
Chen J, Backus KH, Deitmer JW (
Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (
Dallwig R, Deitmer JW (
Dallwig R, Vitten H, Deitmer JW (
Deitmer JW, Verkhratsky AJ, Lohr C (
Dolmetsch RE, Xu K, Lewis RS (
Edwards FA, Konnerth A, Sakmann B, Takahashi T (
Fatatis A, Russell JT (
Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. (
Fiacco TA, McCarthy KD (
Garaschuk O, Linn J, Eilers J, Konnerth A (
Harris-White ME, Zanotti SA, Frautschy SA, Charles AC (
Hirase H, Qian L, Bartho P, Buzsáki G (
Hofer T, Venance L, Giaume C (
Innocenti B, Parpura V, Haydon PG (
Jung S, Pfeiffer F, Deitmer JW (
Kimelberg HK, Cai Z, Rastogi P, Charniga CJ, Goderie S, Dave V, Jalonen TO (
Klein J, Iovino M, Vakil M, Shinozaki H, Loffelholz K (
Konnerth A, Llano I, Armstrong CM (
Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY (
Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M (
Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C (
Mistry R, Golding N, Challiss RA (
Moldrich RX, Giardina SF, Beart PM (
Morita M, Higuchi C, Moto T, Kozuka N, Susuki J, Itofusa R, Yamashita J, Kudo Y (
Mulligan SJ, MacVicar BA (
Nakahara K, Okada M, Nakanishi S (
Nett WJ, Oloff SH, McCarthy KD (
Otani S, Daniel H, Takita M, Crepel F (
Parri HR, Gould TM, Crunelli V (
Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ (
Porter JT, McCarthy KD (
Porter JT, McCarthy KD (
Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H (
Schoepp DD, Johnson BG, Salhoff CR, Wright RA, Goldsworthy JS, Baker SR (
Schools GP, Kimelberg HK (
Seifert G, Steinhäuser C (
Shao Y, McCarthy KD (
Shuttleworth TJ, Thompson JL (
Smolders I, Lindekens H, Clinckers R, Meurs A, O'Neill MJ, Lodge D, Ebinger G, Michotte Y (
Sneyd J, Tsaneva-Atanasova K, Yule DI, Thompson JL, Shuttleworth TJ (
Strahonja-Packard A, Sanderson MJ (
Tomi M, Funaki T, Abukawa H, Katayama K, Kondo T, Ohtsuki S, Ueda M, Obinata M, Terasaki T, Hosoya K (
Woodhall G, Gee CE, Robitaille R, Lacaille JC (
Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW (
Zhang Q, Fukuda M, Van Bockstaele E, Pascual O,. Haydon PG (
Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G (
Zur Nieden R, Deitmer JW (