-
PDF
- Split View
-
Views
-
Cite
Cite
Gaël Chételat, Victor L. Villemagne, Kerryn E. Pike, Kathryn A. Ellis, Pierrick Bourgeat, Gareth Jones, Graeme J. O’Keefe, Olivier Salvado, Cassandra Szoeke, Ralph N. Martins, David Ames, Colin L. Masters, Christopher C. Rowe, the Australian Imaging Biomarkers and Lifestyle Study of ageing (AIBL) Research Group, Independent contribution of temporal β-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease, Brain, Volume 134, Issue 3, March 2011, Pages 798–807, https://doi.org/10.1093/brain/awq383
Close - Share Icon Share
Abstract
The relationship between β-amyloid deposition and memory deficits in early Alzheimer’s disease is unresolved, as past studies show conflicting findings. The present study aims to determine the relative contribution of regional β-amyloid deposition, hippocampal atrophy and white matter integrity to episodic memory deficits in non-demented older individuals harbouring one of the characteristic hallmarks of Alzheimer’s disease, i.e. with β-amyloid pathology. Understanding these relationships is critical for effective therapeutic development. Brain magnetic resonance imaging and [11C]Pittsburgh Compound B-positron emission tomography scans were obtained in 136 non-demented individuals aged over 60 years, including 93 healthy elderly and 43 patients with mild cognitive impairment. Voxel-based correlations were computed between a memory composite score and grey matter volume, white matter volume and β-amyloid deposition imaging datasets. Hierarchical linear regression analyses were then performed using values extracted in regions of most significant correlations to determine the relative contribution of each modality to memory deficits. All analyses were conducted pooling all groups together as well as within separate subgroups of cognitively normal elderly, patients with mild cognitive impairment and individuals with high versus low neocortical β-amyloid. Brain areas of highest correlation with episodic memory deficits were the hippocampi for grey matter volume, the perforant path for white matter volume and the temporal neocortex for β-amyloid deposition. When considering these three variables together, only hippocampal volume and temporal β-amyloid deposition provided independent contributions to memory deficits. In contrast to global β-amyloid deposition, temporal β-amyloid deposition was still related to memory independently from hippocampal atrophy within subgroups of cognitively normal elderly, patients with mild cognitive impairment or cases with high neocortical β-amyloid. In the pre-dementia stage of Alzheimer’s disease, subtle episodic memory impairment is related to β-amyloid deposition, especially in the temporal neocortex, and independently from hippocampal atrophy, suggesting that both factors should be independently targeted in therapeutic trials aimed at reducing cognitive decline.
Introduction
How β-amyloid deposition interferes with memory performance in the pre-dementia stage of Alzheimer’s disease is still debated. As cognition is the major outcome of clinical trials, elucidating this question is crucial to therapeutic developments. The development of new radiotracers such as 11C-Pittsburgh compound B (PiB) has facilitated research, enabling the visualization of β-amyloid deposition in vivo using PET. There is, however, no consensus to date regarding the relationship between episodic memory performance and β-amyloid deposition in non-demented individuals. Lower episodic memory performances have been reported in individuals with, versus without β-amyloid deposition within patients with mild cognitive impairment (Wolk et al., 2009; Rowe et al., 2010), but no difference was found in another study (Jack et al., 2008) or within normal elderly (Jack et al., 2008; Hedden et al., 2009; Rowe et al., 2010). As regard to studies assessing the correlations between β-amyloid deposition and memory scores, the findings are similarly inconclusive. A significant correlation was found when pooling healthy elderly and/or patients with mild cognitive impairment with patients with Alzheimer’s disease (Pike et al., 2007; Jack et al., 2008; Villemagne et al., 2008; Tolboom et al., 2009; Forsberg et al., 2010), but the results are inconsistent when assessed within normal elderly or mild cognitive impairment separately (Pike et al., 2007; Mormino et al., 2009; Sperling et al., 2009; Storandt et al., 2009; Forsberg et al., 2010; Resnick et al., 2010). For a strong conclusion to be drawn, participants with Alzheimer’s disease and non-demented individuals should not be pooled together as patients with Alzheimer’s disease would probably drive the correlation because they all have a high level of β-amyloid deposition and marked memory deficits when compared with normal elderly or mild cognitive impairment. Furthermore, to obtain a more comprehensive view on the underlying mechanisms, it seems crucial to take into account other factors known to play a role in episodic memory deficits such as hippocampal atrophy (Chételat et al., 2003; Mormino et al., 2009) or white matter integrity (Fellgiebel et al., 2005; Kalus et al., 2006), so as to determine the relative contribution of β-amyloid deposition. This question has been addressed in an earlier study (Mormino et al., 2009), showing that the effect of global neocortical β-amyloid deposition on episodic memory is mediated by hippocampal atrophy in non-demented individuals. However, regional β-amyloid deposition has not been considered, which may change the conclusion; while hippocampal atrophy could be a better predictor of memory loss than global β-amyloid deposition, it is possible that β-amyloid deposition in a specific region of the brain would be more specifically involved in episodic memory deficits. Although not taking hippocampal atrophy into account, regional analyses of PiB in relation to memory have been performed in other studies (Forsberg et al., 2008; Tolboom et al., 2009; Rentz et al., 2010) but only considering one region based on a priori hypotheses (e.g. the precuneus) or sampling very large regions (e.g. the whole frontal, temporal or parietal cortex).
The present study aims to determine the relative contribution of regional β-amyloid deposition, hippocampal atrophy and white matter integrity to episodic memory deficits in non-demented individuals harbouring one of the characteristic hallmarks of Alzheimer’s disease (β-amyloid pathology) using a voxel-based approach and considering the whole brain.
Materials and methods
Participants
One hundred and seventy individuals aged over 60 years were included in the study, including 93 healthy elderly subjects, 43 patients with mild cognitive impairment and 34 patients diagnosed with Alzheimer’s disease. All were participants of the Australian Imaging Biomarkers and Lifestyle Study of Ageing (Ellis et al., 2009) and had both magnetic resonance and PiB-PET scans at the Austin Hospital (Melbourne). All subjects underwent clinical and neuropsychological examination, and allocation of individuals to a diagnostic group and exclusion of ineligible individuals was performed by a clinical review panel based on the screening interview and neuropsychological assessment and according to internationally agreed criteria, namely, patients with mild cognitive impairment met Petersen’s consensus criteria for amnestic mild cognitive impairment (Petersen and Morris, 2005) while patients with Alzheimer’s disease met standard National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) clinical criteria for probable Alzheimer’s disease (McKhann et al., 1984). The full methodology for the cohort recruitment and evaluation is detailed elsewhere (Ellis et al., 2009). The neuropsychological examination included the Mini-Mental State Examination, Wechsler Test of Adult Reading, the California Verbal Learning Test (2nd edition), Rey Complex Figure Test, 30-item Boston Naming Test, Digit Span subtest of the Wechsler Adult Intelligence Scale (3rd edition), verbal category fluency (animals and boys’ names) and Stroop tests. A composite episodic memory score was calculated by taking the average of the z-scores for the Rey Complex Figure Test and California Verbal Learning Test-II long-delay recall (Pike et al., 2007).
Demographics, apolipoprotein E status and memory performances for each group are reported in Table 1. Approval for the study was obtained from the Austin Health Human Research Ethics Committee, and written informed consent for participation was obtained for each subject prior to the scans. All evaluations were performed, on average, within 2 months (mean interval between the first and the last examination was 58 ± 66 days).
Demographics and cognitive scores for each group
| . | Healthy elderly (n = 93) . | Mild cognitive impairment (n = 43) . | Alzheimer’s disease (n = 34) . |
|---|---|---|---|
| Males (%) | 47 | 53 | 44 |
| ApoE4+ (%) | 34 | 49 | 74 |
| High-PiB (%) | 26 | 58 | 100 |
| Age (years) | 74.3 ± 7.1 | 75.1 ± 7.2 | 75.0 ± 7.9 |
| Education | 13.7 ± 3.3 | 12.0 ± 3.5 | 11.5 ± 3.0 |
| MMSE | 29.1 ± 1.1 | 27.1 ± 2.0 | 21.6 ± 5.3 |
| Episodic memorya | −0.1 ± 0.8 | −2.1 ± 1.0 | −3.4 ± 0.5 |
| . | Healthy elderly (n = 93) . | Mild cognitive impairment (n = 43) . | Alzheimer’s disease (n = 34) . |
|---|---|---|---|
| Males (%) | 47 | 53 | 44 |
| ApoE4+ (%) | 34 | 49 | 74 |
| High-PiB (%) | 26 | 58 | 100 |
| Age (years) | 74.3 ± 7.1 | 75.1 ± 7.2 | 75.0 ± 7.9 |
| Education | 13.7 ± 3.3 | 12.0 ± 3.5 | 11.5 ± 3.0 |
| MMSE | 29.1 ± 1.1 | 27.1 ± 2.0 | 21.6 ± 5.3 |
| Episodic memorya | −0.1 ± 0.8 | −2.1 ± 1.0 | −3.4 ± 0.5 |
For each variable, mean and standard deviation are indicated, except for males, ApoE4+ and high-PiB indicating the percentage of males in the group, the percentage of subjects in the group having at least one ε4 allele (information missing for two healthy elderly subjects and four patients with mild cognitive impairment), and the percentage of subjects in the group classified as high-PiB (see text for criteria), respectively.
a Episodic memory is expressed as z-scores.
MMSE = Mini-Mental State Examination.
Demographics and cognitive scores for each group
| . | Healthy elderly (n = 93) . | Mild cognitive impairment (n = 43) . | Alzheimer’s disease (n = 34) . |
|---|---|---|---|
| Males (%) | 47 | 53 | 44 |
| ApoE4+ (%) | 34 | 49 | 74 |
| High-PiB (%) | 26 | 58 | 100 |
| Age (years) | 74.3 ± 7.1 | 75.1 ± 7.2 | 75.0 ± 7.9 |
| Education | 13.7 ± 3.3 | 12.0 ± 3.5 | 11.5 ± 3.0 |
| MMSE | 29.1 ± 1.1 | 27.1 ± 2.0 | 21.6 ± 5.3 |
| Episodic memorya | −0.1 ± 0.8 | −2.1 ± 1.0 | −3.4 ± 0.5 |
| . | Healthy elderly (n = 93) . | Mild cognitive impairment (n = 43) . | Alzheimer’s disease (n = 34) . |
|---|---|---|---|
| Males (%) | 47 | 53 | 44 |
| ApoE4+ (%) | 34 | 49 | 74 |
| High-PiB (%) | 26 | 58 | 100 |
| Age (years) | 74.3 ± 7.1 | 75.1 ± 7.2 | 75.0 ± 7.9 |
| Education | 13.7 ± 3.3 | 12.0 ± 3.5 | 11.5 ± 3.0 |
| MMSE | 29.1 ± 1.1 | 27.1 ± 2.0 | 21.6 ± 5.3 |
| Episodic memorya | −0.1 ± 0.8 | −2.1 ± 1.0 | −3.4 ± 0.5 |
For each variable, mean and standard deviation are indicated, except for males, ApoE4+ and high-PiB indicating the percentage of males in the group, the percentage of subjects in the group having at least one ε4 allele (information missing for two healthy elderly subjects and four patients with mild cognitive impairment), and the percentage of subjects in the group classified as high-PiB (see text for criteria), respectively.
a Episodic memory is expressed as z-scores.
MMSE = Mini-Mental State Examination.
Neuroimaging data acquisition
Sagittal T1-weighted magnetic resonance images were acquired using a standard 3D-magnetization prepared rapid gradient echo sequence at 3T, with in-plane resolution 1 × 1 mm, slice thickness 1.2 mm, repetition time/echo time/inversion time = 2300/2.98/900 ms, flip angle 9° and field of view 240×256 and 160 slices.
The PiB-PET scans were acquired using a Phillips Allegro™ PET camera. Each participant was injected with 370 MBq of PiB and a 30 min acquisition in 3D mode was performed starting 40 min after injection of PiB. A transmission scan was performed for attenuation correction. PET images were reconstructed using a 3D RAMLA algorithm. Summed images for the 40–70 min timeframe were used in this study.
Neuroimaging data processing
The procedure for neuroimaging data handling and transformation is fully detailed elsewhere (Chételat et al., 2010,a, b). Briefly, magnetic resonance data were spatially normalized and segmented onto grey matter, white matter and cerebrospinal fluid partitions using the voxel-based morphometry 5 toolbox implemented in Statistical Parametric Mapping 5 (Ashburner and Friston, 2000). Grey matter and white matter partitions were modulated to correct for non-linear warping so that values in resultant images were expressed as volume corrected for brain size. Images were then masked to remove remaining non-grey matter or non-white matter voxels and smoothed (12 mm full-width at half-maximum). PiB-PET data were coregistered to their corresponding magnetic resonance image, corrected for partial volume effect using a modified Müller–Gartner approach (Müller–Gärtner et al., 1992), spatially normalized applying the parameters defined from their corresponding magnetic resonance image and scaled using the mean PiB value in the cerebellum grey matter. The resulting PiB-PET data–expressed as standardized uptake value ratios––were used to obtain the individual mean global neocortical PiB value used in regression analyses (see below). Spatially normalized PiB-PET data were then smoothed (12 mm full-width at half-maximum) to be used in voxel-based analyses.
Statistical analyses
As the main goal of this study was to determine the relative contribution of characteristic Alzheimer’s disease-related brain alterations to episodic memory deficits in non-demented individuals, grey matter, white matter and PiB-PET images were first compared between Alzheimer’s disease and healthy elderly subjects. A mask was obtained from each analysis corresponding to the regions of significant changes in Alzheimer’s disease compared with healthy elderly, and each mask was used to restrict the corresponding correlation analysis.
Second, voxel-based correlations were assessed between the composite episodic memory score and grey matter, white matter and PiB images, respectively, restricting the analyses to the regions showing significant changes in Alzheimer’s disease versus healthy elderly.
For each modality (i.e. grey matter, white matter and PiB), the mean value in the cluster of highest correlation with memory identified in the previous analysis was extracted for each subject using differential statistical thresholds (see below). Multiple regression analyses were then performed to assess the relationships between the three modalities within these regions, successively entering each modality value as the dependant variable. Hierarchical multiple regression models were then performed with the composite episodic memory score as the dependant variable and grey matter and white matter volumes and PiB measures in the selected regions alternatively entered as independent predictors to determine their relative contribution. Forward stepwise regression models were performed with the composite episodic memory score as the dependant variable, regional grey matter, white matter and PiB measures as predictive variables and with age, gender and education forced into the model. All analyses were conducted on the whole non-demented population (including both the healthy elderly and patients with mild cognitive impairment), as well as in separate subgroups, i.e. within the healthy elderly, within the mild cognitive impairment, and within the high versus low PiB non-demented individuals, separately. The same analyses were also performed using the global neocortical PiB value instead of the regional PiB measure for comparison. Age, gender and years of education were introduced as covariates in all statistical models.
All neuroimaging data analyses were performed with Statistical Parametric Mapping 5 using ANOVAs for group comparisons and multiple regressions to assess the relationships with memory. A P (family-wise error-corrected for multiple comparisons) <0.05 threshold (and cluster size >1000 voxels) was used for all voxel-based analyses. If nothing significant was observed using this very strict threshold, a less stringent threshold was applied (Puncorrected <0.001) to reduce the risk of false-negatives. Analyses were only performed in the expected direction (i.e. Alzheimer’s disease < healthy elderly and positive correlations for grey matter and white matter atrophy; Alzheimer’s disease > healthy elderly and negative correlations for β-amyloid deposition).
Results
Profiles of grey matter and white matter atrophy and β-amyloid deposition in Alzheimer’s disease
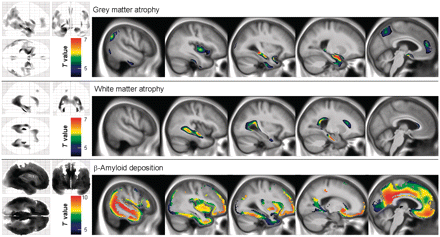
The results of the comparisons between grey matter, white matter and PiB data in Alzheimer’s disease versus healthy elderly are provided in Fig. 1. Expected patterns of alteration were found with (i) grey matter atrophy mainly located in the hippocampus and temporal neocortex extending to temporo-parietal and temporo-occipital areas as well as the precuneus and anterior cingulate cortex; (ii) white matter atrophy mainly involving the cingulum bundle, perforant path and corpus callosum and (iii) brain areas of highest β-amyloid deposition including the posterior cingulate-precuneus area, anterior cingulate and medial frontal cortex as well as lateral temporal and temporo-parietal regions.
Brain areas of significant grey matter atrophy, white matter atrophy and increased PiB uptake in patients with Alzheimer’s disease (n = 34) compared with healthy elderly subjects (n = 93). All results are displayed at P < 0.05 (family-wise error-corrected), k > 1000.
Regression analyses in the non-demented population
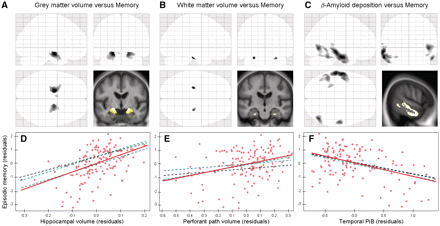
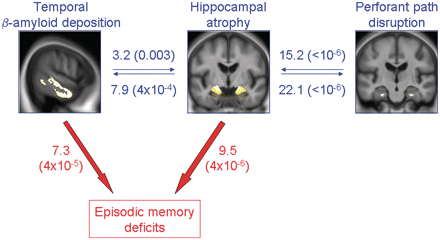
Significant correlations between episodic memory performances and grey matter volume were confined to the right and left hippocampi. As regard to white matter, there was no correlation surviving the P < 0.05 family-wise error-corrected threshold but significant correlations were found in the perforant path, bilaterally, when setting the threshold to P < 0.001. Finally, significant correlations were found between memory and β-amyloid deposition predominantly in the inferior and middle temporal neocortex, but also in the anterior and posterior cingulate and prefrontal cortex. Differential statistical thresholds were then applied to the statistical maps of correlation with composite episodic memory score to extract, for each modality, the corresponding values in the region of highest significance, i.e. the hippocampus for grey matter, the perforant path for white matter and the temporal neocortex (encompassing the inferior and middle temporal lobe and the lingual gyrus) for β-amyloid deposition (Fig. 2). The multiple regression analyses performed between these three measures showed significant relationships between hippocampal volume and perforant path volume (T = 6.54; P < 10 − 6) and between hippocampal volume and temporal PiB (T = −2.99; P = 0.003), while there was no relationship between temporal PiB and perforant path volume (T = −0.26; P < 0.8; Fig. 3).
Results of the voxel-based regression analyses showing (A) the significant relationships between episodic memory and grey matter volume at P < 0.05 (family-wise error-corrected), (B) white matter volume at P < 0.001 (uncorrected) and (C) PiB at P < 0.005 (family-wise error-corrected) within the whole non-demented population (n = 136), displayed as statistical parametric mapping ‘glass brain’ and superimposed onto a representative anatomical section from the group mean magnetic resonance image. Differential statistical thresholds were applied to select areas of strongest correlation for each modality. Bottom: Partial regression plots of the relationship between memory and each modality entered in separate models with residuals being plotted for each variable to adjust for age, gender and years of education. Hippocampal volume (D) was significantly associated with memory when entered in the model separately (red solid slope) or together with perforant path volume (blue dashed slope), or temporal PiB (green dashed slope) or both (black dashed slope). Perforant path volume (E) was significantly associated with memory when entered in the model separately (red) or together with temporal PiB (green), but not when entered together with hippocampal volume (blue), or both hippocampal volume and temporal PiB (black). Temporal PiB (F) was significantly associated with memory when entered in the model separately (red) or together with hippocampal volume (blue), or perforant path volume (green) or both (black). Corresponding R2,T and P values are reported in Table 2.
Illustration of the relationships between hippocampal atrophy, perforant path disruption and temporal PiB (blue), and between these three variables and episodic memory deficits (red) in the whole non-demented sample (n = 136). Values indicate ΔR2 (corresponding P-value), i.e. the added value of the variable to the model in percent (Table 2).
When episodic memory was considered as the dependant variable and each modality was entered separately into the model, the relationship was significant for all three modalities [Fig. 2 (red solid slopes) and Table 2 (Model 1)], consistent with the voxel-based analyses. The modalities were then entered two-by-two in the model to determine whether each modality independently contributed to episodic memory deficits when taking into account the effect of another modality [Fig. 2 (blue and green dashed slopes) and Table 2 (Model 2)]. When entering both temporal PiB and hippocampal volume, or temporal PiB and perforant path volume, into the model, each modality was still found to be significantly associated with episodic memory, i.e. each variable added predictive ability to the model. By contrast, when entering both hippocampus and perforant path volumes into the model, only the hippocampus volume still remained significantly associated with memory while the relationship with the perforant path was no longer significant. Finally, when entering all three modalities into the model, both temporal PiB and hippocampal volume remained significantly associated with memory suggesting they both provided independent contributions, while the relationship with the perforant path was not significant [Fig. 2 (black dashed slopes) and Table 2 (Model 3)]. Forward stepwise regression analysis led to the same conclusion with the hippocampal volume being the first variable to enter the model (T = 6.07; P < 10−6), followed by the temporal PiB (T = −4.24; P = 4 × 10−5) with an overall R2 value of 47%.
Results of the hierarchical multiple regression models with the composite episodic memory score as the dependent variable and hippocampal atrophy, perforant path atrophy and temporal PiB alternatively (Model 1) or simultaneously (Models 2 and 3) entered as predictive variables (in addition to age, gender and years of education) within the whole non-demented sample
| . | R2 (%) . | . | ΔR2 (%) . | T-value . | P-value . |
|---|---|---|---|---|---|
| Model 1 | |||||
| Hippocampal atrophy | 40 | 16.8 | 6.07 | <10−6 | |
| Perforant path atrophy | 31.5 | 8.3 | 3.99 | =10−4 | |
| Temporal PiB | 37.8 | 14.6 | −5.55 | <10−6 | |
| Model 2 | |||||
| Hippocampal atrophy + perforant path atrophy | 40.8 | Hcp | 9.3 | 4.52 | =10−5 |
| PP | 0.8 | 1.29 | =0.2 | ||
| Hippocampal atrophy + temporal PiB | 47.3 | Hcp | 9.5 | 4.84 | =4 × 10−6 |
| Temp-PiB | 7.3 | −4.24 | =4 × 10−5 | ||
| Perforant path atrophy + temporal PiB | 42.8 | PP | 5 | 3.37 | =0.001 |
| Temp-PiB | 11.3 | −5.08 | =10−6 | ||
| Model 3 | |||||
| Hippocampal atrophy + perforant path atrophy + temporal PiB | 48 | Hcp | 5.2 | 3.58 | =5 × 10−4 |
| PP | 0.7 | 1.28 | =0.2 | ||
| Temp-PiB | 7.2 | −4.22 | =5 × 10−5 |
| . | R2 (%) . | . | ΔR2 (%) . | T-value . | P-value . |
|---|---|---|---|---|---|
| Model 1 | |||||
| Hippocampal atrophy | 40 | 16.8 | 6.07 | <10−6 | |
| Perforant path atrophy | 31.5 | 8.3 | 3.99 | =10−4 | |
| Temporal PiB | 37.8 | 14.6 | −5.55 | <10−6 | |
| Model 2 | |||||
| Hippocampal atrophy + perforant path atrophy | 40.8 | Hcp | 9.3 | 4.52 | =10−5 |
| PP | 0.8 | 1.29 | =0.2 | ||
| Hippocampal atrophy + temporal PiB | 47.3 | Hcp | 9.5 | 4.84 | =4 × 10−6 |
| Temp-PiB | 7.3 | −4.24 | =4 × 10−5 | ||
| Perforant path atrophy + temporal PiB | 42.8 | PP | 5 | 3.37 | =0.001 |
| Temp-PiB | 11.3 | −5.08 | =10−6 | ||
| Model 3 | |||||
| Hippocampal atrophy + perforant path atrophy + temporal PiB | 48 | Hcp | 5.2 | 3.58 | =5 × 10−4 |
| PP | 0.7 | 1.28 | =0.2 | ||
| Temp-PiB | 7.2 | −4.22 | =5 × 10−5 |
R2 (%) = R2 value in percent for the overall model; ΔR2 (%) = percent increase in the R2 value induced by adding the variable into the model (in bold when statistically significant; P < 0.05).
Hcp = hippocampal atrophy; PP = perforant path atrophy; Temp PiB = temporal PiB.
Results of the hierarchical multiple regression models with the composite episodic memory score as the dependent variable and hippocampal atrophy, perforant path atrophy and temporal PiB alternatively (Model 1) or simultaneously (Models 2 and 3) entered as predictive variables (in addition to age, gender and years of education) within the whole non-demented sample
| . | R2 (%) . | . | ΔR2 (%) . | T-value . | P-value . |
|---|---|---|---|---|---|
| Model 1 | |||||
| Hippocampal atrophy | 40 | 16.8 | 6.07 | <10−6 | |
| Perforant path atrophy | 31.5 | 8.3 | 3.99 | =10−4 | |
| Temporal PiB | 37.8 | 14.6 | −5.55 | <10−6 | |
| Model 2 | |||||
| Hippocampal atrophy + perforant path atrophy | 40.8 | Hcp | 9.3 | 4.52 | =10−5 |
| PP | 0.8 | 1.29 | =0.2 | ||
| Hippocampal atrophy + temporal PiB | 47.3 | Hcp | 9.5 | 4.84 | =4 × 10−6 |
| Temp-PiB | 7.3 | −4.24 | =4 × 10−5 | ||
| Perforant path atrophy + temporal PiB | 42.8 | PP | 5 | 3.37 | =0.001 |
| Temp-PiB | 11.3 | −5.08 | =10−6 | ||
| Model 3 | |||||
| Hippocampal atrophy + perforant path atrophy + temporal PiB | 48 | Hcp | 5.2 | 3.58 | =5 × 10−4 |
| PP | 0.7 | 1.28 | =0.2 | ||
| Temp-PiB | 7.2 | −4.22 | =5 × 10−5 |
| . | R2 (%) . | . | ΔR2 (%) . | T-value . | P-value . |
|---|---|---|---|---|---|
| Model 1 | |||||
| Hippocampal atrophy | 40 | 16.8 | 6.07 | <10−6 | |
| Perforant path atrophy | 31.5 | 8.3 | 3.99 | =10−4 | |
| Temporal PiB | 37.8 | 14.6 | −5.55 | <10−6 | |
| Model 2 | |||||
| Hippocampal atrophy + perforant path atrophy | 40.8 | Hcp | 9.3 | 4.52 | =10−5 |
| PP | 0.8 | 1.29 | =0.2 | ||
| Hippocampal atrophy + temporal PiB | 47.3 | Hcp | 9.5 | 4.84 | =4 × 10−6 |
| Temp-PiB | 7.3 | −4.24 | =4 × 10−5 | ||
| Perforant path atrophy + temporal PiB | 42.8 | PP | 5 | 3.37 | =0.001 |
| Temp-PiB | 11.3 | −5.08 | =10−6 | ||
| Model 3 | |||||
| Hippocampal atrophy + perforant path atrophy + temporal PiB | 48 | Hcp | 5.2 | 3.58 | =5 × 10−4 |
| PP | 0.7 | 1.28 | =0.2 | ||
| Temp-PiB | 7.2 | −4.22 | =5 × 10−5 |
R2 (%) = R2 value in percent for the overall model; ΔR2 (%) = percent increase in the R2 value induced by adding the variable into the model (in bold when statistically significant; P < 0.05).
Hcp = hippocampal atrophy; PP = perforant path atrophy; Temp PiB = temporal PiB.
The relationships between hippocampal volume, perforant path volume and temporal β-amyloid deposition in non-demented individuals, and their respective contribution to memory deficits are summarized in Fig. 3.
Regression analyses within separate subgroups
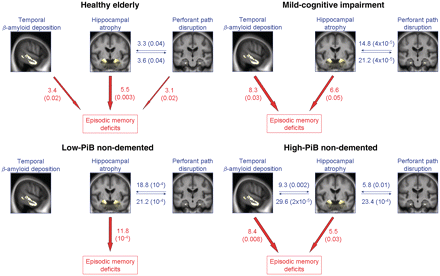
The same analyses performed within the healthy elderly or within the subjects with mild cognitive impairment separately led to similar findings (Fig. 4 and Supplementary Material), with the exception that the relationship between hippocampal atrophy and temporal PiB was not significant (T = −1.39; P = 0.2 in the healthy elderly; T = −0.92; P = 0.36 in the mild cognitive impairment), and the perforant path volume independently contributed to episodic memory decline in addition to hippocampal atrophy and temporal PiB in the healthy elderly (T = 2.31; P = 0.02). Note that, when separating healthy elderly on the basis of subjective cognitive impairment as described earlier (Chételat et al., 2010a, b), the relationship between temporal PiB and memory was only significant in those elderly subjects with subjective cognitive impairment (n = 49) while the hippocampus volume significantly contributed to the model in both groups, and is the only significant contributor in those elderly subjects without subjective cognitive impairment (n = 44). When assessed within the low-PiB non-demented population, temporal PiB was no longer related to hippocampal atrophy (T = −0.11; P = 0.9) or to episodic memory (T = 0.46; P = 0.6), so that only hippocampal atrophy significantly contributed to memory decline (T = 4.03; P = 10−4). When assessed within the high-PiB non-demented cases, the relationships were identical to those obtained within the entire non-demented population (Fig. 4 and Supplementary Material).
Illustration of the relationships between hippocampal atrophy, perforant path disruption and temporal PiB (blue), and between these three variables and episodic memory deficits (red) assessed within the separate subgroups. Values indicate ΔR2 (corresponding P-value), i.e. the added value of the variable to the model in percent (Supplementary Tables).
Regression analyses using the global neocortical PiB value
The relationship between global PiB and memory was weaker than that between temporal PiB and memory in any model and any group. More specifically, global PiB remained significantly associated with episodic memory after accounting for the effect of hippocampal atrophy when considering the whole non-demented population, but the independent contribution of global PiB (ΔR2 = 5.1; P = 0.001) was weaker than that of temporal PiB (ΔR2 = 7.3; P = 4 × 10−5). Moreover, when considering both hippocampal volume and global PiB within each subgroup separately, global PiB did not significantly contribute to the model (T = −0.47; P = 0.6 in the healthy elderly; T = −2; P = 0.053 in the mild cognitive impairment; T = 0.8; P = 0.4 in the low-PiB non-demented; T = −1.37; P = 0.2 in the high-PiB non-demented).
Regression analyses with medial orbitofrontal and precuneus/posterior cingulate PiB values
Because significant correlations between memory and regional PiB were also found with statistical parametric mapping in the precuneus/posterior cingulate and medial orbitofrontal areas, the mean PiB values were extracted in these two regions (termed posterior cingulate and frontal, respectively; Supplementary Fig. 1) and multiple regression analyses were performed in the whole non-demented population to further explore the independent contribution of each regional value to memory performances.
First, only PiB variables were considered. When accounting for the effects of age, gender and education, the frontal (ΔR2 = 10.4; P = 10−5) and posterior cingulate (ΔR2 = 13.1; P = 10–6) PiB both provided a significant independent contribution. When also accounting for the effect of global neocortical PiB, medial frontal PiB did not provide independent contribution to the model (ΔR2 = 0.7; P = 0.2), while posterior cingulate PiB did (ΔR2 = 5.3; P = 0.001), though less significantly than temporal PiB (ΔR2 = 11.3; P = 10–6). When considered together with temporal PiB, neither frontal PiB (ΔR2 = 1.4; P = 0.09) nor posterior cingulate PiB (ΔR2 = 0; P = 0.96) provided a significant contribution, while the contribution of temporal PiB was independent from that of frontal PiB (ΔR2 = 5.6; P = 0.001) but not from that of posterior cingulate PiB (ΔR2 = 1.5; P = 0.08). When entering all regional PiB values (i.e. temporal, frontal and posterior cingulate) together in the same model (with age, gender and education) to predict memory performances, only temporal PiB provided a significant independent contribution (ΔR2 = 2.8; P = 0.02). Similar findings were obtained in a stepwise regression analysis with the three regional PiB values as predictors (and age, gender and education forced into the model), with only temporal PiB entering into the model.
Second, hippocampal atrophy was added to the models. Both posterior cingulate and medial frontal PiB remained significantly associated with episodic memory after accounting for the effect of hippocampal atrophy, with the independent contribution of posterior cingulate PiB (ΔR2 = 7.3; P = 4 × 10–5) being comparable with that of temporal PiB, while the contribution of medial frontal PiB (ΔR2 = 5.2; P = 0.001) was weaker. Forward stepwise regression analysis with the three regional PiB values as well as the hippocampal volume and the perforant path as predictors (and age, gender and education forced into the model) led to the same findings as those obtained without frontal and posterior cingulate PiB, i.e. the hippocampal volume was the first variable to enter the model followed by the temporal PiB.
Finally, when considering both hippocampal volume and each regional PiB values within the separate subgroups, frontal PiB did not significantly contribute to the model in any group (T = −0.57; P = 0.6 in the healthy elderly; T = −1.88; P = 0.07 in the mild cognitive impairment; T = 0.28; P = 0.8 in the low-PiB non-demented; T = –1.18; P = 0.2 in the high-PiB non-demented), while posterior cingulate PiB did within the mild cognitive impairment group (T = –0.47; P = 0.6 in the healthy elderly; T = –2.52; P = 0.02 in the mild cognitive impairment; T = –0.95; P = 0.3 in the low-PiB non-demented; T = –1.8; P = 0.08 in the high-PiB non-demented).
To summarize these findings, the contribution of frontal PiB is similar to that of global neocortical PiB and weaker than that of temporal or posterior cingulate PiB. The relative contributions of temporal PiB and posterior cingulate PiB are not independent from one another, and are comparable when considered together with the hippocampus volume or within mild cognitive impairment. Temporal PiB is, however, a better predictor than posterior cingulate PiB when taking into account global PiB, or all regional PiB values, as well as within the other subgroups (healthy elderly and high-PiB non-demented subjects).
Discussion
The most important finding in this study is that lower episodic memory performances in the pre-dementia stage of Alzheimer’s disease is related to β-amyloid deposition, especially in the temporal neocortex, and independently from hippocampal atrophy. This confirms the idea proposed by Jack et al. (2008) that both of these pathological insults contribute to the cognitive functioning of individuals. Still, previous studies assessing the relationships between β-amyloid deposition and memory almost all used global neocortical PiB and showed discrepant findings, suggesting that the relationship between global β-amyloid deposition and memory is weak. Furthermore, this relationship was no longer significant when considering the effect of hippocampal atrophy (Mormino et al., 2009), suggesting that it was indirect. The present study consistently showed that, when considering hippocampal atrophy, the relationship between global PiB and memory is weak within the whole sample and non-significant within each subgroup. By contrast, temporal PiB was strongly related to memory deficits, even when taking into account the effect of hippocampal atrophy, and considering the healthy elderly, the mild cognitive impairment, or the high-PiB non-demented subgroups separately. This is a new and important finding as it suggests that hippocampal atrophy and β-amyloid deposition in the temporal neocortex should be considered as independent targets in therapeutic trials aimed at reducing memory deficits in the pre-dementia stage. These results also highlight the relevance of considering regional instead of global β-amyloid deposition, especially when assessing the relationship with memory.
Note however, that when separating among healthy elderly subjects those with versus those without subjective cognitive impairment, the relationship between temporal PiB and memory was only significant within the subjective cognitive impairment (while the hippocampus volume was found to be positively related to memory in both groups). Similarly, the relationship between hippocampal atrophy and temporal PiB was significant in the subjective cognitive impairment only (data not shown). These differences between elderly subjects with versus those without subjective cognitive impairment are consistent with a previous study where we showed that atrophy is significantly related to global and regional PiB in the subjective cognitive impairment only (Chételat et al., 2010,a). By contrast, high PiB was associated with greater hippocampal volume in healthy elderly subjects without subjective cognitive impairment (Chételat et al., 2010b), which was interpreted as reflecting a higher brain reserve in these subjects (see below). Consistent with this latter study, the lack of relationships in the elderly without subjective cognitive impairment in the present findings further suggests that there may be a delay before (temporal) β-amyloid deposition is associated with hippocampal atrophy and memory disturbances. This interpretation would in turn support the hypotheses of an indirect effect of β-amyloid deposition on memory as well as of alternative mechanisms for hippocampal atrophy, as discussed below.
As mentioned earlier (Rentz et al., 2010), the posterior cingulate cortex would appear to be a good candidate for a specific relationship between regional PiB and memory given the high level of β-amyloid deposition in this region and its well-known implication in episodic memory. Our findings have confirmed the implication of this region showing a strong link between posterior cingulate β-amyloid deposition and memory performance. They also indicate that, except in particular conditions where the posterior cingulate and the temporal PiB provide comparable contribution (for instance within the subjects with mild cognitive impairment), the temporal PiB is generally a better predictor of memory performances than the posterior cingulate. This predominant implication of the temporal PiB is consistent with the previous studies that assessed this region and found a significant correlation between temporal PiB and memory (Forsberg et al., 2008; Tolboom et al., 2009) or longitudinal cognitive decline (Resnick et al., 2010). Moreover, in the study using a similar memory index as the one used in the present study (i.e. a composite memory z-score), the PiB value in the temporal cortex showed the most significant relationships with memory deficits (P = 0.0064; as compared with P = 0.04 for the posterior cingulate and P = 0.03 for the frontal cortex; Forsberg et al., 2008). It is unlikely that the relationship between temporal PiB and episodic memory performance found here reflects a direct effect of β-amyloid deposition on memory. Indeed: (i) the relationship between both factors in post-mortem studies is weak or non-existent; (ii) the delay between β-amyloid deposition and the appearance of first symptoms is considerable; and (iii) the temporal neocortex itself is not known to play a central role in episodic memory processing. Instead, we believe that this relationship may reflect an indirect effect of temporal β-amyloid deposition on the structure and function of remote brain regions, especially those involved in memory, such as the hippocampus. Consistent with this hypothesis, in a previous study (Bourgeat et al., 2010), we highlighted a specific relationship between hippocampal volume and β-amyloid deposition in the inferior temporal lobe and we showed in this study, that this interaction is responsible for a significant part of episodic memory deficits. The present study also shows that both factors provide an independent contribution to memory impairment, suggesting that hippocampal atrophy is not only due to temporal β-amyloid deposition, and is not the only way temporal β-amyloid deposition leads to episodic memory deficits. Hippocampal/parahippocampal activity or connectivity disruption may also mediate β-amyloid-related memory impairment, as suggested by evidence of an association between β-amyloid deposition and functional connectivity within the default network and especially between regions of the memory network (Hedden et al., 2009).
The relationships between episodic memory and hippocampal volume loss as well as perforant path disruption were not unexpected given the well known implication of these structures in memory and in Alzheimer’s disease-related memory deficits (Chételat et al., 2003; Fellgiebel et al., 2005; Kalus et al., 2006). Besides, the present study shows that, in contrast to temporal β-amyloid deposition, the contribution of perforant path disruption is not independent from that of hippocampal atrophy. This suggests that the relationship between memory and perforant path disruption is mediated by hippocampal atrophy.
Note that when considering both hippocampal atrophy and temporal PiB, ∼50% of the variance of memory performance remains unexplained. This could reflect the fact that all measures are indirect and imprecise: memory tests are impure and incomplete measures of episodic memory capacity, atrophy does not directly reflect neuronal loss (Zarow et al., 2005), and PiB does not directly measure the concentration of the more toxic soluble forms of β-amyloid (Maezawa et al., 2008). In addition, memory performance depends on multiple factors (Jack et al., 2008) not all measured in the present study. These include psychological and emotional factors, with changing mood and motivation states contributing to fluctuation in memory performance; and cognitive/brain reserve, which significantly modifies the impact of neuronal changes on cognitive performances (Coffey et al., 1999; Bennett et al., 2003; Fjell et al., 2006; Fotenos et al., 2008; Querbes et al., 2009; Chételat et al., 2010b; Rentz et al., 2010). While years of education have been accounted for in the present study, more sophisticated proxies of cognitive reserve, such as measures of social, physical and intellectual occupation or environmental/lifestyle factors, would probably allow a more accurate acknowledgement of this factor. Neuropathological factors, other than hippocampal atrophy and temporal β-amyloid deposition, also contribute to memory performance such as atrophy and β-amyloid deposition in other brain areas, and hypometabolism (Chételat et al., 2003) or changes in brain activity during the task (including both impairments and compensatory mechanisms) (Sperling et al., 2010).
The present study is limited by the cross-sectional nature of the investigations, which may have limited sensitivity because of potential sample effects, although the main demographic variables were corrected for. More importantly, this prevents assessment of the temporal sequence of events; therefore, we cannot make inferences on the causal relationships between the different events (e.g. Villain et al., 2010). Finally, the specific relationship found here between memory and regional PiB cannot be extrapolated as an early marker of Alzheimer’s disease. Only a longitudinal study with cognitive changes measured to reflect conversion to Alzheimer’s disease could answer this question.
In conclusion, this study suggests that memory decline in non-demented individuals harbouring one of the characteristic hallmarks of Alzheimer’s disease (Aβ pathology) is partly due to the combined and independent effects of hippocampal atrophy and temporal β-amyloid deposition. Further studies may allow identification of the sequence of events and determination of the relative effects of these factors in a model considering other potential memory-influencing factors.
Funding
The study was supported by the Commonwealth Scientific Industrial Research Organization (CSIRO) through the Australian Imaging, Biomarkers and Lifestyle flagship study of aging (AIBL), the Austin Hospital Medical Research Foundation, Neurosciences Victoria and the University of Melbourne.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Ms Brigitte Landeau, Prof. Michael Woodward, Dr John Merory, Dr Peter Drysdale, Dr Henri Tochon-Danguy, Dr Rachel Mulligan, Dr Uwe Ackerman, Dr Gordon Chan, Dr Kenneth Young, Dr Sylvia Gong, Dr Greg Savage, Dr Paul Maruff, Dr David Darby, Ms Tiffany Cowie, Mr Alex Bahar-Fuchs, Ms Asawari Killedar, Mr David Baxendale, Ms Denise El-Sheikh, Ms Svetlana Pejoska, Ms Tanya Petts, Mr Parnesh Raniga, Mr Oscar Acosta, Mr Jurgen Fripp, Mr Tim Saunder, Ms Jessica Sagona and Mr Jason Bradley for their assistance with this study. We are also indebted to the volunteers who participated in this study.
Abbreviations
References
Author notes
*The members of the Australian Imaging Biomarkers and Lifestyle Study of ageing (AIBL) Research Group are found in http://www.aibl.csiro.au/partners.html.