-
PDF
- Split View
-
Views
-
Cite
Cite
Rod C. Scott, Martin D. King, David G. Gadian, Brian G. R. Neville, Alan Connelly, Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study, Brain, Volume 126, Issue 11, November 2003, Pages 2551–2557, https://doi.org/10.1093/brain/awg262
Close - Share Icon Share
Abstract
Mesial temporal sclerosis (MTS) is the most common lesion in patients who require epilepsy surgery, and ∼50% of patients with MTS have a history of prolonged febrile convulsion (PFC) in childhood. The latter led to the hypothesis that convulsive status epilepticus, including PFC, can cause MTS. Our recently published data on children investigated within 5 days of a PFC showed that children investigated by MRI within 48 h of a PFC had large hippocampal volumes and prolongation of T2 relaxation time. Patients investigated >48 h from a PFC had large hippocampal volumes and normal T2 relaxation time. These data are strongly suggestive of hippocampal oedema that is resolving within 5 days of a PFC, but do not exclude the possibility of a pre‐existing hippocampal lesion. Fourteen children from the original study had follow‐up investigations carried out 4–8 months after the acute investigations. Of the 14 patients, four have had further seizures. Two had short febrile convulsions, one had PFC and one had non‐febrile seizures. There was a significant reduction in hippocampal volume and T2 relaxation time between the first and second investigations, and there is now no difference in hippocampal volume or T2 relaxation time in patients compared with a control population. Moreover, there is a significant increase in hippocampal volume asymmetry in patients at follow‐up when compared with initial data. Five out of 14 patients had asymmetry outside the 95th percentile for control subjects and, of these, three had one hippocampal volume outside the lower 95% prediction limit for control subjects. A reduction in hippocampal volume or T2 relaxation time, into or below the normal range between the first and second scans, indicates that the earlier findings are temporary and are strongly suggestive of hippocampal oedema as the abnormality in the initial investigations. The change in hippocampal symmetry in the patient group is consistent with injury and neuronal loss associated with a PFC, especially in the three individuals who now have a single small hippocampus. However, as there is no T2 relaxation time abnormality, the hippocampi do not meet the criteria for MTS. There may be a lag period of several years between a PFC and the onset of epilepsy, and therefore some of these patients may be developing MTS. Alternatively, the asymmetry could represent return (post‐acute oedema) to a pre‐existing hippocampal abnormality similar to that identified in family members of patients with MTS and a history of PFC.
Introduction
Prolonged febrile convulsion (PFC), defined as a seizure lasting at least 30 min, which is associated with a fever not of CNS origin, in a neurologically normal child between 6 months and 5 years of age, is the most frequent type of convulsive status epilepticus in children under the age of 5 years and, although the outcome is largely perceived to be excellent, there is a long‐standing debate on whether PFC can cause mesial temporal sclerosis (MTS) (Cavanagh and Meyer, 1956). MTS is the most common lesion identified in patients who require surgery for the treatment of epilepsy and, of the patients who have resective surgery in the treatment of MTS, only ∼70% will be expected to become seizure free. Up to 50% of patients with MTS have a history of PFC in childhood, suggesting that there may be an association, but studies that enrol patients with MTS, rather than patients with PFC, cannot confirm a causative relationship. Characterization of the relationships between PFC and MTS is important as this may lead to strategies to prevent MTS, and may be an effective way of reducing the numbers of patients requiring epilepsy surgery. Thus, prospective studies of children who have had a PFC are required. Prospective evidence for a causative association between PFC and MTS in humans is still in the early stages.
A causative association between status epilepticus and hippocampal injury has been shown in animal models (Ben Ari, 1985; Cavalheiro, 1995; Fountain and Lothman, 1995), but it remains uncertain whether a similar pathophysiological sequence can occur in humans. There are some human MRI studies that attempt to address this. Progressive increases in hippocampal T2 signal hyperintensity have been identified in a child with status epilepticus lasting 6 weeks. The child subsequently died and, at post‐mortem, neuronal necrosis was identified bilaterally in CA1, CA3 and in the hilus subregions of the hippocampus (Stafstrom et al., 1996). Hippocampal abnormalities have also been identified in patients with less severe episodes of status epilepticus. T2 signal hyperintensity has been seen following status epilepticus, and two of the five patients in that study developed MTS (Tien and Felsberg, 1995). Hippocampal volume asymmetry, consistent with unilateral hippocampal oedema, has been identified in four out of 15 children with lateralized febrile convulsions, and two of these children then developed hippocampal atrophy (VanLandingham et al., 1998). In that study, the methodology did not allow the identification of bilateral abnormalities, and therefore it remains uncertain whether any children with generalized convulsions had evidence of bilateral hippocampal oedema.
In animal models, the hippocampal injury occurs in the context of a previously normal hippocampus. This may not be the case in humans who may have subtle hippocampal abnormalities prior to the occurrence of a PFC. Previous reports have shown hippocampal asymmetries on hippocampal volumetry that have been suggested to represent pre‐existing lesions such as hippocampal dysgenesis. Such asymmetry has been identified in siblings of patients with MTS, many of whom had never had a seizure and none had had an episode of status epilepticus (Fernandez et al., 1998). Patients who underwent MRI investigations a median of 11 days from the time of an early complicated childhood convulsion were shown to have asymmetry of hippocampal volume but not of T2 relaxation time (Grunewald et al., 2001). The authors suggested that this asymmetry was not consistent with hippocampal oedema and was more likely to be due to a pre‐existing hippocampal abnormality. Alternatively, those findings could represent hippocampal injury and neuronal loss at the time of the PFC.
Our recently published data (Scott et al., 2002) suggest that children investigated with MRI within 48 h of a generalized PFC had quantitative evidence of large hippocampi and prolongation of T2 relaxation time. Patients investigated between 48 h and 5 days from a PFC had large hippocampal volumes and normal T2 relaxation time. These hippocampal abnormalities were not asymmetrical in either of the groups. This combination of findings is consistent with hippocampal oedema, the T2 component of which is resolving within a 5‐day period. However, these data do not exclude the possibility of a pre‐existing lesion such as hippocampal dysgenesis (Grunewald, 2002). It is of importance to clarify the pathological basis of our previously described abnormalities, as confirmation of acute hippocampal injury is a necessary first step for development of appropriate neuroprotective strategies.
Thus, the aims of the current MRI study are: (i) To determine whether hippocampal volumes and T2 relaxation times have changed 4–8 months after a PFC compared with hippocampal volumes and T2 relaxation times acquired within 5 days of a PFC. (ii) To determine whether hippocampal volume and T2 relaxation time acquired 4–8 months after PFC are different from hippocampal volume and T2 relaxation time in control subjects. (iii) To determine whether there has been a change in the degree of asymmetry in hippocampal volume or T2 relaxation time, 4–8 months after a PFC, compared with data acquired within 5 days of a PFC.
Methods
Patients who underwent MRI investigations within 5 days of a PFC were requested to have follow‐up MRI investigations 4–8 months following the PFC. This study was approved by the Great Ormond Street Hospital and Institute of Child Health Local Research Ethics Committee. Consent for enrolment into the study, comprising early and follow‐up investigations, was given by a parent of the child. The patients were assessed clinically for any evidence of seizures, developmental delay or behavioural abnormalities. They then underwent the same MRI investigations as in the original study (Scott et al., 2002), again under sedation. The investigations included T2‐weighted axial and coronal (perpendicular to the long axis of the hippocampus) images, a three‐dimensional fast low angle shot (3D FLASH) data set for visual assessment and for hippocampal volumetry, and T2 relaxometry, as reported previously (Scott et al., 2002). The investigations were carried out on a Siemens 1.5 T Vision whole body system.
The 3D FLASH parameters were: repetition time, TR = 16.8 ms; echo time, TE = 5.7 ms; number of excitations, NEX = 1; flip angle = 21°; matrix size 200 × 256; and 160 partitions in the third dimension with a partition thickness of 1 mm, as reported previously (Scott et al., 2002). The data were reformatted into contiguous axial and coronal images. T2‐weighted images (TE 90 ms/TR 4600 ms) were obtained in the coronal plane at 90° to the long axis of the hippocampus and also in the tilted axial plane orthogonal to this. The images were reviewed by two neuroradiologists, with emphasis on identification of abnormalities in the hippocampi and temporal lobes. However, abnormalities outside these regions were also sought.
Hippocampal volumetry was performed using the images obtained using the FLASH sequence. The data set was transferred to a PC and analysed using MEDx, version 3.3 (Sensor Systems Inc., VA). The data were reformatted in the tilted coronal plane perpendicular to the long axis of the hippocampus. Each hippocampal slice, 1 mm in thickness, was then measured. A three‐dimensional contour was derived from which the hippocampal volume was calculated. For the measurement of intracranial volume, the data set was reformatted in the sagittal plane. The first slice was randomly selected, following which every fifth slice was systematically measured using landmarks previously described (Van Paesschen et al., 1997). A three‐dimensional contour was derived and the volume calculated.
Hippocampal T2 maps were calculated from 16 images obtained with echo times ranging from 22 to 262 ms using a modified Carr–Purcell–Meiboom–Gill sequence, as described previously (Scott et al., 2001). The map is calculated by fitting a single exponential to the signal from each of the 16 spin echoes. The thickness of the selected plane was 8 mm and its orientation was in a tilted coronal plane along the anterior border of the brainstem perpendicular to and at the level of the body of the hippocampus. The T2 relaxation time in milliseconds is displayed as pixel intensity on a map and the hippocampal T2 relaxation time was read using the largest possible region of interest placed within the hippocampus, avoiding boundaries where partial volume effects with CSF may occur.
Statistical analysis
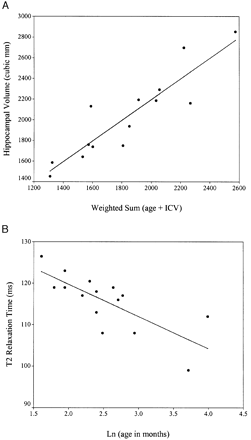
SPSS for Windows (version 10) (SPSS Inc., Chicago, IL) was used for the analysis. To address whether hippocampal volume and T2 relaxation time are different at 4–8 months follow‐up compared with such data obtained within 5 days of a PFC, a paired sample t test was carried out after adjustment for age and/or intracranial volume. For the adjustment, the predicted hippocampal volume or T2 relaxation time for control subjects was obtained for each patient age and/or intracranial volume using linear regression (see Fig. 1). In order to adjust the raw patient measure for the effect of age and/or intracranial volume, the predicted hippocampal volume or T2 relaxation time was subtracted from the observed hippocampal volume or T2 relaxation time. This difference is defined as the adjusted hippocampal volume or the adjusted T2 relaxation time as appropriate. The adjusted hippocampal volume was used for the paired sample t test. For the adjusted T2 relaxation time data, the paired sample t test was carried out as a regression calculation to take account of whether the patient’s initial scan was performed within 48 h of a PFC, or whether they were scanned >48 h but <5 days from the PFC (Scott et al., 2002).
Multiple linear regression and general linear modelling were carried out to identify differences in T2 relaxation time and hippocampal volume between patient and control groups after adjustment for age and/or intracranial volume, as described previously (Scott et al., 2002). Variables investigated were age and intracranial volume. Interaction terms were investigated and found to be unnecessary. A log transformation was used on the T2 relaxometry data and revealed the best distribution of residuals. The mean of right and left values was used in the regression analysis. A hippocampal value that falls outside the 95% prediction limits for control subjects is considered abnormal.
Hippocampal side‐to‐side asymmetry was characterized by calculation of an asymmetry index (AI) defined as
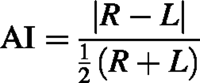
where R is the right‐sided hippocampal T2 relaxation time or hippocampal volume, and L is the left‐sided hippocampal T2 relaxation time or hippocampal volume. Differences in AI between patient and control groups were investigated using a two‐sample Kolmogorov–Smirnov test. To determine whether there is a difference between patient and control populations in the tails of the distributions, the Moses extreme reactions test was carried out. On an individual basis, an abnormal AI was defined as one that is greater than the 95th percentile for control subjects. A paired sample t test was carried out to determine whether the AI had changed between the first and second investigations in the patient population.
Results
Fourteen patients were enrolled into the study and had follow‐up MRI investigations at a median age of 22 months (range, 14–31 months). The PFC was observed to be generalized in all cases, although, as the onset was usually not witnessed, it is possible that some had a focal onset. The second scan was carried out a mean of 5.5 months (range, 4–8 months) after the acute scan. Of the 14 patients, four had further seizures; two had a short febrile convulsion, one had a PFC and one had non‐febrile seizures. This last child has started to show evidence of developmental delay at the age of 2 years. There is no evidence of a focal abnormality in her inter‐ictal EEG. An ictal EEG has not yet been carried out. None of the other children in the study have clinical evidence of developmental stasis or regression, and none have had an abnormal change in behaviour following the PFC.
Fourteen control subjects with a median age of 12 months (range, 5–65 months) had hippocampal volumetry and 15 control subjects with a median age of 12 months (range, 5–65 months) had T2 relaxometry. As it is not ethical to sedate young children in order to obtain control data, a reference population was obtained from children undergoing scans for other reasons, e.g. skin lesions suggestive of neurocutaneous disorders, those undergoing staging of malignancies, and those with eye or ear abnormalities. These children were all neurologically normal and had normal MRI on visual assessment.
On visual assessment of the follow‐up MRI scans, two patients had an abnormality identified, which had been present on the earlier scans in both cases. One patient had an arachnoid cyst, and one had evidence of hippocampal asymmetry.
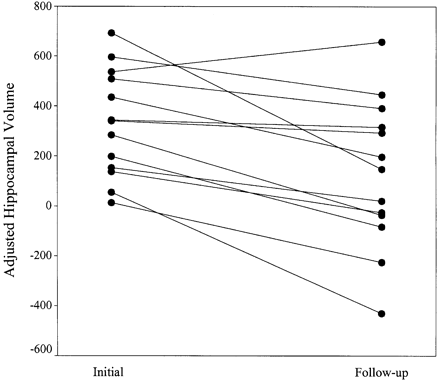
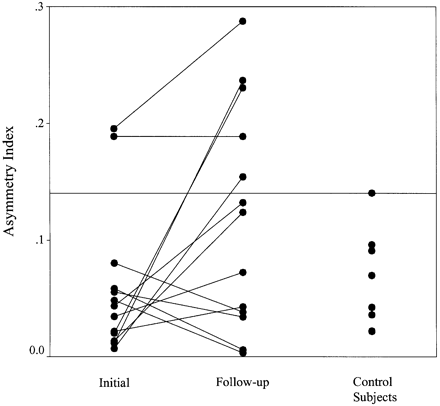
Hippocampal volume was a mean of 202 mm3 [95% confidence interval (CI), 92–311 mm3] smaller in the follow‐up scans compared with the initial hippocampal volumes after adjustment for age and intracranial volume (P = 0.002, Fig. 2). On group analysis, there was no significant difference in hippocampal volume between patient and control populations [–12 mm3, 95% confidence limit (CL) –224 to 200 mm3, P = 0.90)], after adjustment for age and intracranial volume. In the patients, there was also evidence for an increase in asymmetry of hippocampal volume when the follow‐up hippocampal AI was compared with the initial hippocampal AI (P = 0.044, paired t test; Fig. 3), although there was no evidence for hippocampal volume asymmetry in the patient group compared with control subjects (P = 0.15, two‐sample Kolmogorov–Smirnov). On the Moses extreme reactions test, there was a significant difference in the tails of the distribution of AI for the patients compared with the control subjects (P = 0.006). Five of the 14 patients had an abnormal AI (i.e. greater than the 95th percentile for control subjects, Fig. 3). After adjustment of the individual hippocampal volumes for age and intracranial volume, three of these patients had one hippocampus outside the lower 95% prediction limit. Two patients with abnormal AI had both hippocampi within the 95% prediction limits. In one, the smaller hippocampus was close to the lower prediction limit and in the other the volumes lay symmetrically within the 95% prediction limits. Two patients had abnormal AI at the time of the initial MRI investigations and both are included in the five with abnormal AI at follow‐up. One of these is the patient in whom the asymmetry was noted on visual assessment. There was no individual who, at the early time point, had a hippocampal volume below the lower 95% prediction interval.
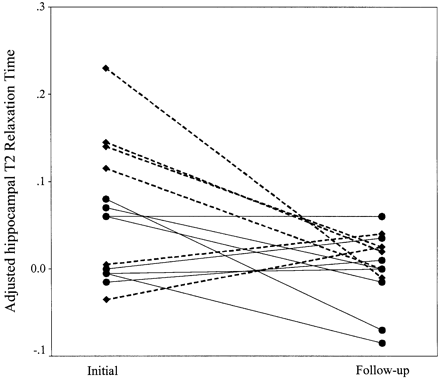
T2 relaxation time was shorter at follow‐up compared with data acquired within 5 days of a PFC after adjustment for age, and adjustment for whether the patient was investigated within 48 h or between 48 h and 5 days from the PFC (P = 0.018, Fig. 4). As the T2 relaxation time data were transformed to normalize residuals, means and confidence intervals are not reported. There is no group evidence for differences in side‐to‐side asymmetry of T2 relaxation time in patient and control populations (P = 0.73, two‐sample Kolmogorov–Smirnov; P = 0.27, Moses extreme reactions test). One patient had an abnormal AI. Neither value lies outside the 95% prediction limits, although the lower value is close to the lower 95% prediction limit. None of the patients with an abnormal AI for hippocampal volume had an abnormality of the AI for T2 relaxation time. On group analysis, there was no difference in T2 relaxation time in patients compared with control subjects (P = 0.99). There is no difference in AI between the first and the second scans in the patient group (P = 0.86, paired sample t test).
Discussion
There continues to be debate as to whether PFC has a causative association with later MTS and associated temporal lobe epilepsy. Characterization of such an association would provide a framework for the development of neuroprotective strategies that may prevent the development of MTS, which in turn may reduce the number of patients requiring epilepsy surgery. In starting to address this association, we previously have reported MRI data suggesting that hippocampal oedema can be identified within 2 days of a PFC, and that it may be resolving within 5 days of the acute event (Scott et al., 2002). This conclusion was based on the hippocampi being found to be bilaterally large and the T2 relaxation time to be prolonged if patients were investigated within 48 h of a PFC, but normal if patients were investigated between 48 h and 5 days from the PFC. It has been suggested that the large hippocampi could represent a pre‐existing hippocampal abnormality such as hippocampal dysgenesis (Grunewald, 2002), although such a suggestion does not appear consistent with the observed acute changes in our T2 relaxometry data.
To clarify the implications of the acute findings, it is important to establish the longer term evolution of the hippocampal measures. The results from the current follow‐up study of the patients investigated acutely show that hippocampal volume and T2 relaxation time had decreased between the early and the follow‐up investigations, and that hippocampal volume and T2 relaxation time acquired at follow‐up are not different from those in a control population. These results provide strong support for the suggestion that patients with PFC develop acute hippocampal oedema that resolves within several months of the acute event. They are consistent with a pre‐existing enlargement of hippocampi in the group as a whole only if significant neuronal injury (leading to neuronal loss) occurred at the time of a PFC in the majority of cases. However, the observed reduction in T2 relaxation time between the two scans makes this interpretation less likely. Nevertheless, each of these interpretations of our data requires the conclusion that PFC is associated with an acute hippocampal insult, and therefore contributes to the hypothesis that PFC can cause hippocampal neuronal injury.
The other main finding in the current study provides information on the evolution of the acute hippocampal injury. There is increasing hippocampal asymmetry between the early and follow‐up scans. It has been shown previously that MTS in patients with a history of PFC has markedly greater hippocampal volume asymmetry compared with MTS in patients with no history of PFC (Scott et al., 2001). In contrast to these findings in patients with established MTS, our previous results suggest that the acute injury associated with PFC is largely bilateral (Scott et al., 2002). Given that only a small minority of the patients with acute injury are likely to develop MTS, it is possible that such MTS results from an insult to a selectively vulnerable hippocampus, leading to particularly marked asymmetry. None of the patients, including the one with non‐febrile seizures, have hippocampal abnormalities that meet the MRI criteria for MTS. However, in humans with MTS associated with PFC, there is a lag period of up to several years between the PFC and the development of non‐febrile seizures (Annegers et al., 1987). Since the temporal evolution of hippocampal oedema to MTS remains uncertain, it is possible that the findings in this study are consistent with ‘developing’ MTS. This may be the case in the three individuals who had an abnormal degree of hippocampal asymmetry in conjunction with one hippocampus that was small with respect to a control population. The asymmetry is consistent with data reported previously (Grunewald et al., 2001). The functional consequence of such subtle hippocampal injury is uncertain. However, severe neuropsychological deficits have been recognized in patients who have had severe hippocampal injury associated with status epilepticus (Oxbury et al., 1997). Therefore, it will be important to determine whether less severe neuropsychological deficits can be recognized in our cohort of patients but, given their age, such testing would be premature at present.
An alternative explanation for the observed hippocampal asymmetry is that it is pre‐existing, i.e. that in our patients the hippocampi were asymmetrical prior to the PFC, became symmetrical in the presence of oedema and then returned to their pre‐PFC condition. Direct evidence of the presence of a hippocampal abnormality prior to a PFC is unlikely to be obtained systematically; children who have a PFC would not usually have had appropriate MRI investigations prior to the PFC. Nevertheless, there is some indirect evidence that there may be prior hippocampal abnormalities in some cases. For example, such asymmetry has been identified in family members of patients with MTS and a history of PFC, despite these family members never having had status epilepticus (Fernandez et al., 1998). In contrast, however, in a series of three sets of twins in which one twin in each set had MTS associated with PFC, no hippocampal asymmetry was identified in any twin who had not had a PFC (Jackson et al., 1998). Hippocampal volume asymmetry, similar to that identified in the current study, has also been identified within a median of 11 days from a complicated early childhood convulsion (Grunewald et al., 2001). The authors suggested that the short time from an acute event to the identification of hippocampal asymmetry supported the view that the asymmetry was pre‐existing, although neuronal injury and loss in response to PFC cannot be excluded in this case.
A combination of these interpretations is of course also possible. There may be a subtle hippocampal abnormality present prior to a PFC. The PFC then results in a spectrum of hippocampal injury that may alter the degree of asymmetry identified prior to the PFC, but only leads to the clinical and pathological syndrome of MTS in a small number of selectively vulnerable patients.
Acknowledgements
We wish to thank Tim Cox and Kling Chong who reviewed the MRI films, and the paediatricians who referred patients to be enrolled in the study. Research at the Institute of Child Health and Great Ormond Street Hospital for Children NHS Trust benefits from R&D funding received from the NHS Executive. The study was also supported financially by the Wellcome Trust.
Fig. 1 (A) Hippocampal volume in control subjects with the least squares regression line used in the adjustment of patient hippocampal volume (see Statistical analysis section). The coefficients for the weighted sum were obtained using linear regression. ICV = intracranial volume. (B) T2 relaxation times in control subjects showing the least squares regression line used in the adjustment of patient T2 relaxation time (see statistical analysis section)
Fig. 2 Adjusted hippocampal volume (adjustment defined as the difference between the predicted hippocampal volume for a given age and intracranial volume in control subjects, and the patient hippocampal volume; see Statistical analysis section) in patients investigated within 5 days of a prolonged febrile convulsion (initial) and 4–8 months later (follow‐up). Each pair of dots joined by a line represents a single patient.
Fig. 3 Asymmetry index (see Statistical analysis section for calculation of AI) in patients investigated within 5 days of a prolonged febrile convulsion (initial), patients investigated 4–8 months later (follow‐up) and control subjects. Each pair of dots joined by a line represents a single patient. The reference line is at the 95th percentile for control subjects.
Fig. 4 Adjusted T2 relaxation time (adjustment defined as the difference between the predicted T2 relaxation time for a given age in control subjects, and the patient T2 relaxation time; see Statistical analysis section) in patients investigated within 2 days of a prolonged febrile convulsion (initial; diamonds and dotted line), between 48 h and 5 days (initial; dots and solid lines) and 4–8 months later (follow‐up). Each pair of dots joined by a line represents a single patient.
References
Annegers JF, Hauser WA, Shirts SB, Kurland LT. Factors prognostic of unprovoked seizures after febrile convulsions.
Ben Ari Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy.
Cavanagh JB, Meyer A. Aetiological aspects of Ammon’s horn sclerosis associated with temporal lobe epilepsy.
Fernandez G, Effenberger O, Vinz B, Steinlein O, Elger CE, Dohring W, et al. Hippocampal malformation as a cause of familial febrile convulsions and subsequent hippocampal sclerosis.
Fountain NB, Lothman EW. Pathophysiology of status epilepticus.
Grunewald R. Childhood seizures and their consequences for the hippocampus.
Grunewald RA, Farrow T, Vaughan P, Rittey CD, Mundy J. A magnetic resonance study of complicated early childhood convulsion.
Jackson GD, McIntosh AM, Briellmann RS, Berkovic SF. Hippocampal sclerosis studied in identical twins.
Oxbury S, Oxbury J, Renowden S, Squier W, Carpenter K. Severe amnesia: an usual late complication after temporal lobectomy.
Scott RC, Gadian DG, Cross JH, Wood SJ, Neville BG, Connelly A. Quantitative magnetic resonance characterization of mesial temporal sclerosis in childhood.
Scott RC, Gadian DG, King MD, Chong WK, Cox TC, Neville BG, et al. Magnetic resonance imaging findings within 5 days of status epilepticus in childhood.
Stafstrom CE, Tien RD, Montine TJ, Boustany R. Refractory status epilepticus associated with progressive magnetic imaging signal change and hippocampal neuronal loss.
Tien RD, Felsberg GJ. The hippocampus in status epilepticus: demonstration of signal intensity and morphologic changes with sequential fast spin‐echo MR imaging.
Van Paesschen W, Revesz T, Duncan JS, King MD, Connelly A. Quantitative neuropathology and quantitative magnetic resonance imaging of the hippocampus in temporal lobe epilepsy.