-
PDF
- Split View
-
Views
-
Cite
Cite
Armando Bertone, Laurent Mottron, Patricia Jelenic, Jocelyn Faubert, Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity, Brain, Volume 128, Issue 10, October 2005, Pages 2430–2441, https://doi.org/10.1093/brain/awh561
Close - Share Icon Share
Abstract
Visuo-perceptual processing in autism is characterized by intact or enhanced performance on static spatial tasks and inferior performance on dynamic tasks, suggesting a deficit of dorsal visual stream processing in autism. However, previous findings by Bertone et al. indicate that neuro-integrative mechanisms used to detect complex motion, rather than motion perception per se, may be impaired in autism. We present here the first demonstration of concurrent enhanced and decreased performance in autism on the same visuo-spatial static task, wherein the only factor dichotomizing performance was the neural complexity required to discriminate grating orientation. The ability of persons with autism was found to be superior for identifying the orientation of simple, luminance-defined (or first-order) gratings but inferior for complex, texture-defined (or second-order) gratings. Using a flicker contrast sensitivity task, we demonstrated that this finding is probably not due to abnormal information processing at a sub-cortical level (magnocellular and parvocellular functioning). Together, these findings are interpreted as a clear indication of altered low-level perceptual information processing in autism, and confirm that the deficits and assets observed in autistic visual perception are contingent on the complexity of the neural network required to process a given type of visual stimulus. We suggest that atypical neural connectivity, resulting in enhanced lateral inhibition, may account for both enhanced and decreased low-level information processing in autism.
Introduction
Autism is a variant phenotype with a neurogenetic basis, defined by negative symptoms affecting social interaction, communication and imagination, as well as by positive symptoms, namely repetitive patterns of behaviours and interests and cognitive strengths (APA, 1994). Given the diagnostic and adaptive importance of social manifestations, it is not surprising that the investigation of underlying neural dysfunction in autism has been for the most part symptom-driven. Accordingly, most neurofunctional research in autism is primarily concerned with assessing higher-level cognitive and/or social capacities. Recent imaging studies indicating atypical brain activation during face processing (Critchley et al., 2000; Schultz et al., 2000; Pierce et al., 2001; Hubl et al., 2003), or during voice processing (Gervais et al. 2004), as well as impaired mentalizing ability (Castelli et al., 2002) and modified language integration (Just et al., 2004) exemplify this research direction. Although different with regard to the nature of the dysfunction they describe (cortical rededication of brain regions devoted to face processing, Schultz et al., 2000; reduced feedback modulation between higher and lower cortical areas, Castelli et al., 2002, Frith, 2003; decreased connectivity between cortical regions, Just et al., 2004, McAlonan et al. 2004), all these models converge towards an atypical large-scale neural connectivity in autism, i.e. impoverished integration of information between cortical regions involved in their respective tasks (Frith, 2003; Just et al., 2004). This would be either restricted to the subcomponents of the ‘social brain’, or generalized to the entire brain.
However, and as alluded to by Belmonte et al. (2004), the emphasis on higher-level, symptomology-related functioning has resulted in the over-looking of atypical perceptual processing in autism. Top-down models for autistic patterns of cognitive performance are based on the assumption that lower-level perceptual information processing in autism is intact, or that enhanced performance is due to the hyper-functioning of an otherwise typical low-level perceptual processing system. However, atypical processing of low-level perceptual information is also a characteristic feature of autism (Happé, 1999). The performance of persons with autism on tasks necessitating the detection of a static visual target embedded in a larger field has been found to be either enhanced (Plaisted et al., 1999; O'Riordan et al., 2001; Caron et al., 2004; Pellicano et al., 2005) or more locally oriented (Shah and Frith, 1983, 1993; Jolliffe et al., 1997; Mottron and Belleville, 1999; Lahaie et al., 2005) when compared to typically developing observers. Hypotheses explaining such perceptual assets in autism include superior processing of low-level static information (Plaisted et al., 1998; Mottron and Burack, 2001) or a by-product of limited integration of low-level information in higher-order operations (Frith, 2003).
Recent research has shown that visual information processing in autism presents a dichotomous picture, with intact or enhanced performance on tasks necessitating static spatial information processing, and inferior performance regarding dynamic information analysis. Persons with autism are consistently less sensitive to a variety of complex motion stimuli which include full-field radiating flow fields (Gepner et al., 1995), adapted global motion stimuli (Spencer et al., 2000), random dot kinematograms (Milne et al., 2002), biological motion stimuli (Blake et al., 2003) and texture-defined motion patterns (Bertone et al., 2003). All the aforementioned complex motion stimuli are processed in motion-sensitive, extra-striate areas located within the dorsal visual pathway (i.e. Goodale and Milner, 1992) and necessitate feed-forward integrative processing to be perceived (Watamaniuk and Sekular, 1992; Wilson et al., 1992; Neri et al., 1998; Bertone et al., 2003).
Such findings of decreased complex motion have been attributed, for the most part, to either a motion-processing deficit per se (Gepner et al., 1995), or to a dorsal stream dysfunction (Spencer et al., 2000; Milne et al., 2002; Blake et al., 2003) since motion-sensitive areas operate within this pathway (Ungerleider and Mishkin, 1982; Merigan et al., 1991; Goodale and Milner, 1992; Merigan and Maunsell, 1993; Milner and Goodale, 1995). We refer to this interpretation as the pathway-specific hypothesis. This interpretation has been supported by concurrent demonstrations of intact global form analysis and typical perception of the global aspect of hierarchical stimuli (Ozonoff et al., 1994; Plaisted et al., 1999; Spencer et al., 2000; Blake et al., 2003; Mottron et al., 2003), believed to be mediated by mechanisms operating within the ventral pathway (Gallant et al., 1993, 1996; Wilson et al., 1997, 1998; Wilkinson et al., 2000).
Bertone et al. (2003) proposed alternatively that decreased complex motion sensitivity in autism might be explained by the reduced efficiency of neuro-integrative mechanisms operating at a perceptual level in autism. We refer to this interpretation as the complexity-specific hypothesis. They measured sensitivity to two types of motion stimuli, differing in the amount of neuro-integrative analysis required to perceive the motion. The pathway-specific hypothesis would predict a decrease in sensitivity to any motion stimuli, regardless of the amount of neuro-integrative processing involved in its processing. Simple, first-order motion (luminance-defined) perception was found unaffected for persons with autism, but there was a selective decrease for complex, second-order (or texture-defined) motion perception (Chubb and Sperling, 1988; Cavanagh and Mather, 1989). The first versus second-order dissociation was impossible to account for in the framework guiding previous investigations (Gepner et al., 1995; Spencer et al., 2000; Milne et al., 2002; Blake et al., 2003) since motion sensitivity was measured for only complex motion types (i.e. assessing functioning of motion-sensitive mechanisms at only one level along the dorsal pathway). Since the processing of simple motion is carried out by standard motion analysis at early levels within the dorsal pathway, Bertone et al. (2003) suggested that their results (and others demonstrating inferior autistic sensitivity to complex motion) might be better explained by a complexity-specific hypothesis. This hypothesis predicts that even low-level visual information necessitating complex neuro-integrative resources should be affected in autism. Therefore, a similar explanation, for decreased perceptual integration, may account for local bias in static stimuli, and for defective perception for dynamic, second-order information.
In order to further differentiate between these two hypotheses and to more precisely characterize visuo-perceptual profile of abilities in autism, two experiments were performed. Static information processing in autism was assessed by using stimuli that varied in the complexity of the presented information. This was accomplished by measuring the ability of high-functioning persons with autism (HFA) and typically developing (TD) observers to identify the orientation of both simple (first-order) and complex (second-order) static gratings. Importantly, and in relation to the anatomical substrate of the pathway-specific hypothesis, these two types of stimuli are processed at two different levels of the ventral stream. The pathway-specific hypothesis would predict that the complexity of the presented stimulus would not affect performance for the HFA group since it contends that only dynamic information processing (mediated by dorsal stream functioning) is affected in autism. Conversely, the complexity-specific hypothesis would predict a decrease in HFA performance for the second-order condition only, since it contends that inefficient neuro-integrative functioning preferentially affects complex information analysis in autism, regardless of whether the presented visual information is static or dynamic. In addition, we also evaluated the functional integrity of the sub-cortical visual processing using a flicker contrast sensitivity task with stimuli that preferentially evaluated the magnocellular and parvocellular systems. Accordingly, previous findings of decreased global motion stimuli sensitivity in autism have been interpreted as evidence of deficient pre-cortical (i.e. magnocellular) processing of dynamic information (Milne et al., 2002).
Methods
Participants
Thirteen HFA individuals with normal intelligence (average Wechsler FSIQ = 100.4, SD = 13.6) were recruited from a specialized clinic for persons with autism. A diagnosis of autism was obtained using the algorithm of the Autism Diagnostic Interview (ADI; Lord et al., 1994) combined with the Autistic Diagnostic Observation Schedule General (ADOS-G; Lord et al., 2000). Current and retrospective standardized assessments were conducted by a trained researcher (LM) who obtained reliability on these instruments. All HFA had a score above the ADI/ADOS cut-off in the four areas relevant for diagnosis (social, communication, restricted interest and repetitive behaviour, and age of symptom onset). Thirteen TD participants were recruited from the community as a comparison group. These were screened for a past or current history of psychiatric, neurological or other medical disorders and all had a typical academic background and development (mean IQ = 108.2, SD = 13.1). The groups were matched as closely as possible in terms of laterality, gender and chronological age and full-scale IQ. The mean chronological age of the control and autism groups was 22.3 (SD = 6.1) and 20.5 years (SD = 4.3), respectively. All participants had normal or corrected-to-normal vision (Table 1). Informed written consent was obtained from all participants.
Participant characteristics for HFA and TD participants
| Participant characteristic . | HFA participants . | TD participants . | ||
|---|---|---|---|---|
| Number | 13 | 13 | ||
| Age (y : m) | ||||
| Mean | 22 : 3 | 20 : 5 | ||
| SD | 6.1 | 4.3 | ||
| Range | 11.0–31.0 | 14.0–27.0 | ||
| FSIQ | ||||
| Mean | 100.4 | 108.2 | ||
| SD | 13.6 | 13.1 | ||
| Range | 82–120 | 90–137 | ||
| Participant characteristic . | HFA participants . | TD participants . | ||
|---|---|---|---|---|
| Number | 13 | 13 | ||
| Age (y : m) | ||||
| Mean | 22 : 3 | 20 : 5 | ||
| SD | 6.1 | 4.3 | ||
| Range | 11.0–31.0 | 14.0–27.0 | ||
| FSIQ | ||||
| Mean | 100.4 | 108.2 | ||
| SD | 13.6 | 13.1 | ||
| Range | 82–120 | 90–137 | ||
Participant characteristics for HFA and TD participants
| Participant characteristic . | HFA participants . | TD participants . | ||
|---|---|---|---|---|
| Number | 13 | 13 | ||
| Age (y : m) | ||||
| Mean | 22 : 3 | 20 : 5 | ||
| SD | 6.1 | 4.3 | ||
| Range | 11.0–31.0 | 14.0–27.0 | ||
| FSIQ | ||||
| Mean | 100.4 | 108.2 | ||
| SD | 13.6 | 13.1 | ||
| Range | 82–120 | 90–137 | ||
| Participant characteristic . | HFA participants . | TD participants . | ||
|---|---|---|---|---|
| Number | 13 | 13 | ||
| Age (y : m) | ||||
| Mean | 22 : 3 | 20 : 5 | ||
| SD | 6.1 | 4.3 | ||
| Range | 11.0–31.0 | 14.0–27.0 | ||
| FSIQ | ||||
| Mean | 100.4 | 108.2 | ||
| SD | 13.6 | 13.1 | ||
| Range | 82–120 | 90–137 | ||
Apparatus
For all testing, stimulus presentation and data collection were controlled by a Power Macintosh G4 microcomputer and presented on a 14-inch AppleVision color monitor refreshed at a rate of 75 cycles per second (Hz). The screen resolution was 1152 × 870 pixels. The VPixx©(www.vpixx.com) graphics program controlled stimulus generation and animation. The luminance of the monitor was gamma-corrected (implemented with a colour calibration within the VPixx© program) to minimize the non-linearities in the display. Calibration and luminance readings were verified using a Minolta CS-100 Chroma Meter colorimeter on a regular basis.
Psychophysical tasks
Orientation-identification task
In order to differentiate between the pathway-specific and complexity-specific hypotheses, all participants completed two psychophysical tasks. The orientation-identification task assessed static information processing, using stimuli that varied in complexity and are processed by mechanisms operating at different levels along the ventral stream. For this task, the pathway-specific hypothesis would predict no differences in sensitivity between TD and HFA groups since only dorsal visual stream-mediated dynamic information processing is argued to be dysfunctional in autism (Spencer et al., 2000; Milne et al., 2002; Blake et al., 2003). On the other hand, the complexity-specific hypothesis would predict a selective decrease in sensitivity for the complex (second-order), but not the simple (first-order) gratings for the HFA group, since it contends that complex information processing, whether static or dynamic, is affected in autism.
The static stimuli used in the orientation-identification task are a static version of the motion stimuli used in the translational condition of the Bertone et al. (2003) study. As described below, these static stimuli were constructed in the same manner as their dynamic counterparts (i.e. Ledgeway and Smith, 1994; Bertone and Faubert, 2003), by either adding or multiplying greyscale noise to a modulating sinewave (velocity = 0). Static and dynamic forms of first- and second-order information are initially processed in parallel by separate feed-forward mechanisms, using similar principles of detection (Chubb and Sperling, 1988; Wilson et al., 1992; Baker, 1999). This processing is exemplified by filter-rectify-filter analysis where the first stage filters, operating within V1, extract first-order orientation or motion direction, whereas second-order orientation or motion information is detected at a second stage of filtering at a coarser spatial scale (in areas V2/V3), but only after rectification of the second-order signals (e.g. Chubb and Sperling, 1988; Wilson et al., 1992; Sperling et al., 1994; Sutter et al., 1995; Smith et al., 1998; Wilson, 1998; Nishida et al., 1997; Baker, 1999; Bertone and Faubert, 2003). For this reason, first-order static information can also be considered ‘simple’ whereas second-order static information is considered more ‘complex’ since it recruits more extensive neural circuitry as well as additional processing prior to orientation identification.
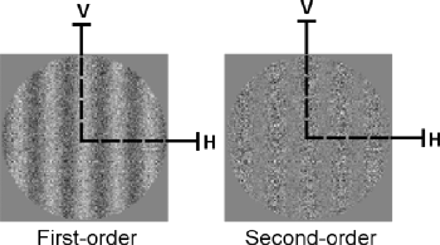
Stimuli
Schematic representation of static stimuli used for experiment 1. First- and second-order stimuli are presented in their vertical (V) orientation. Both static and dynamic forms of first- and second-order information are initially processed in parallel by separate passive mechanisms using similar principles of detection. Specifically, first stage filters, operating within V1, extract first-order orientation or motion direction whereas second-order information is detected at a second stage of filtering at a coarser spatial scale (in areas V2/V3), but only after full-wave rectification of the second-order signals (Wilson et al., 1992; Chubb and Sperling, 1988, Cavanagh and Mather, 1989; Sperling et al., 1994; Baker, 1999). For this reason, first-order information can be considered to be simple and second-order information complex because the latter type recruits more extensive neural circuitry as well as additional processing prior to detection.
Procedure
Participants were tested individually in a dimly lit room. Procedural instructions were given verbally to each participant prior to each experimental block. Before the actual testing, practice trials were completed so that the participants could familiarize themselves with fixation, stimuli presentation and responding. Each participant was then presented with static first- and second-order stimuli oriented either vertically or horizontally for 750 ms. They were then required to identify the orientation of each stimulus by pressing one of two buttons on a keypad (2 alternative forced-choice). For each testing session, first- and second-order stimuli were presented in random order ten times in either orientation at each level of modulation (for a total of twenty trials at each level of modulation). Psychometric functions were then fitted to the responses for each condition in order to obtain orientation-identification thresholds at a 75% correct level of performance. Throughout testing, the participants were reminded to fixate at the centre of each pattern. The experimenter remained present throughout testing and initiated successive trials.
Flicker contrast sensitivity task
Efficient ventral and dorsal visual stream functioning is dependent on afferent parvocellular and magnocellular input, respectively, originating from retinal and thalamic levels of information processing (i.e. Shapley, 1990; Merigan and Maunsell, 1990). Magnocellular neurons respond preferentially to low-spatial/high-temporal frequency defined stimuli (best suited for dynamic information processing), whereas parvocellular neurons respond preferentially to high-spatial/low-temporal frequency defined stimuli (best suited for static information processing) (Tohlurst, 1975; Derrington and Lennie, 1984; Merigan and Maunsell, 1990, 1993; Shiller et al., 1990; Merigan et al., 1991; Lynch et al., 1992; Chapman et al., 2004). The purpose of this task was to assess the integrity of pre-cortical (magno- and parvocelluar) visual functioning in autism. Pre-cortical magnocellular functioning in autism has been assessed by Pellicano et al. (2005) using a flicker task (minimum contrast needed to detect a stimulus flickering at 10 Hz). They demonstrated no significant difference in sensitivity between autism and control groups; parvocellular functioning was also evaluated in the present study.
Stimuli
As was the case for the orientation-discrimination task, flicker stimuli were presented to the participants within a circular region at the centre of the display that had a diameter of 10° when viewed from a distance of 57 cm. The mean luminance of the remainder of the display during testing was 17.70 cd/m2 [u′ = 0.1912, v′ = 0.4456 in CIE (Commission Internationale de l'Eclairage) u′ v′ colour space] where Lmin and Lmax were 0.01 and 35.40 cd/m2, respectively. A two-alternative temporal forced choice (2ATFC) paradigm was used to measure the minimum contrast needed to detect a 0.5 c.p.d. sinusoidal luminance grating counterphasing at a rate of 6 Hz (magnocellular condition) and a 6 c.p.d. sinusoidal luminance grating counterphasing at a rate of 1 Hz (parvocellular condition).
Procedure
For both magno- and parvocellular conditions, participants were presented with trials consisting of flickering stimuli of a certain luminance contrast for 750 ms, followed (or preceded) by a stimulus containing no flicker information (i.e. static grey region). Participants were required to identify the trial that contained the flickering stimuli (i.e. first or second presentation). Luminance contrast was the physical variable being manipulated for each condition using an adaptive PEST (parameter estimation by sequential testing) routine (Pentland, 1980). This method was chosen over the more conventional staircase procedures because the PEST has been shown to significantly reduce the number of trials necessary to determine a threshold compared to staircase methods at a given level of accuracy (Taylor et al., 1983). A session ended when the PEST routine converged on the 81% level on a psychometric Weibull function (Weibull, 1951), representing the flicker contrast thresholds for each condition, which were then transformed into flicker contrast sensitivity measures. For the PEST routine to end for each condition, a preset level of accuracy (95% confidence interval at threshold was within 0.1 log units of the PESTed threshold) had to be met. The maximum number of trials allowed, fixed at one hundred for each condition, was never met. The total time taken for each participant to complete both orientation-discrimination and flicker sensitivity tasks took, on average, ∼60 min.
Results
Orientation-identification task: enhanced and diminished autistic performance depends on stimulus complexity
Orientation-identification thresholds for HFA and TD participants were measured using static gratings differing only in the attribute defining their orientation; luminance for the first-order condition and texture for the second-order condition (Fig. 1). Results revealed two very different patterns of HFA performance, contingent on the complexity of the stimuli used in each condition.
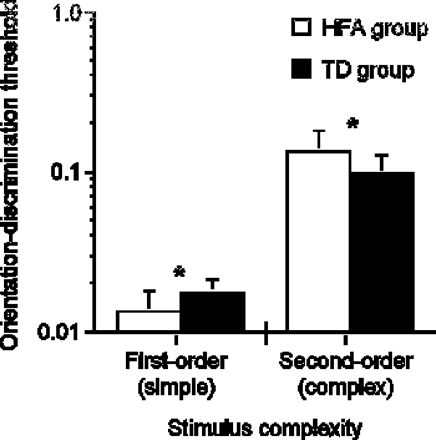
As shown in Fig. 2, HFA orientation-identification thresholds were significantly lower for the first-order condition when compared to the TD participants [F (1,24) = 7.872, P = 0.0098]. These findings represent another demonstration of superior performance in tasks necessitating elementary visuo-spatial information processing (i.e. position discrimination, visual search, etc.), albeit at a lower level of processing. In contrast, HFA thresholds were significantly higher for the same task using complex second-order stimuli [F (1,24) = 5.042, P = 0.0342], representing the first demonstration of a perceptual visual deficit for a static task in autism. Taken as a whole, these findings suggest that enhanced autistic performance on visuo-spatial tasks is complexity dependent, and that persons with autism are selectively less sensitive to complex visual information, whether it is static or dynamic in nature. These results will be discussed in the context of a complexity-specific account of visuo-perceptual processing in autism in later sections.
Orientation-discrimination thresholds as a function of stimulus complexity for HFA and TD participants. Since first- and second-order stimuli are constructed using different image attributes, the absolute difference between first- and second-order thresholds is uninformative. Error bars represent 1 standard deviation.
Flicker sensitivity task: unaffected pre-cortical visual functioning in autism
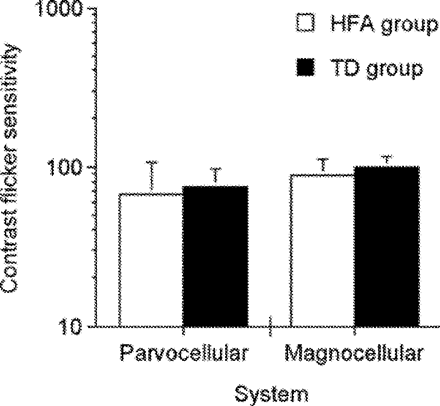
Flicker contrast sensitivity did not differ significantly between HFA and TD participants for either magnocellular [F(1,24) = 1.729, P = 0.2009] or parvocellular [F(1,24) = 0.451, P = 0.5810] conditions (see Fig. 3). These findings are consistent with those of Pellicano et al. (2005). In addition to demonstrating intact simple motion perception in autism (Bertone et al., 2003), the current findings do not support a pathway-specific account of perceptual abnormalities in autism. Accordingly, a dorsal pathway dysfunction resulting in decreased complex motion perception would entail a disruption of afferent magnocellular visual inputs (Chapman et al., 2004).
Contrast flicker sensitivity measures for parvocellular and magnocellular functioning for HFA and TD groups. Error bars represent 1 standard deviation.
The finding of unaffected parvocellular (pre-cortical) functioning suggests that enhanced and diminished performance on the orientation-discrimination task result from atypical processing at early cortical levels in autism, and is not the result of abnormal pre-cortical input.
Discussion
Pathway—versus complexity—specific information processing hypotheses in autism
The present study represents the first evaluation of ventral stream processing in autism at two levels of neural complexity, assessed by measuring orientation-discrimination thresholds for simple luminance- and complex texture-defined stimuli for both HFA and TD observers. By demonstrating that complex static information processing is selectively impaired in autism, we propose that atypical visual information analysis in autism is best described by a complexity-specific account. Regardless of whether the visual information is dynamic (Bertone et al., 2003) or static (current findings), diminished neuro-integrative functioning at a perceptual level preferentially affects complex information analysis. We are able to forward this suggestion because both ventral (current study) and dorsal visual stream (Bertone et al., 2003) functioning have now been evaluated at two levels of complexity, using static and dynamic stimuli differing solely and comparably in level of complexity. Our proposition contradicts two underpinnings of the pathway-specific hypothesis: impairment of dorsal stream and integrity of ventral stream. These two aspects will be discussed separately.
Impairment of dorsal stream
We contend that previous demonstrations of decreased motion sensitivity, attributed either to motion impairments or to dorsal stream dysfunction (Gepner et al., 1995; Spencer et al., 2000; Milne et al., 2002; Blake et al., 2003), may be re-interpreted according to the complexity-specific hypothesis. Measuring complex motion sensitivity in isolation does not allow for differentiation between pathway and complexity-specific hypotheses. Accordingly, the integrity of first-order movement perception evident in Bertone et al. (2003) indicates that it is not movement per se which is impaired. This argument has recently been supported by the results of Pellicano et al. (2005) who demonstrated that only complex motion processing (global motion) is affected in autism, while no dysfunction of early visual processing was demonstrated using a flicker contrast sensitivity task. These findings were interpreted as intact lower-level dorsal stream functioning in autism (Bertone et al., 2003), and evidence against a generalized ‘dorsal stream deficit’. Pellicano et al.'s (2005) results are congruent with the complexity-specific hypothesis, since deficits in motion processing were only found at higher (i.e. processed by extra-striate mechanisms) but not lower (pre-cortical or magnocellular) levels of analysis along the dorsal visual stream, possibly reflecting neuro-integrative dysfunction in autism (Bertone et al., 2003). In addition, there has yet to be a direct demonstration of either a physiological or anatomical abnormality of thalamic magnocellular structures in autism, as has been demonstrated in FXS (Kogan et al., 2004b).
Integrity of ventral stream
The pathway-specific hypothesis is supported by a differentiation between impaired movement perception and preserved static perception in autism. Previous studies proposed that ventral stream processing is unremarkable in autism by demonstrating a typical detection of circular forms composed of locally-oriented line segments (Spencer et al., 2000; Blake et al., 2003). We propose that the argued static/dynamic dissociation reported in these studies may be stimulus dependent, since static circular stimuli were not equivalent to their complex dynamic counterparts (which were not circular in nature) in terms of processing requirements [see Bertone (2004) for complete discussion] and, therefore, differ in terms of their sensitivity to neuro-integrative dysfunction. Integrating complex visual information, whether static or dynamic, is more efficient when local information is organized in a circular manner due to specialized analysis (Freeman and Harris, 1992; Kovács and Julesz, 1993; Wilson et al., 1997; Wilkinson et al., 1998; Kovács et al., 1999; Burr and Santoro, 2001; Achtman et al., 2003). Within the context of experimental approaches used by Spencer et al. (2000) and Blake et al. (2003), one can argue that the decrease in sensitivity for the complex motion condition (and not the complex form condition) may have been, at least in part, due to the fact that only one condition used circular stimuli (complex form condition). As mentioned by Pellicano et al. (2005), the detection of complex form contours from individual oriented line elements may be achieved at earlier levels than previously believed i.e. orientation selective mechanisms operating in V1 [see Hess et al. (2003) for a review]. Although such complex motion and form tasks selectively assess dorsal and ventral stream processing, respectively, they do not in effect assess functioning in either stream at the same level of neural complexity. We believe that the static stimuli used in the present study (i.e. stationary first- and second-order gratings) are better matched to their dynamic counterparts in terms of how ‘efficiently’ they are processed (see Kogan et al., 2004a), allowing for a more accurate assessment of dorsal and ventral stream processing in autism. In conclusion, neuro-integrative processing can be demonstrated to be dysfunctional in both ventral and dorsal visual streams in autism, as long as the stimuli used to assess the functional integrity of both streams are not circular in nature.
The neural origin of enhanced static information processing in autism
Our results demonstrate that the performance of HFA participants is inferior at discriminating the orientation of second-order gratings but superior for first-order gratings. This dichotomous performance contrasts two types of information differing only in the physical attribute orientation, luminance versus texture. In typical participants, the first- versus second-order distinction is associated with two distinct levels of integration among visual areas (V1 only versus V1 + V2/V3). Two alternative and partially overlapping interpretations of this pattern of findings, involving neural versus regional level of organization, will be discussed.
A first interpretation of this pattern of performance would be that functional regions involved in the visual system operate better in isolation than in synchrony. It can be summarized as ‘superior when autonomous, inferior when synchronized’. According to this interpretation, first-order patterns would be processed at a higher level of performance because they may be analysed within a single brain region (or a brain region more limited in surface), whereas second-order information requires communication among regions to be automatic and constant. This trend of interpretation is inspired by the model initially proposed by Castelli et al. (2002) for higher-order performances in autism. Castelli et al. found diminished functional synchrony between extra-striate areas and the superior temporal sulcus during a theory of mind task, while the early visual processing areas (extra-striate cortex) were activated normally in individuals with autism. A so-called ‘under connectivity’ between regions involved in syntactic processing was also recently established using fMRI (Just et al., 2004). During the processing of complex syntactic sentences, HFA individuals presented with a reduced functional synchrony, measured by the correlation between the average time course of all the activated voxels in each member of pairs of anatomically defined ROIs implicated in language processing. Finally, a recent study using diffusion tensor imaging (Barnea-Goraly et al., 2004) revealed a reduction of white matter tracts adjacent to the grey matter of associative cortex (temporo-parietal junctions, superior temporal sulcus) and between prefrontal and temporal cortices. Some neural network models of visual processing implicate feedback or top-down connectivity as being an important part of information coding in V1 (Rao and Ballard, 1999; Angelucci et al., 2002). These models are defined by the existence of feedback connections between higher (i.e. V2, V3, V4, MT) and lower (i.e. V1) visual areas that result in the amplification and focused activity of neurons in lower-order areas, implicated in such perceptual processing as orientation selectivity and figure ground differentiation (Hupé et al., 1998; Bullier et al., 2001). This ‘superior when autonomous, inferior when synchronized’ hypothesis receives, therefore, increasing support from studies on large-scale communication/synchrony among brain regions. Apart from the current findings, it has, however, not yet found support from sub-regions implicated in low level processing.
A second trend of interpretation is that the same atypical neural system mediating orientation information processing in autism may have two opposite perceptual consequences, depending on the complexity of the information provided. We propose that this dichotomous performance is best explained by an atypical neural connectivity mediating the extraction of low-level orientation information within the visual processing hierarchy in autism (Cohen, 1994; Gustaffson, 1997a,b; McLelland, 2000; Grice et al., 2001; Brock et al., 2002; Bertone et al., 2003, 2004). The efficient orientation selectivity and detection of neurons in the primary visual cortex (V1) is largely dependent on cortical lateral interactions between orientation-selective neurons (Andrews, 1965). Therefore, the type of abnormal connectivity the most congruent with enhanced sensitivity to simple luminance-defined gratings is that of strong or excessive lateral inhibition [This model is also consistent with the hypothesis of increased latent inhibition, proposed by K. Plaisted (in prep.)], as first suggested by Gustafsson (1997a, b). Gustafsson's model is based on a ‘feature map’ model of cortical functioning where neurons selective to a certain orientation are arranged in columns. These columns are optimally activated (increased neuronal activity within each column) when a specific orientation (i.e. vertical) is presented (Kohonen, 1995). Lateral inhibition allows proximal columns to be activated by similar orientations, resulting in a bell-shaped tuning curve function (or ‘Mexican hat’ function) in the orientation domain, for each orientation-selective column. For typically developing individuals, a possible functional role of lateral inhibition is to sharpen the orientation tuning of each of these columns (Sillito et al., 1995; Gilbert et al., 1996; Gilbert, 1998; Somers et al., 2002). We propose that increasing lateral inhibition would narrow the range of a particular orientation activating each column, resulting in an improved ability for both the detection of oriented stimuli and the discrimination between different orientations. As suggested by Gustafsson (1997a, b), narrowing the tuning of each column would facilitate ‘discrimination’ between orientations (resulting in enhanced edge detection), whereas widening their tuning curves would facilitate ‘generalization’. Increased lateral inhibition would, therefore, produce an increased performance on the orientation-discrimination task stimuli for the HFA group, since luminance-defined stimuli can be analysed by a single orientation-selective neuron with a narrowly tuned receptive field in V1. Physiological support for this suggestion has come form recent neuropathological studies demonstrating numerous and more narrow minicolumns (i.e. columns of orientation selective neurons) in the autistic brain (Casanova et al., 2002a, b).
Although the narrowing of microcolumns may be responsible for enhanced static information processing in autism, why did not persons with autism demonstrate enhanced sensitivity to the simple motion stimuli (i.e. translational condition) used in the Bertone et al. (2003) study? A possible explanation would be based on the differential filtering properties of early mechanisms mediating static and dynamic information. Human motion-detectors operating in the primary visual cortex have a very broad orientation tuning (broadband detectors) (i.e. Anderson and Burr, 1991; Georgeson and Scott-Samuel, 2000). In contrast, a subset of neurons in V1 are extremely selective for orientation, in part the result of both lateral and feed-back connections. Therefore, the processing of simple dynamic information may be less affected by atypical neural connectivity such as strong or excessive lateral inhibition. To our knowledge, there has yet to be a clear demonstration of an ‘autistic advantage’ or enhanced autistic performance on a task necessitating dynamic information analysis.
Enhanced edge detection caused by increased lateral inhibition may also be implicated in other findings of improved autistic performance on visuo-spatial tasks, inasmuch as these tasks involve the discrimination of luminance-defined stimuli, mediated by low-level perceptual processing (Plaisted, 1999; O'Riordan and Plaisted, 2000; O'Riordan et al., 2001; Caron et al., 2004). As suggested by Casanova et al. (2003), if the enhanced discrimination on visuo-spatial tasks in autism is indeed the result of altered low-level information processing, its neural origin is most likely at the level of the microcolumn, where visual information is initially filtered/processed before being fed forward to higher-level visual mechanisms. Interestingly, enhanced low-level perceptual functioning has also been demonstrated in the auditory modality (Mottron and Burack, 2000). Although speculative, a similar explanation may be provided for enhanced low-level auditory perception, since neural organization within the primary auditory cortex has a columnar arrangement similar to that of the primary visual cortex (Abeles and Goldstein, 1970). Increased lateral inhibition between frequency-specific columns may, therefore, result in an increased temporal resolution, with the benefit of enhanced pitch sensitivity in autism (Bonnel et al., 2003) and diminished local-to-local interference (Foxton et al., 2003).
Conversely, the same neural atypicality may have a detrimental effect on other types of low-level information in autism, such as complex texture-defined static information. Neurons comprising feature-specific columns in V1 selectively respond to oriented edges defined by changes in luminance, such as the simple luminance-defined, first-order stimuli used in our task (Fig. 1). In contrast, enhanced edge detection mediated by lateral inhibition for complex texture-defined information has been demonstrated, but only after additional information processing (i.e. full-wave rectification, see legend of Fig. 1) (Lu et al., 1996). After such processing, the resulting texture-defined spatial information is much coarser in nature, as defined by the filter-rectify-filter hypothesis (Chubb and Sperling, 1988; Cavanagh and Mather, 1989; Wilson et al., 1992; Sperling et al., 1994; Baker, 1999). It is, therefore, less likely that the ‘narrowing’ of the orientation-selective, luminance-driven columns in V1 improve orientation-discrimination of texture-defined stimuli for the HFA group. On the contrary, the same altered local neural networks in autism may hinder the processing for more complex types of static information necessitating integration via lateral connections between orientation-selective V1 neurons analysing nearby spatial locations (Field et al., 1993) since a larger neural circuitry is involved.
In conclusion, the primary function of lateral and feedback connectivity within low-level visual areas is to identify, modulate and optimize neural signals belonging to elementary visual features (i.e. orientation). These signals are subsequently fed forward and integrated by specialized mechanisms operating in higher visual areas. It is possible that both lateral and feedback connectivity are atypical in autism, since each type of connectivity is implicated in orientation selectivity. Lateral and feedback connectivity are differentially involved in integrating signals within (lateral connections) and between (feedback) visual areas (Angelucci et al., 2002). However, our data show that atypical connectivity may be implicated initially within low-level visual areas rather than (or in addition to) between higher and lower visual areas (i.e. between V1 and specialized visual areas such as the superior temporal cortex or visual association areas). In this sense, atypical autistic visual information processing, and probably, visually related abnormal behaviour manifested by persons with autism, may be related to low-level perceptual differences to a greater extent than previously believed (Belmonte et al., 2004).
Enhanced simple versus impaired complex perceptual performance: specific to autism?
We have measured first and second order information processing along each visual pathway in order to successfully characterize the perceptual functioning in other neuro-developmental conditions characterized by visually related symptoms (Habak and Faubert, 2000; Bertone et al., 2003; Kogan et al., 2004a). As shown in Table 2, such investigations using the same stimuli and experimental paradigm have resulted in different patterns of performance, specific to each condition and consistent with their respective phenotype. Therefore, a hypothesis regarding abnormal neural connectivity as differentiating autism from other conditions manifesting decreased complex motion sensitivity can be forwarded.
Schematic representation of the sensitivity compared to control participants for normally-ageing persons (Habak and Faubert, 2000), persons with fragile X syndrome (FXS; Kogan et al., 2004a) and HFA [dorsal (Bertone et al., 2003) and present results] using the same task
| . | Normal ageing . | . | FXS . | . | HFA . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Simple . | Complex . | Simple . | Complex . | Simple . | Complex . | |||
| Ventral | = | ↓↓ | = | ↓↓ | ↑↑ | ↓↓ | |||
| Dorsal | ↓↓ | ↓↓ | ↓↓ | ↓↓ | = | ↓↓ | |||
| . | Normal ageing . | . | FXS . | . | HFA . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Simple . | Complex . | Simple . | Complex . | Simple . | Complex . | |||
| Ventral | = | ↓↓ | = | ↓↓ | ↑↑ | ↓↓ | |||
| Dorsal | ↓↓ | ↓↓ | ↓↓ | ↓↓ | = | ↓↓ | |||
Equal signs (=) and double arrows (↓↓, ↑↑) represent no difference and significant difference (respectively) in sensitivity between control and clinical groups at the P = 0.05 level.
Schematic representation of the sensitivity compared to control participants for normally-ageing persons (Habak and Faubert, 2000), persons with fragile X syndrome (FXS; Kogan et al., 2004a) and HFA [dorsal (Bertone et al., 2003) and present results] using the same task
| . | Normal ageing . | . | FXS . | . | HFA . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Simple . | Complex . | Simple . | Complex . | Simple . | Complex . | |||
| Ventral | = | ↓↓ | = | ↓↓ | ↑↑ | ↓↓ | |||
| Dorsal | ↓↓ | ↓↓ | ↓↓ | ↓↓ | = | ↓↓ | |||
| . | Normal ageing . | . | FXS . | . | HFA . | . | |||
|---|---|---|---|---|---|---|---|---|---|
. | Simple . | Complex . | Simple . | Complex . | Simple . | Complex . | |||
| Ventral | = | ↓↓ | = | ↓↓ | ↑↑ | ↓↓ | |||
| Dorsal | ↓↓ | ↓↓ | ↓↓ | ↓↓ | = | ↓↓ | |||
Equal signs (=) and double arrows (↓↓, ↑↑) represent no difference and significant difference (respectively) in sensitivity between control and clinical groups at the P = 0.05 level.
Additional support for this argument is evidenced in a recent study assessing both dynamic and static information processing in another type of developmental disorder, fragile X syndrome (FXS). In a study employing the orientation-discrimination task used in the present study, Kogan et al. (2004a) found a selective decrease in sensitivity for the second-order static condition for the FXS group (see Table 2). Based on this finding, Kogan et al. (2004a) suggest that in addition to pervasive deficits regarding motion processing in FXS (consistent with abnormal magnocellular neuropathology in FXS, see Kogan et al., 2004b), integrative cortical dysfunction is also present in FXS, affecting both dynamic and static complex information processing in this condition. However, the finding of enhanced sensitivity to simple static information remains specific to autism. Conversely, decreased sensitivity to complex motion stimuli (i.e. global motion) has been demonstrated in at least a dozen other neurological disorders (Regan, 1991; Trick and Silverman, 1991; Gilmore et al., 1994; Trick et al., 1994, Cornelissen et al., 1995, 1998; Atkinson et al., 1997; Gunn et al., 2002; Chen et al., 2003; Mapstone et al., 2003; Kogan et al., 2004b; McKendrick and Badcock, 2004).
Conclusion: integration within and between regions in autism
The present results are interpreted as behavioural evidence of altered ‘local’ neural networks in autism, possibly affecting the low-level processing of elementary stimulus features such as spatial frequency, orientation and contrast. Given the fact that these abnormal networks are the initial components of standard larger-scale networks responsible for higher-order information analysis, subsequent larger-scale networks integrating across specific stimulus features would also be modified in autism (McClelland, 2000; Grice et al., 2001; Brock et al., 2002; Bertone et al., 2003; Just et al., 2004). At least in the context of the present experimental paradigm, excessive lateral inhibition seems to be a promising candidate to account for the perceptual consequences of abnormal neural connectivity. This hypothesis is congruent both with superior visuo-static information processing and with neuro-integrative dysfunction. Other systems-based explanations have been forwarded to account for dichotomous abilities in autism for both perceptual (enhanced perceptual functioning, Mottron and Burack, 2001; temporal binding deficit hypothesis, Brock et al., 2002) language (underconnectivity hypothesis; Just et al., 2004) or relation between high and low level cognitive processes (weak coherence hypothesis, Frith 1989; reduced feed back flow of information, Frith 2003). Although different in respect of the purported synaptic dysfunction, these hypotheses predict impaired information processing if it is contingent on integrating information between specialized networks located in different brain regions and enhanced processing when limited within local networks. The current demonstration of both enhanced and diminished information processing in autism using the same task indicate that atypical connectivity can affect different levels of processing within the same ‘local’ network and is not necessarily contingent on reduced inter-network interactions.
This study was supported by a student fellowship to A.B. from the Canadian Institute for Health Research (CIHR) and grants from the CIHR to J.F. (No. 14777) and L.M. (MT No. 14322). We would like to thank all the participants for their involvement in this project. Special thanks to Michelle Dawson and Kate Plaisted.
References
Abeles M, Goldstein MH. Functional architecture in cat primary cortex: columnar organization and organization according to depth.
American Psychological Association. Diagnostic and statistical manual of mental disorders. DSM-IV 4th edn. Washington (DC): American Psychological Association;
Anderson SJ, Burr DC. Spatial properties of directionally selective mechanisms in human vision.
Angelucci A, Levitt JB, Walton EJS, Hupé JM, Bullier J, Lund JS. Circuits for local and global signal integration in primary visual cortex.
Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams' syndrome.
Baker CL Jr. Central neural mechanisms for detecting second-order motion.
Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging.
Belmonte MK, Cook Jr EH, Anderson GM, Rubenstein JLR, Greenough WT, Beckel-Mitchener A, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy.
Bertone A. A psychophysical investigation of visual motion processing among high-functioning persons with autism. Doctoral thesis, Université de Montréal, Canada;
Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a “complex” issue.
Bertone A, Mottron L, Faubert J. Autism and schizophrenia: different neurobiological etiology, similar perceptual consequence?
Blake R, Turner LM, Smoski MJ, Pozdol SL, Stine WL. Visual recognition of biological motion is impaired in children with autism.
Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: a signal detection study.
Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and ‘dorsal-stream vulnerability’.
Brock J, Brown CC, Boucher J, Rippon G. The temporal binding hypothesis of autism.
Bullier J, Hupe JM, James AC, Girard. The role of feedback connections in shaping the responses of visual cortical neurons.
Burr DC, Santoro L (2001). Temporal integration of optic flow, measured by contrast and coherence thresholds.
Caron M-J, Mottron L, Rainville C, Chouinard S. Do high functioning persons with autism present superior spatial abilities?
Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism.
Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Neuronal density and architecture (grey level index) in the brains of autistic patients.
Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism.
Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrom and brain mechanisms for the attribution of mental states to animated shapes.
Chapman C, Hoag R, Giaschi D. The effect of disrupting human magnocellular pathway on global motion perception.
Chen Y, Nakayama K, Levy D, Matthysse S, Holzman P. Processing of global, but not local, motion direction is deficient in schizophrenia.
Chubb C, Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception.
Cohen IL. An artificial neural network analogue of learning in autism.
Cornelissen PL, Hansen PC, Gilchrist I, Cormack F, Essex J, Frankish C. Coherent motion detection and letter position encoding.
Cornelissen P, Richardson A, Mason A, Fowler S, Stein J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls.
Critchley HD, Daly EM, Bullmore ET, Williams SC, van Amelsvoort T, Robertson DM, et al. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions.
Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque.
Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local “association field”.
Foxton JM, Stewert ME, Barnard L, Rodgers J, Young AH, O'Brian G, et al. Absence of auditory ‘global interference’ in autism.
Freeman TCA, Harris MG. Human sensitivity to expanding and rotating motion: effects of complementary masking and directional structure.
Frith C. What do imaging studies tell us about the neural basis of autism? Novartis Found Symp
Gallant JL, Braun J, van Essen DC. Selectivity for polar, hyperbolic, and Cartesian gratings in the macaque visual cortex.
Gallant JL, Connor CE, Rakshit S, Lewis JW, van Essen DC. Neural responses to polar, hyperbolic, and Cartesian gratings in area V4 of the macaque monkey.
Georgeson MA, Scott-Samuel NE. Spatial resolution and receptive field height of motion sensors in human vision.
Gepner B, Mestre D, Masson G, de Schonen S. Postural effects of motion vision in young autistic children.
Gervais H, Belin P, Boddaert N, Leboyer M, Coez A et al. Abnormal cortical voice processing in autism.
Gilbert CD, Das A, Ito M, Kapadia M, Westheimer G. Spatial integration and cortical dynamics.
Gilmore GC, Wenk HE, Naylor LA, Koss E. Motion perception and Alzheimer's disease.
Goodale MA, Milner AD. Separate visual pathways for the perception of action.
Grice SJ, Spratling MW, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome.
Gustafsson L. Inadequate cortical feature maps: a neural circuit theory of autism.
Gustafsson L. Excessive lateral feedback synaptic inhibition may cause autistic characteristics.
Gunn A, Cory E, Atkinson J, Braddick OJ, Wattam-Bell J, Guzzetta A, Cioni G. Dorsal and ventral stream sensitivity in normal development hemiplegia.
Habak C, Faubert J. Larger effect of aging on the perception of higher-order stimuli.
Hess RF, Hayes A, Field DJ. Contour integration and cortical processing.
Hubl D, Bolte S, Feineis-Mattthews S, Lanfermann H, Federspiel A, Strik W, et al. Functional imbalance of visual pathways indicates alternative face processing strategies in autism.
Hupe JM, James AC, Payne BR, Lomber SG, Girard P, Bullier J. Cortical feedback improves discrimination between figure and background by V1, V2 and V3 neurons.
Jolliffe T, Baron-Cohen S. Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test?
Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity.
Kogan CS, Bertone A, Cornish K, Boutet I, DerKaloustian VM, Andermann E, et al. Integrative cortical dysfunction and pervasive motion perception deficit in fragile X syndrome.
Kogan CS, Boutet I, Cornish K, Zangenehpour S, Mullen KT, Holden JA, et al. Differential impact of the FMR1 gene on visual processing in fragile X syndrome.
Kovács I, Julesz B. A closed curve is much more than an incomplete one: effect of closure in figure-ground segmentation.
Kovács I, Kozma P, Fehér Á, Benedek G. Late maturation of visual spatial integration in humans.
Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, Saumier D. Face perception in high-functioning autistic adults: evidence for superior processing of face parts, not for a configural face processing deficit.
Ledgeway T, Smith AT. Evidence for separate motion-detecting mechanisms for first and second order motion in human vision.
Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism.
Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders.
Lu Z-L, Sperling G. Second-order illusions: Mach Bands, Chevreul, and Craik-O'Brien-Cornsweet.
Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: getting lost between aging and AD.
McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism.
McLelland JL. The basis of hypersensitivity in autism: a preliminary suggestion based on properties of neural nets.
Milne E, Sweetenham J, Hansen P, Campbell R, Jeffries H, Plaisted K. High motion coherence thresholds in children with autism.
Minshew NJ, Goldstein G, Siegel DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder.
Merigan WH, Byrne CE, Maunsell JH. Does primate motion perception depend on the magnocellular pathway?
Merigan WH, Maunsell JH. Macaque vision after magnocellular lateral geniculate lesions.
Merigan WH, Maunsell JHR. How parallel are the primate visual pathways?
Mottron L, Belleville S, Ménard E. Local bias in autistic subjects as evidenced by graphic tasks: perceptual hierarchization or working memory deficit.
Mottron L, Peretz I, Ménard E. Local and global processing of music in high-functioning persons with autism.
Mottron L, Burack J. Enhanced perceptual functioning in the development of persons with autism. In: Burack JA, Charman T, Yirmiya N, Zelazo PR, editors. The development of autism: perspectives from theory and research. Hillside, NJ: Erlbaum;
Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms.
Nishida S, Ledgeway T, Edwards M. Dual multiple-scale processing for motion in the human visual system.
O'Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism.
Ozonoff S, Strayer DL, McMahon WM, Filloux F. Executive function abilities in autism and Tourette's syndrome: an information processing approach.
Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak central coherence.
Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI.
Plaisted K, O'Riordan M, Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task.
Plaisted K, Sweetenham J, Reese L. Children with autism show local precedence in a divided attention task and global precedence in a selective attention task.
Rao RP, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects.
Regan D, Kothe AC, Sharpe JA. Recognition of motion-defined shapes in patients with multiple sclerosis and optic neuritis.
Ring HA, Baron-Cohen S, Wheelright S, Williams SC, Brammer M, Andrew C, et al. Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance.
Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome.
Shah A, Frith U. An islet of ability in autistic children: a research note.
Shah A, Frith U. Why do autistic individuals show superior performance on the block design task?
Shapley R. Visual sensitivity and parallel retinocortical channels.
Shiller PH, Logothesis NK, Charles ER. Role of colour-opponent and broad-band channels in vision.
Sillito AM, Grieve KL, Jones HE, Cudeiro J, Davis J. Visual cortical mechanisms detecting focal orientation discontinuities.
Smith AT, Greenlee MW, Singh KD, Kraemer FM, Henning J. The processing of first- and second-order motion in human visual cortex assessed by functional magnetic resonance imaging (fMRI).
Somers D, Dragoi V, Sur M. Orientation selectivity and its modulation by local and long-range connections in the visual cortex. In: Payne BR, Peters A, editors. The cat primary visual cortex. San Diego: Academic;
Spencer J, O'Brien J, Riggs K, Braddick O, Atkinson J, Wattam-Bell J. Motion processing in autism: evidence for a dorsal stream deficiency.
Sperling G, Chubb C, Solomon JA, Lu ZL. Full-wave and half-wave processes in second-order motion and texture.
Sutter A, Sperling G, Chubb C. Measuring the spatial frequency selectivity of second-order texture mechanisms.
Taylor MM, Forbes, Creelman CD. PEST reduces bias in forced choice psychophysics.
Trick GL, Silverman SE. Visual sensitivity to motion: age-related changes and deficits in senile dementia of the Alzheimer's type.
Trick GL, Kaskie B, Steinman SB. Visual impairment in Parkinson's disease: deficits in orientation and motion discrimination.
Watamaniuk SN, Sekular R. Temporal and spatial integration in dynamic random-dot stimuli.
Wilkinson F, James TW, Wilson HR, Gati JS, Menon RS, Goodale MA. An fMRI study of selective activation of human extrastriate form vision areas by radial and concentric gratings.
Wilkinson F, Wilson HR, Habak C. Detection and recognition of radial frequency patterns.
Wilson HR. Non-Fourier cortical processes in texture, form, and motion. In: Ulinski PS, Jones EG, (Eds), Cerebral cortex: models of cortical circuitry, New York: Plenum.
Wilson HR, Ferrera VP, Yo C. A psychophysically motivated model for two-dimensional motion perception.
Wilson HR, Wilkinson F. Detection of global structure in Glass patterns: implications for form vision.
Author notes
1Visual Psychophysics and Perception Laboratory, École d'optométrie, Université de Montréal, C. P. 6128 Succursale Centre Ville, Montréal, H3C-3J7, Canada and 2Clinique Spécialisée de l'Autisme, Hôpital Rivière-des-Prairies, 7070 boulevard Perras, Montréal, H1E-1A4, Canada