-
PDF
- Split View
-
Views
-
Cite
Cite
M. Caulo, J. Van Hecke, L. Toma, A. Ferretti, A. Tartaro, C. Colosimo, G. L. Romani, A. Uncini, Functional MRI study of diencephalic amnesia in Wernicke–Korsakoff syndrome, Brain, Volume 128, Issue 7, July 2005, Pages 1584–1594, https://doi.org/10.1093/brain/awh496
Close - Share Icon Share
Abstract
Anterograde amnesia in Wernicke–Korsakoff syndrome is associated with diencephalic lesions, mainly in the anterior thalamic nuclei. Whether diencephalic and temporal lobe amnesias are distinct entities is still not clear. We investigated episodic memory for faces using functional MRI (fMRI) in eight controls and in a 34-year-old man with Wernicke–Korsakoff syndrome and diencephalic lesions but without medial temporal lobe (MTL) involvement at MRI. fMRI was performed with a 1.5 tesla unit. Three dual-choice tasks were employed: (i) face encoding (18 faces were randomly presented three times and subjects were asked to memorize the faces); (ii) face perception (subjects indicated which of two faces matched a third face); and (iii) face recognition (subjects indicated which of two faces belonged to the group they had been asked to memorize during encoding). All activation was greater in the right hemisphere. In controls both the encoding and recognition tasks activated two hippocampal regions (anterior and posterior). The anterior hippocampal region was more activated during recognition. Activation in the prefrontal cortex was greater during recognition. In the subject with Wernicke–Korsakoff syndrome, fMRI did not show hippocampal activation during either encoding or recognition. During recognition, although behavioural data showed defective retrieval, the prefrontal regions were activated as in controls, except for the ventrolateral prefrontal cortex. fMRI activation of the visual cortices and the behavioural score on the perception task indicated that the subject with Wernicke–Korsakoff syndrome perceived the faces, paid attention to the task and demonstrated accurate judgement. In the subject with Wernicke–Korsakoff syndrome, although the anatomical damage does not involve the MTL, the hippocampal memory encoding has been lost, possibly as a consequence of the hippocampal–anterior thalamic axis involvement. Anterograde amnesia could therefore be the expression of damage to an extended hippocampal system, and the distinction between temporal lobe and diencephalic amnesia has limited value. In the subject with Wernicke–Korsakoff syndrome, the preserved dorsolateral prefrontal cortex activation during incorrect recognition suggests that this region is more involved in either the orientation or attention at retrieval than in retrieval. The lack of activation of the prefrontal ventrolateral cortex confirms the role of this area in episodic memory formation.
Introduction
Wernicke encephalopathy is an acute neurological disorder characterized by ataxia, vestibular dysfunction, a variety of ocular motility abnormalities, a confusional state and drowsiness, which can progress to a chronic amnesic state called the Korsakoff syndrome. Both disorders are caused by thiamine deficiency and are found mainly in chronic alcoholics but also in cases of persistent vomiting, gastric carcinoma or other disturbances of the alimentary tract (Victor et al., 1989).
The cardinal feature of the Korsakoff syndrome is severe and disproportionate anterograde memory loss in an otherwise alert and responsive patient. Patients suffering from this amnesic syndrome show degeneration of diencephalic regions: the mammillary bodies and periventricular regions, especially the anterior thalamic nucleus (Harding et al., 2000). Anterograde amnesia resulting from diencephalic damage resembles amnesia following medial temporal lobe (MTL) damage (Aggleton and Brown, 1999).
Whether these two types of amnesia should be considered as independent is still a matter of debate. The extensive anatomical connections between the temporal lobe, the mammillary bodies and the thalamus on the one hand and the position of the diencephalon connecting the MTL and the frontal lobe on the other suggest a crucial role of the diencephalon in the network underlying memory formation (Vann and Aggleton, 2004). Both the MTL and the diencephalon could be considered part of a single system for acquiring new declarative memories. Therefore, both amnesic syndromes could be variants of a disorder involving the same cerebral network (Aggleton and Brown, 1999). Alternatively, damage to the diencephalic structures could result in widespread dysfunction of cortical regions, disrupting memory formation and retrieval without involvement of the MTL (Mair, 1994).
We used functional MRI (fMRI) to study memory processes for facial episodic recognition to clarify the relationship between the MTL and diencephalic amnesia in eight healthy volunteers and in a subject with Wernicke–Korsakoff (WK) syndrome with diencephalic damage but without MTL involvement.
Material and methods
Subjects
A 34-year-old, non alcoholic, right-handed man with oesophageal stenosis after surgery for cancer, persistent vomiting and severe malnutrition was admitted because of gait unsteadiness and confusional state. Neurological examination performed 3 days later showed a global confusional state and drowsiness, dysarthria, ophthalmoparesis, nystagmus and ataxia. A clinical diagnosis of Wernicke encephalopathy was made and thiamine treatment (100 mg/day intramuscularly) initiated. After 5 days the patient was alert and attentive but presented retrograde amnesia and severe anterograde amnesia. Five months after onset, ataxia and ophthalmoparesis had disappeared, retrograde amnesia shrank, but dense anterograde amnesia was still present. A neuropsychological examination was performed 4 weeks and 5 months after onset using the following tests: the Mini-Mental State Examination (MMSE), the Prose Memory test, the Rey Complex Figure, Crovitz test, the Wechsler Adult Intelligence Scale (WAIS) Information subtest, the Gollin Incomplete Pictures Test, and the Wechsler Adult Intelligence Scale—Revised (WAIS-R) Digit Symbol subtest. Results (raw scores) are reported in Table 1. At 4 weeks the low score obtained on the MMSE revealed reduced global cognitivity. Poor performances on the Prose test and the Rey Complex Figure delayed recall test documented a severe anterograde episodic memory loss. The good performance observed in the immediate recall on the Rey Complex Figure test could be explained by the relative preservation of attention and visuospatial/constructional abilities. Immediate and non-declarative memory was preserved, as proved by the scores in the Gollin and Digit Symbol tests. The score obtained in the WAIS Information subtest demonstrated intact semantic memory storage. A low score on the Crovitz test showed an important deficit in remote autobiographical memory, also observed during the clinical interview, which revealed retrograde amnesia spanning approximately 2 years. At 5 months the global level of cognitive functions returned within the normal range. We did not find significant changes on the declarative (verbal and non-verbal) memory test as a whole, although a slight improvement in relative scores was evident. The scores on semantic and non-declarative tests were slightly improved. A better performance on the Crovitz test was concordant with the improvement in retrograde amnesia, which had declined, as inferred by clinical interview, and spanned approximately 10 months.
Neuropsychological tests and scores 4 weeks and 5 months after onset
. | 4 weeks . | 5 months . |
|---|---|---|
| MMSE (range 0–30) | 22 | 27 |
| Prose Memory test (range 0–28) | 1.5 | 4 |
| *Rey Complex Figure (range 0–36) | Immediate 29 | Immediate 31 |
| Delayed 3 | Delayed 4 | |
| Gollin Incomplete Pictures Test (range 0–60) | 50 | 55 |
| WAIS-R Digit Symbol Subtest (range 0–90) | 79 | 82 |
| WAIS Information Subtest (range 0–29) | 17 | 21 |
| Crovitz test (range of mean score for each episode 0–3) | 0.5 | 1.5 |
. | 4 weeks . | 5 months . |
|---|---|---|
| MMSE (range 0–30) | 22 | 27 |
| Prose Memory test (range 0–28) | 1.5 | 4 |
| *Rey Complex Figure (range 0–36) | Immediate 29 | Immediate 31 |
| Delayed 3 | Delayed 4 | |
| Gollin Incomplete Pictures Test (range 0–60) | 50 | 55 |
| WAIS-R Digit Symbol Subtest (range 0–90) | 79 | 82 |
| WAIS Information Subtest (range 0–29) | 17 | 21 |
| Crovitz test (range of mean score for each episode 0–3) | 0.5 | 1.5 |
Copy was correctly performed in both examinations.
Neuropsychological tests and scores 4 weeks and 5 months after onset
. | 4 weeks . | 5 months . |
|---|---|---|
| MMSE (range 0–30) | 22 | 27 |
| Prose Memory test (range 0–28) | 1.5 | 4 |
| *Rey Complex Figure (range 0–36) | Immediate 29 | Immediate 31 |
| Delayed 3 | Delayed 4 | |
| Gollin Incomplete Pictures Test (range 0–60) | 50 | 55 |
| WAIS-R Digit Symbol Subtest (range 0–90) | 79 | 82 |
| WAIS Information Subtest (range 0–29) | 17 | 21 |
| Crovitz test (range of mean score for each episode 0–3) | 0.5 | 1.5 |
. | 4 weeks . | 5 months . |
|---|---|---|
| MMSE (range 0–30) | 22 | 27 |
| Prose Memory test (range 0–28) | 1.5 | 4 |
| *Rey Complex Figure (range 0–36) | Immediate 29 | Immediate 31 |
| Delayed 3 | Delayed 4 | |
| Gollin Incomplete Pictures Test (range 0–60) | 50 | 55 |
| WAIS-R Digit Symbol Subtest (range 0–90) | 79 | 82 |
| WAIS Information Subtest (range 0–29) | 17 | 21 |
| Crovitz test (range of mean score for each episode 0–3) | 0.5 | 1.5 |
Copy was correctly performed in both examinations.
Anatomical MRI studies were performed using standard (spin-echo, turbo spin-echo and fluid-attenuated inversion recovery; FLAIR) sequences 7 days, 4 weeks and 5 months after the onset of symptoms. During the last two sessions, fMRI studies with the blood oxygenation level-dependent contrast (BOLDc) technique were performed using a block paradigm to test memory for faces. Eight healthy right-handed men (30–40 years) were recruited as controls for the fMRI study. All subjects gave informed consent. This study had received prior approval from our institutional Ethics Committee.
Functional MRI
Recordings
BOLDc functional imaging was performed using a Siemens Magnetom Vision scanner with 1.5 T- and T2*-weighted echo-planar imaging (EPI). Sequences parameters were: repetition time (TR) 2 s, echo time (TE) 60 ms, matrix size 64 × 64, field of view 256 mm, in-plane voxel size 4 × 4 mm, flip angle 90°, slice thickness 5 mm, and no gap. Foam padding was added to the standard head coil in order to minimize involuntary subject movement.
Functional volumes consisted of 16 transaxial slices parallel to the anterior commissure–posterior commissure (AC–PC) line and including the medial and posterior temporal, frontal and occipital cortexes. A high-resolution structural volume was acquired at the end of each session with a 3D MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence (matrix 256 × 256; field of view 256 mm; slice thickness 1 mm; no gap; in-plane voxel size 1 × 1 mm; flip angle 12°; TR 9.7 ms; TE 4 ms).
fMRI paradigm
The fMRI study was a block-design study. Each scanning session consisted of five runs containing five blocks of 24 s. Participants performed a face-encoding task during the first three runs, a face discrimination task during the fourth run and a face recognition task during the last run. For each of the three tasks the subjects were required to choose between two options (dual-choice), as previously described by Haxby and colleagues in a PET study (Haxby et al., 1996). The dual-choice blocks (‘on’ condition) were alternated in an ‘off–on’ fashion, in which the ‘off’ condition lasted 24 s and consisted of repeating, alternating pressing of the two buttons.
Stimuli for the dual-choice tasks consisted of one upper and two lower white frames, in which either a greyscale neutral face or a nonsense pattern, according to the task type, was presented. The photos with a neutral facial expression were obtained with the consent of volunteers from our institute. During each block, six stimuli were presented for 4 s each. The stimuli for the face-encoding task were a nonsense pattern in the upper frame and an alternating face and a nonsense pattern in the lower frames: the subject was required to press the button that corresponded to the lower frame which contained the face (Fig. 1A). The stimuli for the face discrimination task were a master face pattern in the upper frame and two faces in the lower frames: the subject was required to press the button that corresponded to the lower frame that contained the matching face (Fig. 1B). The stimuli for the face recognition task were a nonsense pattern in the upper frame and two faces in the lower frames: the subject was required to press the button that corresponded to the lower frame that contained the face that had already been presented during the encoding tasks (Fig. 1C). Subjects were asked during the tasks to always express a choice. For the control task all three frames contained the nonsense pattern and no perceptual or memory judgement were required.
Examples of faces used as stimuli for the encoding (A), perception (B) and recognition (C) tasks.
Stimuli were projected by means of an LCD projector and two perpendicular mirrors on a translucent glass plate placed on the back of the scanner bore. An additional mirror was attached to the head coil inside the magnet bore. This permitted the subjects to see the stimuli. The LCD projector was driven by a personal computer placed in the console room.
The nonsense pattern was obtained by scrambling one face using a commercial package for picture manipulation (swirl function of Adobe® Photoshop® CS).
Behavioural data (correct or incorrect responses) were obtained by analysing which of the two buttons was activated (left or right index fingers). These buttons were connected via fibre-optic cables to the same computer that produced the stimuli. Behavioural data were recorded using the Psychotoolbox of the Matlab® program (Mathworks, Natick, MA, USA).
The subject and the controls received training the day before and immediately prior to fMRI in order to ensure a correct execution of the session. Task instructions were repeated orally between runs and projected on the screen 4 s before the onset of each task block. This repetitive procedure was necessary because of the increased probability that the subject would otherwise forget the instructions.
fMRI data analysis
Raw data were analysed using Brain Voyager 4.9 software (Brain Innovation, The Netherlands). Preprocessing of functional scans included motion correction and removal of linear trends from voxel time series. A 3D motion correction was performed. Preprocessed functional volumes of each subject were co-registered with the corresponding structural data set. Since the 2D functional and 3D structural measurements were acquired in the same session, the co-registration transformation was determined using the Siemens slice position parameters of the functional images and the position parameters of the structural volume. Structural and functional images were transformed into Talairach space coordinates (Talairach and Tournoux, 1988). The Talairach transformation was performed in two steps. In the first step the structural and functional data sets were rotated in the AC-PC plane. In the second step the extreme points of the cerebrum were specified, defining 12 subvolumes which were scaled into Talairach space using a piecewise affine and continuous transformation. Functional volumes were resampled at a voxel size of 3 × 3 × 3 mm.
Statistical analysis was performed individually and for the control group using the general linear model (Friston et al., 1995) in order to reveal cortical regions showing a significantly higher BOLD signal during the three tasks with respect to the off condition. The boxcar waveform representing the task and control conditions was convolved with an empirically founded haemodynamic response function in order to account for the haemodynamic delay (Boynton et al., 1996).
Individual and group statistical maps were thresholded at P < 0.0004 at the voxel level and a cluster size of at least four voxels was required in order to obtain a corrected (for multiple comparisons) significance level: the probability of a false detection for the entire functional volume was P < 0.05, as estimated by Monte Carlo simulation (3dFWHM and AlphaSim routines of AFNI package; Forman et al., 1995; Cox, 1996). Regions of interest (ROI) were determined for each control and for the WK subject, considering the mask obtained from the union of voxels activated in each condition separately.
This analysis was restricted to the ROI of activated cortical regions that have been indicated in the literature as having a major role in face memory processing: the prefrontal cortex (ventrolateral and dorsolateral), MTL structures and a visual cortical region (fusiform gyrus) (Kelley et al., 1988; Clark et al., 1996; Haxby et al., 1996; Kanwisher et al., 1997). The BOLD signal of the insular and medial prefrontal cortex was also analysed. The regional comparison of activation for the control group was performed using analysis of variance (ANOVA) for repeated measures. The dependent variable of the ANOVA analysis was the BOLD signal relative variation between the task and off conditions; the factors were the memory task (encoding, perception, recognition) and the selected ROI. The Duncan test was used for post hoc comparisons. Activation in the subject was considered significantly different when it was greater than 2 SD with respect to controls.
In addition a voxel-wise analysis was performed by concatenating controls' and the WK subject's voxel time courses to look for cortical regions showing differences between conditions (encoding versus perception, and recognition versus perception) in the WK patient but not in controls and vice versa. In this analysis we used a less stringent threshold (P < 0.001, uncorrected) in order to exclude false negatives.
Results
Anatomical MRI
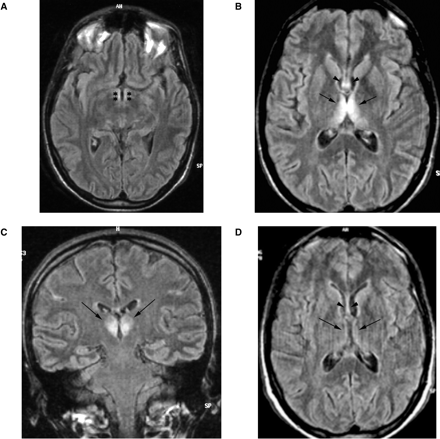
Signal changes involving the mammillary bodies, the medial thalamic region and the fornix, in the absence of any signal abnormalities in MTL structures, were observed 1 week after clinical onset (Fig. 2A). The 5-month follow-up study demonstrated almost complete resolution of the lesions and absence of signal changes in the MTL (Fig. 2B). An expert neuroradiologist made a visual qualitative evaluation of brain atrophy using morphological MRI sequences. No signs of generalized or focal MTL atrophy were detected in the 4-week and 5-month MRI studies.
Images from axial and coronal FLAIR sequence of the WK subject. Seven-day MRI study demonstrates signal abnormalities of the mammillary bodies (A) (stars), the medial aspect of the thalami (B, C) (arrows) and the fornix (B) (arrowheads). Five months later signal changes have almost completely disappeared (D) (arrows and arrowheads).
fMRI findings
Controls
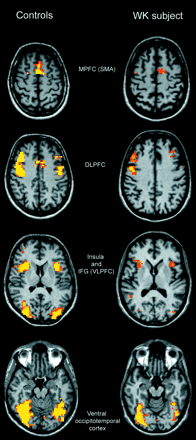
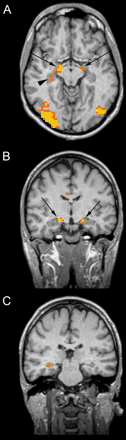
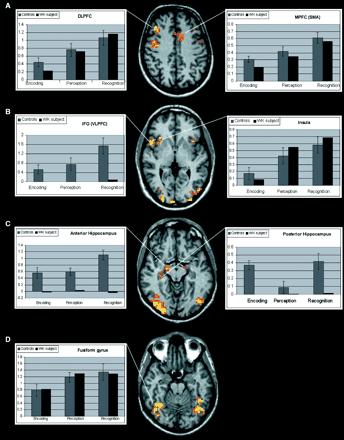
Analysis of fMRI data comparing the on and off conditions revealed that activated areas were bilateral during each task but with relative right lateralization. The control group map of cumulative cortical activation for all tasks revealed enhancement of several cortical regions: a region in the medial surface of the frontal lobe (medial prefrontal cortex, MPFC) in a position generally attributed to the supplementary motor area (Fig. 3); two confluent bilateral regions, one anterior and one posterior, in the dorsolateral prefrontal cortex (DLPFC) and a cluster of activation in the low convexity involving the pars opercularis of the right inferior frontal gyrus (IFG) (ventrolateral prefrontal cortex, VLPFC) and the insular cortex (Fig. 3). Given the close position of the insula and inferior frontal gyrus and of the two regions of activation of the DLPFC, the group analysis showed confluent activations, whereas analysis of the individual subjects distinguished two distinct cortical areas. The insular cortex was also activated in the left hemisphere. Regions of cortical activation were present in the bilateral ventral occipitotemporal cortex and included the fusiform gyrus (Fig. 3). In this region it was also necessary to look at individual activations in order to distinguish the fusiform gyrus from the rest of the extrastriatal cortex. In the MTL, the hippocampus was activated in two distinct regions, one anterior and one posterior (Fig. 4).
Activated areas during the ‘on’ condition in controls (left column) and the WK subject (right column). Structural and functional images are shown using the right–left radiological convention.
Activated areas in the hippocampal region in controls during the ‘on’ condition. Structural and functional images are shown using the right–left radiological convention. The images are oriented so that the long axis of the hippocampus is parallel to the axial section. In A the hippocampus is activated in two distint regions: anterior (arrows) and posterior (arrowhead). B and C show coronal slices passing trought the anterior (B) and posterior hippocampal activations (C). The anterior aspect of the left hippocampus is also activated during the ‘on’ condition (A).
WK subject
Because at the 5-month follow-up the severity of anterograde amnesia the fMRI activations and the behavioural data were similar to the study performed at 4 weeks, we merged the data from the two fMRI examinations.
At the selected threshold, the activations showed marked right hemisphere predominance. The areas of activation in the DLPFC, MPFC, insula and ventral occipitotemporal cortex (including the fusiform gyrus) resembled those observed in the control group (Fig. 3). The list of activated areas and corresponding Talairach coordinates is reported in Table 2.
Cumulative activated areas during all three tasks in controls and the WK subject
| Region . | Control group . | . | WK subject . | . | ||
|---|---|---|---|---|---|---|
. | Talairach coordinates (x, y, z) . | Brodmann area . | Talairach coordinates (x, y, z) . | Brodmann area . | ||
| Right middle frontal gyrus (DLPFC) | −47, −24, −31 | 9 | −42, −29, −31 | 9 | ||
| Right middle frontal gyrus (DLPFC) | −47, −6, −36 | −45, −5, −32 | ||||
| Left middle frontal gyrus (DLPFC) | −44, −25, −34 | 9 | −44, −27, −36 | 9 | ||
| Left middle frontal gyrus (DLPFC) | −45, −6, −37 | −40, −7, −31 | ||||
| Right inferior frontal gyrus (VLPFC) | −45, −18, −4 | – | ||||
| Left inferior frontal gyrus (VLPFC) | −48, −14, −3 | – | ||||
| Right insula | −33, −17, −6 | −28, −20, −11 | ||||
| Left insula | −39, −16, −5 | −34, −15, −11 | ||||
| Right hippocampus | −15, −10, −10 | 28 | – | |||
| Right hippocampus | −24, −26, −8 | 28 | – | |||
| Left hippocampus | −18, −11, −10 | 28 | – | |||
| Right superior frontal gyrus (MPFC) | −5, −10, −44 | 6, 32 | −5, −9, −46 | 6, 32 | ||
| Left superior frontal gyrus (MPFC) | −4, −11, −44 | 6, 32 | −4, −11, −46 | 6, 32 | ||
| Right fusiform gyrus | −29, −55, −16 | −30, −52, −16 | ||||
| Left fusiform gyrus | −33, −60, −13 | −36, −53, −17 | ||||
| Right middle temporal gyrus | −32, −74, −15 | 39 | −32, −77, −16 | 39 | ||
| Left middle temporal gyrus | −37, −78, −16 | 39 | −39, −79, −12 | 39 | ||
| Right inferior temporal gyrus | −40, −72, −1 | −39, −74, −1 | ||||
| Left inferior temporal gyrus | −48, −72, −1 | −40, −77, −1 | ||||
| Left lingual gyrus | −3, −80, −6 | 18 | −2, −77, −11 | 18 | ||
| Right middle occipital gyrus | −41, −75, −7 | −38, −75, −7 | ||||
| Left middle occipital gyrus | −45, −75, −7 | −46, −74, −10 | ||||
| Right inferior occipital gyrus | −26, −88, −7 | −27, −88, −6 | ||||
| Region . | Control group . | . | WK subject . | . | ||
|---|---|---|---|---|---|---|
. | Talairach coordinates (x, y, z) . | Brodmann area . | Talairach coordinates (x, y, z) . | Brodmann area . | ||
| Right middle frontal gyrus (DLPFC) | −47, −24, −31 | 9 | −42, −29, −31 | 9 | ||
| Right middle frontal gyrus (DLPFC) | −47, −6, −36 | −45, −5, −32 | ||||
| Left middle frontal gyrus (DLPFC) | −44, −25, −34 | 9 | −44, −27, −36 | 9 | ||
| Left middle frontal gyrus (DLPFC) | −45, −6, −37 | −40, −7, −31 | ||||
| Right inferior frontal gyrus (VLPFC) | −45, −18, −4 | – | ||||
| Left inferior frontal gyrus (VLPFC) | −48, −14, −3 | – | ||||
| Right insula | −33, −17, −6 | −28, −20, −11 | ||||
| Left insula | −39, −16, −5 | −34, −15, −11 | ||||
| Right hippocampus | −15, −10, −10 | 28 | – | |||
| Right hippocampus | −24, −26, −8 | 28 | – | |||
| Left hippocampus | −18, −11, −10 | 28 | – | |||
| Right superior frontal gyrus (MPFC) | −5, −10, −44 | 6, 32 | −5, −9, −46 | 6, 32 | ||
| Left superior frontal gyrus (MPFC) | −4, −11, −44 | 6, 32 | −4, −11, −46 | 6, 32 | ||
| Right fusiform gyrus | −29, −55, −16 | −30, −52, −16 | ||||
| Left fusiform gyrus | −33, −60, −13 | −36, −53, −17 | ||||
| Right middle temporal gyrus | −32, −74, −15 | 39 | −32, −77, −16 | 39 | ||
| Left middle temporal gyrus | −37, −78, −16 | 39 | −39, −79, −12 | 39 | ||
| Right inferior temporal gyrus | −40, −72, −1 | −39, −74, −1 | ||||
| Left inferior temporal gyrus | −48, −72, −1 | −40, −77, −1 | ||||
| Left lingual gyrus | −3, −80, −6 | 18 | −2, −77, −11 | 18 | ||
| Right middle occipital gyrus | −41, −75, −7 | −38, −75, −7 | ||||
| Left middle occipital gyrus | −45, −75, −7 | −46, −74, −10 | ||||
| Right inferior occipital gyrus | −26, −88, −7 | −27, −88, −6 | ||||
Cumulative activated areas during all three tasks in controls and the WK subject
| Region . | Control group . | . | WK subject . | . | ||
|---|---|---|---|---|---|---|
. | Talairach coordinates (x, y, z) . | Brodmann area . | Talairach coordinates (x, y, z) . | Brodmann area . | ||
| Right middle frontal gyrus (DLPFC) | −47, −24, −31 | 9 | −42, −29, −31 | 9 | ||
| Right middle frontal gyrus (DLPFC) | −47, −6, −36 | −45, −5, −32 | ||||
| Left middle frontal gyrus (DLPFC) | −44, −25, −34 | 9 | −44, −27, −36 | 9 | ||
| Left middle frontal gyrus (DLPFC) | −45, −6, −37 | −40, −7, −31 | ||||
| Right inferior frontal gyrus (VLPFC) | −45, −18, −4 | – | ||||
| Left inferior frontal gyrus (VLPFC) | −48, −14, −3 | – | ||||
| Right insula | −33, −17, −6 | −28, −20, −11 | ||||
| Left insula | −39, −16, −5 | −34, −15, −11 | ||||
| Right hippocampus | −15, −10, −10 | 28 | – | |||
| Right hippocampus | −24, −26, −8 | 28 | – | |||
| Left hippocampus | −18, −11, −10 | 28 | – | |||
| Right superior frontal gyrus (MPFC) | −5, −10, −44 | 6, 32 | −5, −9, −46 | 6, 32 | ||
| Left superior frontal gyrus (MPFC) | −4, −11, −44 | 6, 32 | −4, −11, −46 | 6, 32 | ||
| Right fusiform gyrus | −29, −55, −16 | −30, −52, −16 | ||||
| Left fusiform gyrus | −33, −60, −13 | −36, −53, −17 | ||||
| Right middle temporal gyrus | −32, −74, −15 | 39 | −32, −77, −16 | 39 | ||
| Left middle temporal gyrus | −37, −78, −16 | 39 | −39, −79, −12 | 39 | ||
| Right inferior temporal gyrus | −40, −72, −1 | −39, −74, −1 | ||||
| Left inferior temporal gyrus | −48, −72, −1 | −40, −77, −1 | ||||
| Left lingual gyrus | −3, −80, −6 | 18 | −2, −77, −11 | 18 | ||
| Right middle occipital gyrus | −41, −75, −7 | −38, −75, −7 | ||||
| Left middle occipital gyrus | −45, −75, −7 | −46, −74, −10 | ||||
| Right inferior occipital gyrus | −26, −88, −7 | −27, −88, −6 | ||||
| Region . | Control group . | . | WK subject . | . | ||
|---|---|---|---|---|---|---|
. | Talairach coordinates (x, y, z) . | Brodmann area . | Talairach coordinates (x, y, z) . | Brodmann area . | ||
| Right middle frontal gyrus (DLPFC) | −47, −24, −31 | 9 | −42, −29, −31 | 9 | ||
| Right middle frontal gyrus (DLPFC) | −47, −6, −36 | −45, −5, −32 | ||||
| Left middle frontal gyrus (DLPFC) | −44, −25, −34 | 9 | −44, −27, −36 | 9 | ||
| Left middle frontal gyrus (DLPFC) | −45, −6, −37 | −40, −7, −31 | ||||
| Right inferior frontal gyrus (VLPFC) | −45, −18, −4 | – | ||||
| Left inferior frontal gyrus (VLPFC) | −48, −14, −3 | – | ||||
| Right insula | −33, −17, −6 | −28, −20, −11 | ||||
| Left insula | −39, −16, −5 | −34, −15, −11 | ||||
| Right hippocampus | −15, −10, −10 | 28 | – | |||
| Right hippocampus | −24, −26, −8 | 28 | – | |||
| Left hippocampus | −18, −11, −10 | 28 | – | |||
| Right superior frontal gyrus (MPFC) | −5, −10, −44 | 6, 32 | −5, −9, −46 | 6, 32 | ||
| Left superior frontal gyrus (MPFC) | −4, −11, −44 | 6, 32 | −4, −11, −46 | 6, 32 | ||
| Right fusiform gyrus | −29, −55, −16 | −30, −52, −16 | ||||
| Left fusiform gyrus | −33, −60, −13 | −36, −53, −17 | ||||
| Right middle temporal gyrus | −32, −74, −15 | 39 | −32, −77, −16 | 39 | ||
| Left middle temporal gyrus | −37, −78, −16 | 39 | −39, −79, −12 | 39 | ||
| Right inferior temporal gyrus | −40, −72, −1 | −39, −74, −1 | ||||
| Left inferior temporal gyrus | −48, −72, −1 | −40, −77, −1 | ||||
| Left lingual gyrus | −3, −80, −6 | 18 | −2, −77, −11 | 18 | ||
| Right middle occipital gyrus | −41, −75, −7 | −38, −75, −7 | ||||
| Left middle occipital gyrus | −45, −75, −7 | −46, −74, −10 | ||||
| Right inferior occipital gyrus | −26, −88, −7 | −27, −88, −6 | ||||
Activated areas in the MTL and VLPFC were not observed at the statistical threshold of P < 0.05 (corrected). Even at a less rigorous threshold (P < 0.001 uncorrected), activated voxels were not detected in these regions. In order to further reduce the possibility of false negatives, the fMRI signals for these cortical areas were derived from ROI determined using the respective ROI obtained from the group analysis of the controls. The Talairach normalization can account only partially for the intersubject anatomical variability. Therefore, in addition to the use of Talairach coordinates we also visually checked the overlapping of the group's ROI with the patient's cortical anatomy on the basis of known anatomical landmarks. In order to avoid a methodological bias, the BOLD response in these regions for each normal subject was also evaluated considering the group ROI, in addition to the evaluation performed using individual ROI.
ANOVA
The two-way ANOVA (factors: ROI, tasks) in the control group was focused on eight ROI in the right hemisphere: anterior and posterior DLPFC, IFG, MPFC, insular cortex, anterior and posterior hippocampus, and fusiform gyrus. A statistically significant main effect was observed for the ROI factor [F(7.49) = 6.22; P < 0.00003] and for the task factor [F(2.14) = 49.79; P < 0.0000004]. Furthermore, a statistically significant interaction [F(14.98) = 3.49; P < 0.0001] between the two factors was observed.
In the prefrontal cortex, the BOLD signal increased during the three memory tasks (encoding, perception, and recognition) (Fig. 5A). The anterior and posterior DLPFC, the MPFC and the insular cortex were significantly more activated during recognition than during encoding. No difference was demonstrated, for the same cortical regions, between recognition and perception except for the MPFC, which was more activated during recognition (Fig. 5A and B). Moreover, the anterior and posterior DLPFC, MPFC and insular cortex showed no significant difference between encoding and perception, except for the posterior DLPFC, which was more activated during perception. The VLPFC activation was significantly greater during recognition than during both encoding and perception (Fig. 5B). In the MTL, the ROI in the anterior hippocampus was significantly more activated during recognition than during encoding and perception but a difference was not observed between encoding and perception. The region of activation in the posterior hippocampus showed a significantly greater BOLD signal during encoding and recognition than during perception, whereas differences did not exist between encoding and recognition (Fig. 5C). The BOLD signal of the fusiform gyrus was significantly greater during recognition and perception than during encoding. Statistically significant differences were not observed between recognition and perception (Fig. 5D). Levels of significance of the post hoc comparison of the BOLD signal change during the three different tasks in each ROI are reported in Table 3.
BOLD % signal changes in different cortical regions during task execution in controls and the WK subject. Structural and functional images are shown using the right–left radiological convention. The y axis shows the BOLD % signal change. The error bars represent standard deviations. The comparison between the activations of the control group and the WK subject demonstrates a similar BOLD signal course exept for the hippocampal region and the IFG, where no activation was observed in the WK subject.
ANOVA results: comparison of BOLD signal percentage changes during the memory tasks for the different ROI: probabilities for post hoc test
. | Insula . | Anterior DLPFC . | Posterior DLPFC . | MPFC . | IFG VLPFC . | Anterior hippocampus . | Posterior hippocampus . | Fusiform gyrus . |
|---|---|---|---|---|---|---|---|---|
| Encoding vs perception | 0.094 | 0.051 | 0.016* | 0.051 | 0.156 | 0.835 | 0.034* | 0.012* |
| Encoding vs recognition | 0.011* | 0.000* | 0.000* | 0.000* | 0.000* | 0.001* | 0.072 | 0.000* |
| Recognition vs perception | 0.334 | 0.051 | 0.229 | 0.000* | 0.000* | 0.001* | 0.028* | 0.258 |
. | Insula . | Anterior DLPFC . | Posterior DLPFC . | MPFC . | IFG VLPFC . | Anterior hippocampus . | Posterior hippocampus . | Fusiform gyrus . |
|---|---|---|---|---|---|---|---|---|
| Encoding vs perception | 0.094 | 0.051 | 0.016* | 0.051 | 0.156 | 0.835 | 0.034* | 0.012* |
| Encoding vs recognition | 0.011* | 0.000* | 0.000* | 0.000* | 0.000* | 0.001* | 0.072 | 0.000* |
| Recognition vs perception | 0.334 | 0.051 | 0.229 | 0.000* | 0.000* | 0.001* | 0.028* | 0.258 |
Significant differences.
ANOVA results: comparison of BOLD signal percentage changes during the memory tasks for the different ROI: probabilities for post hoc test
. | Insula . | Anterior DLPFC . | Posterior DLPFC . | MPFC . | IFG VLPFC . | Anterior hippocampus . | Posterior hippocampus . | Fusiform gyrus . |
|---|---|---|---|---|---|---|---|---|
| Encoding vs perception | 0.094 | 0.051 | 0.016* | 0.051 | 0.156 | 0.835 | 0.034* | 0.012* |
| Encoding vs recognition | 0.011* | 0.000* | 0.000* | 0.000* | 0.000* | 0.001* | 0.072 | 0.000* |
| Recognition vs perception | 0.334 | 0.051 | 0.229 | 0.000* | 0.000* | 0.001* | 0.028* | 0.258 |
. | Insula . | Anterior DLPFC . | Posterior DLPFC . | MPFC . | IFG VLPFC . | Anterior hippocampus . | Posterior hippocampus . | Fusiform gyrus . |
|---|---|---|---|---|---|---|---|---|
| Encoding vs perception | 0.094 | 0.051 | 0.016* | 0.051 | 0.156 | 0.835 | 0.034* | 0.012* |
| Encoding vs recognition | 0.011* | 0.000* | 0.000* | 0.000* | 0.000* | 0.001* | 0.072 | 0.000* |
| Recognition vs perception | 0.334 | 0.051 | 0.229 | 0.000* | 0.000* | 0.001* | 0.028* | 0.258 |
Significant differences.
Comparison of cortical activation of the control group versus the WK subject demonstrated a precise match in the DLPFC, MPFC, insula and fusiform gyrus. However, the patient failed to activate the MTL and the VLPFC during each condition.
The voxel-wise analysis, testing for differences between conditions in the WK subject compared with controls, did not show further regions of activation other than those which entered the ANOVA analysis.
Behavioural data
Controls
During encoding, the correct responses in the face–nonsense judgement were virtually 100% in all subjects, indicating good attention and accurate judgement. Mean correct responses were 88.6 ± 3.8% (range 83–94%) for perception and 83.5 ± 4.2% (range 78–89%) for recognition.
WK subject
Cumulative correct responses of the two fMRI studies were 98% for encoding and 88% for perception. These results were not significantly different from those for controls. Correct responses for recognition were 54%, which was significantly lower than the figure for controls.
Discussion
Patients with WK syndrome present persistent anterograde episodic memory loss with preserved semantic memory, intelligence and learned behaviour (Kopelman, 1995). The severe anterograde memory loss is similar to the amnesia that is associated with MTL lesions in humans and experimental animals (Squire, 1992; Aggleton and Brown, 1999). There are a number of recent fMRI and PET studies indicating that MTL is activated during episodic memory encoding and retrieval. A meta-analysis of 52 PET studies concluded that the anterior MTL is strongly associated with episodic encoding, whereas the posterior MTL is associated with retrieval (Lepage et al., 1998). In contrast, Schacter and Wagner (1999), in their meta-analysis of fMRI studies, indicated a predominance of posterior MTL activation for encoding. Data on retrieval were insufficient to draw any conclusion on rostrocaudal localization. Overall, although there is no general agreement on regional anatomical differences in activation between encoding and retrieval, several authors have reported that MTL activation is observed during both episodic encoding and retrieval (Shacter et al., 1999; Eldridge et al., 2000; Greicius et al., 2003).
We chose recognition memory for faces because faces are novel, unfamiliar and unique in their configuration. Therefore, they are more suitable than words for testing episodic memory (Haxby et al., 1996). Few fMRI studies have investigated facial encoding and recognition. Kelley and colleagues reported a bilateral MTL (greater on the right) and right dorsal frontal cortex activation during encoding (Kelley et al., 1998). Bernard and colleagues tested memory for famous faces using an event-related fMRI study and demonstrated a functional distinction between the anterior and posterior aspects of the hippocampus, the former being involved in successful episodic encoding and the latter with retrieval of semantic information (Bernard et al., 2004).
Haxby and colleagues, using PET, found that facial encoding was associated with increased regional cerebral blood flow (rCBF) in the right MTL and left prefrontal cortex, whereas facial recognition was associated with increased rCBF in an extensive region of the right prefrontal cortex (Haxby et al., 1996). We employed a modified version of the paradigm developed by Haxby and colleagues. Since we were dealing with a patient with memory impairment, we thought that a shorter study would increase the probability that the subject would complete it with adequate collaboration. Therefore we choose a block-design fMRI study, which generally yields more robust activation compared with an event related study of the same duration (Friston et al., 1999).
In our fMRI study we found that in controls a right posterior hippocampal region is activated by both facial encoding and recognition, whereas a more anterior hippocampal region is greatly activated in recognition. The prefrontal cortex (greater on the right) was activated during all tasks, especially during recognition. The discrepancies in the reported studies may be due to the differences in techniques and paradigms employed.
The lateralization within MTL and the prefrontal cortex has been demonstrated to be dependent on the type of material being remembered (Kelley et al., 1998). The left frontal and medial temporal network is predominantly activated by verbal stimuli whereas a contralateral network is activated by non-verbal stimuli, such as faces. Therefore, we will only discuss activations of the right hemisphere.
The cortex of the fusiform gyrus has been demonstrated to be selectively specialized in facial perception (Haxby et al., 1994; Kanwisher et al., 1997); therefore, we focused our analysis on this region. In our controls and the WK subject, the bilateral activations of the cortex of the fusiform gyrus were comparable. In both controls and the WK subject the greater activation of the fusiform gyrus during the perception and recognition compared with the encoding task could be due either to the number of faces employed in each task (one in encoding, two in recognition, three in perception) or to the different attention levels (Wojciulik et al., 1998).
In the WK subject fMRI showed no activation of MTL during encoding, although the behavioural scores indicated that faces were seen, attention was good and judgement was accurate. Moreover, the normal activation of the cortex of the fusiform gyrus excludes haemodynamic impairment of the cortical–visual network as a possible cause of the lack of MTL activation. During recognition, the activations observed in the prefrontal regions were not significantly different from those in controls, except for the inferior frontal cortex. However behavioural data indicated impaired recognition. These findings suggest that MTL activation during encoding is crucial for subsequent recognition memory for faces and that activation of the dorsolateral prefrontal cortex may be more involved in orientation and/or attention at retrieval than in retrieval per se (Dobbins et al., 2003). The inferior frontal cortex is thought to be involved in the elaborative encoding of information into episodic memory, as well as in the maintenance of retrieved information (Fletcher and Henson, 2001; Dobbins et al., 2002). Therefore, its lack of activation seems to be more specifically connected with the defective encoding and unsuccessful retrieval in WK. Although the poor memory performance of the patient and the associated activation deficits in the MTL and the IFC are consistent with recent literature (Bunge et al., 2004), we cannot exclude some additional problems of the patient in retrieving and/or perceiving faces, not detected by our paradigm, that may influence the differences in BOLD signal between patient and controls.
No activation differences were found between subject and controls in the MPFC and the insula. As generally acknowledged, the MPFC is involved in motor behaviour (Picard and Strick, 2001), whereas activation of the insula is found in most tasks requiring visual attention (Woldorff et al., 2004). The lack of differences in these regions, which are involved in the demands of general processing, supports the specificity of the activation deficits found in regions related to memory formation in the WK subject.
Neuropathological studies in alcoholic Korsakoff syndrome have identified the dorsal medial thalamic nuclei as the anatomical site of pathology (Victor and Adams, 1989). Harding and colleagues, using operational criteria to identify alcoholics with and without Korsakoff syndrome (Caine et al., 1997), demonstrated that neuronal loss in the anterior thalamic nuclei is the best predictor of memory loss (Harding et al., 2000). This supports the view that the hippocampal–anterior thalamic axis is critical for episodic memory formation (Aggleton and Brown, 1999). Damage to this axis is responsible for the anterograde amnesia in Korsakoff syndrome, as originally proposed by Delay and Brion (1969). Pathology and MRI studies support the view that hippocampal structures are not damaged in WK syndrome (Squire et al., 1990; Harding et al., 1997; Colchester et al., 2001).
The patient we report is not an alcoholic. MRI showed lesions in the diencephalic structures matching the classical descriptions of Wernicke encephalopathy (Gallucci et al., 1990). MRI did not show signal abnormality or cortical atrophy in the MTL structures in either the 4-week or 5-month studies. There were also no signs of generalized cortical atrophy. Nonetheless, fMRI did not demonstrate hippocampal activation during encoding, the initial step of episodic memory formation. A PET study in two WK patients documented, besides the expected hypometabolism in the diencephalic grey matter, hypometabolism of MTL structures (Reed et al., 2003). This finding was interpreted as a secondary metabolic effect of the diencephalic pathology. Several experimental studies have demonstrated that anterior thalamic damage disrupts normal hippocampal activity (Jenkins et al., 2002; Savage et al., 2003).
In conclusion, in the patient we report here, MRI confirms that Korsakoff syndrome is related to diencephalic lesions and fMRI indicates that anterograde memory loss is due to a failure to encode the aspects of the incoming information at the hippocampal level. These findings support the view that the distinction between diencephalic and MTL amnesias is of limited value. The common denominator of anterograde amnesia is damage to an extended hippocampal system which seems to work as a unitary network (Aggleton and Brown, 1999).
This work was supported in part by a grant from the Italian Ministry of Research to the Center of Excellence on Aging of the University of Chieti.
References
Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis.
Bernard FA, Bullmore ET, Graham KS, Thompson SA, Hodges JR, Fletcher PC. The hippocampal region is involved in successful recognition of both remote and recent famous faces.
Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1.
Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval.
Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke's encephalopathy.
Clark VP, Keii K, Maisog M, Courtney S, Ungerleider LG, Haxby JV. Functional magnetic resonance imaging of human visual cortex during face matching: a comparison with positron emission tomography.
Colchester A, Kingsley D, Larserson D, Kendall B, Bello F, Rush C, et al. Structural MRI volumetric analysis in patients with organic amnesia: Methods of comparative findings across diagnostic groups.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages.
Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory.
Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition.
Eldridge LL, Knowlton BJ, Furmanski CS, Bookeimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval.
Fletcher PC, Henson RN. Frontal lobe and human memory insights from functional neuroimaging.
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach.
Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AN. Stochastic design in event-related fMRI.
Gallucci M, Bozzao A, Splendiani A, Masciocchi C, Passariello R. Wernicke encephalopathy: MR findings in five patients.
Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study.
Harding AJ, Wong A, Svoboda M, Kril JJ, Halliday GM. Chronic alcohol consumption does not cause hippocampal neuron loss in humans.
Harding AJ, Halliday G, Caine D, Kril JJ. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia.
Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and location.
Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain.
Jenkins TA, Dias R, Amin E, Brown MW, Aggleton JP. Fos imaging reveals that lesions in the anterior thalamic nuclei produce widespread limbic hypoactivity in rats.
Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception.
Kelley WM, Miezin F, McDermott K, Buckner RL, Raichle ME, Cohen NJ, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and non verbal memory encoding.
Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model.
Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, et al. FDG-PET findings in the Wernicke–Korsakoff syndrome.
Savage LM, Chang O, Gold PE. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing.
Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval.
Schacter DL, Curran T, Reiman EM, Chen K, Bandy DJ, Frost JT. Medial temporal lobe activation during episodic encoding and retrieval: a PET study.
Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans.
Squire LR, Amaral DG, Press GA. Magnetic resonance imaging of the hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia.
Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one?
Victor M, Adams RD, Collins GH. The Wernicke–Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2nd edn. Philadelphia: FA Davies;
Wojciulik E, Kanwisher N, Driver J. Covert visual attention modulates face-specific activity in the human fusiform gyrus: fMRI study.
Author notes
1Department of Clinical Sciences and Bio-imaging, University ‘G. d'Annunzio’, 2ITAB Institute for Advanced Biomedical Technologies, 3Department of Oncology and Neurosciences and Aging Research Center, Ce.S.I. University ‘G. d'Annunzio’ Foundation Chieti-Pescara, Chieti-Pescara, Italy and 4Collaborative Antwerp Psychiatric Research Institute, CAPRI, University of Antwerp, Antwerp, Belgium