-
PDF
- Split View
-
Views
-
Cite
Cite
E. L. PLOSCHUK, G. A. SLAFER, D. A. RAVETTA, Reproductive Allocation of Biomass and Nitrogen in Annual and Perennial Lesquerella Crops, Annals of Botany, Volume 96, Issue 1, July 2005, Pages 127–135, https://doi.org/10.1093/aob/mci158
Close - Share Icon Share
Abstract
• Background and Aims The use of perennial crops could contribute to increase agricultural sustainability. However, almost all of the major grain crops are herbaceous annuals and opportunities to replace them with more long-lived perennials have been poorly explored. This follows the presumption that the perennial life cycle is associated with a lower potential yield, due to a reduced allocation of biomass to grains. The hypothesis was tested that allocation to perpetuation organs in the perennial L. mendocina would not be directly related to a lower allocation to seeds.
• Methods Two field experiments were carried on with the annual Lesquerella fendleri and the iteroparous perennial L. mendocina, two promising oil-seed crops for low-productivity environments, subjected to different water and nitrogen availability.
• Key Results Seed biomass allocation was similar for both species, and unresponsive to water and nitrogen availability. Greater root and vegetative shoot allocation in the perennial was counterbalanced by a lower allocation to other reproductive structures compared with the annual Lesquerella. Allometric relationships revealed that allocation differences between the annual and the perennial increased linearly with plant size. The general allocation patterns for nitrogen did not differ from those of biomass. However, nitrogen concentrations were higher in the vegetative shoot and root of L. mendocina than of L. fendleri but remained stable in seeds of both species.
• Conclusions It is concluded that vegetative organs are more hierarchically important sinks in L. mendocina than in the annual L. fendleri, but without disadvantages in seed hierarchy.
INTRODUCTION
The use of perennial crops could contribute to increasing agricultural sustainability (Piper, 1998; Glover, 2003) and yield stability in marginal environments (Ploschuk et al., 2001b). Germplasm and crop technologies for the cultivation of at least 20 perennial grain species are available (Suneson et al., 1963; Srinivasan and Brewbaker, 1999; Scheinost et al., 2001; Cox et al., 2002; Weik et al., 2002; DeHaan et al., 2003; Sacks et al., 2003). The belief that a perennial life cycle reduces allocation of biomass to grains and yield potential may explain why there have been few attempts to introduce perennials to marginal environments in which traditional crops yield poorly (Moffat, 1996). It is also believed that to select for higher yields would shorten the plant's lifespan (Ceccarelli and Grando, 1996; Pimm, 1997; Cox et al., 2002).
Seed yield and allocation to reproduction are actually lower in several perennial weeds compared with a closely related annual crop (Benech Arnold et al., 1992). However, the comparison may be ill suited for the objective of searching for alternative perennial grain crops, because annual crops were selected for yield while perennial weeds are not necessarily selected for seed productivity. On the other hand, several studies have shown that yields can be potentially high in perennial crops (Moffat, 1996; Pimm, 1997; DeHaan et al., 2003). Furthermore, it has been shown that selection for increased grain yield does not necessarily come at the expense of other vegetative structures such as roots and perpetuation and propagation organs. In the case of Sorghum, Piper and Kulakow (1995) obtained perennial hybrids and backcrosses of both S. halepense and S. bicolor with non-aggressive propagation rhizomes and found that these intermediate forms, though perennial, yielded similar to the annual parent, although with a lower reproductive allocation. These studies suggest that, at least in some cases, the combination of high seed yield and perennial habit may be possible. Still, the knowledge about annual versus perennial allocation is scarce and information is missing on the reproductive responses of potential perennial grain crops. This is of particular importance in environments characterized by a relatively low availability of resources in which cultivation technology, associated with perennial cycles, may contribute to reduce soil degradation and erosion and increase sustainability.
There is recent evidence that new promising perennial crops can perform well in semi-arid environments which are marginal for the cultivation of more traditional crops (Cox et al., 2002). Potential candidates include species of the genus Lesquerella, with approx. 100 species with annual, biennial and perennial habits (Rollins and Shaw, 1973). Lesquerella is considered a promising industrial crop because it contains hydroxy fatty acids in its seeds (Dierig et al., 1993, 1996). Its present degree of domestication is very incipient. Lesquerella fendleri has been chosen in the USA as the main candidate for domestication because of its high seed and oil yield, low seed dormancy, and low fruit dehiscence (Roetheli et al., 1991). Although the natural populations of this species have been described as both short-lived perennials (Rollins and Shaw, 1973) and annuals (Barclay et al., 1962; Gentry and Barclay, 1962), the cultivated type behaves strictly as an annual (Roseberg, 1993; Ravetta and Soriano, 1998).
Annual crops from L. fendleri and L. angustifolia are being developed under irrigation for Arizona, USA (Dierig et al., 1993) and Patagonia, Argentina (Ploschuk et al., 2003), respectively. However, the iteroparous perennial L. mendocina native to Argentina (Cabrera, 1967; Correa, 1984) can reach similar yields to those of L. fendleri during its first cycle of flowering (Ploschuk et al., 2001a, 2003). Lesquerella mendocina perennial rosettes develop lateral spicate inflorescences every year, including the first year of establishment. On the other hand, in L. fendleri, all stems become reproductive at flowering, including the apical bud (Ploschuk et al., 2001b, 2003; Windauer, 2002), resulting in the death of the plant after the fruit matures.
Several morphological, physiological and phenological traits that could contribute to increase yield stability (across sites and years) have been found in L. mendocina (Ploschuk et al., 2001b), making it a promising perennial alternative to the annual L. fendleri. An initial comparison revealed that L. mendocina has a lower reproductive effort than the annual, but this difference is not associated with yield (Ploschuk et al., 2001a). It could be then hypothesized that allocation to perpetuation organs in the perennial L. mendocina is not directly related to a lower allocation to reproduction compared with the annual L. fendleri. To test this hypothesis, allocation to vegetative and reproductive structures was compared in these two closely related crop species.
Reproductive allocation is usually measured as the ratio between seed and total biomass (Bazzaz and Ackerly, 1992). However, allometric relationships between reproductive and vegetative shoots are considered an appropriate tool to investigate the patterns of reproductive allocation in annual crops (Sadras et al., 1997). Although the definition of allometric coefficients involves logarithmic relationships (Pearsall, 1927), other common methods to describe allocation use simple and meaningful models based on the comparison of slopes from linear relationships between reproductive and vegetative shoots or total biomass (Gardner and Gardner, 1983; Samson and Werk, 1986; Sinclair et al., 1990; Prihar and Stewart, 1991; Moot et al., 1997). Vega et al. (2000), for instance, analysed the relationship between grain yield and vegetative shoot biomass for individual plants of several traditional annual crops, generating a wide range of availability of resources by manipulating plant density. In the work reported in the present paper, the high variability in plant size within Lesquerella species (Ploschuk et al., 2001b) is employed to analyse vegetative and reproductive allocations estimated using both ratios and allometric relationships.
MATERIALS AND METHODS
General experimental design
Two experiments were carried out outdoors, during the winter and spring of 2000 (expt 1) and 2001 (expt 2) at Facultad de Agronomía, Universidad de Buenos Aires, Argentina (34°35′S, 58°29′W). Seeds of Lesquerella fendleri and L. mendocina were initially sown in germination-trays and maintained in a glasshouse (day mean temperature 25°C) until transplantation to the field. The sowing date (3 June for both experiments) was chosen to ensure the synchronization of the flowering phase between the two species (Windauer, 2002; Windauer et al., 2004). Seedlings were transplanted to the field 30 d after sowing (DAS) in both experiments. Experimental units consisted of 1·5 × 2·0 × 0·6 m plots edged with wooden boards, filled with 1000 dm3 of a mixed soil isolated from the ground with polyethylene sheeting. Plant density was 30 plants m−2, 0·2 m between plants, with rows 0·3 m apart. Plots were placed under a rain shelter made from transparent 200-µm polyethylene sheeting. Weeds were removed manually, and Cipermetrine (20 cm3 hL−1) and Benomyl (150 g hL−1) were applied at monthly intervals to prevent damage from insects and fungi, respectively.
Treatments
In expt 1, water stress (−W), low nitrogen availability (−N) and control (C) treatments were established for both species, arranged in a completely randomized design with three replicates (plots) per treatment and species, for a total of 18 plots. Controls received an equivalent to 50 kg ha−1 of P and 25 kg ha−1 of K at 47 DAS, 150 kg ha−1 of N distributed between rows at 48, 61 and 76 DAS, and were watered to field capacity at daily intervals. Water-stressed plots received the same fertilization as the control but water was withheld from 77 DAS (prior to visible flower-bud stage) to 134 DAS (Fig. 1A). Nitrogen-stressed plots received the same amount of water, P and K as the control, but were not fertilized with N.
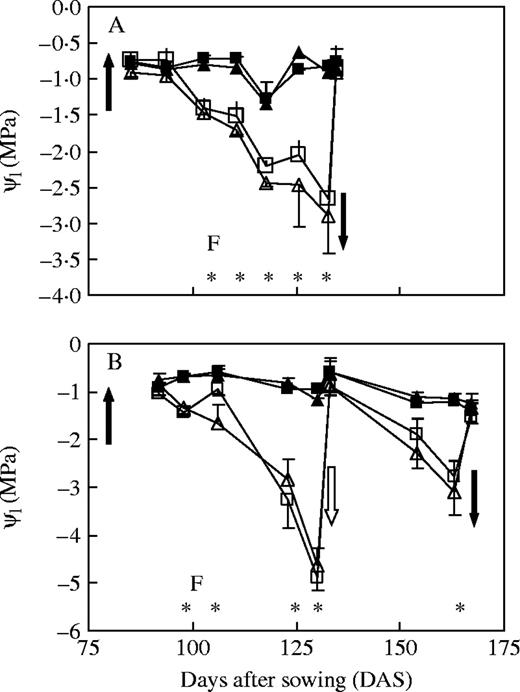
Leaf water potential (ψl) for L. fendleri (squares) and L. mendocina (triangles) plants for controls (closed symbols) and water-stressed (open symbols) treatments, measured at dusk in expts 1 (A) and 2 (B). Closed arrows indicate the onset and end of the period of water restriction. In expt 2 (B) there was an interruption of the stress by a single irrigation (open arrow). The letter ‘F’ stands for the onset of flowering. Vertical lines indicate standard errors and are shown only when larger than symbols. Asterisks denote significant differences between treatments for each date (P < 0·05).
Experiment 2 was carried out with the aim of increasing the intensity and duration of water stress, and only −W and C treatments were applied in an experimental design similar to that of expt 1, but with four replicates per treatment. Nutrient conditions were similar to those of C and −W treatments from expt 1. In this experiment, treatments were set for L. fendleri (LF) and L. mendocina plots with plants sown in that same year (LM1). Water-stress treatments were arranged by withholding water at 87 DAS (prior to visible floral bud stage) until 167 DAS, with a transitory interruption by a single irrigation at 132 DAS (Fig. 1B). Seed production was also analysed in a second growing season (2002) for the perennial species (LM2). During this second cycle of L. mendocina, both C and −W experimental units were maintained under irrigation (then any effect of −W on LM2 was due to effects of the water stress in the previous season).
In both experiments, leaf water potential (ψl) measurements were made at weekly intervals with a Scholander pressure chamber (Scholander et al., 1965). Once significant differences between −W and C treatments were detected, −W crops were irrigated with the equivalent of 20 % of the daily potential evapotranspiration (Priestley and Taylor, 1972), in order to avoid an extreme drop in water potential.
Sampling and analyses
In both experiments, L. fendleri (LF) and L. mendocina (LM1) plants were harvested after physiological maturity, i.e. when fruits were dehiscent at touch and contain reddish seeds. In expt 1, four or five plants per experimental unit were harvested, while sample intensity was increased to ten plants per experimental unit in expt 2. In expt 2, LM2 plants were also harvested (six plants per experimental unit) at physiological maturity of the following reproductive cycle. Plant biomass was divided into vegetative shoot, root, seeds, fruits and reproductive supports (structures of the reproductive organs other than seed/fruit), dried at 70 °C for 48 h and weighed.
Seed-oil concentrations were approx. 20 % for both species and the patterns in carbon content, measured as glucose equivalents (Penning de Vries et al., 1974), were similar to those for biomass in all vegetative and reproductive organs (data not shown). Thus, allocation for vegetative and reproductive organs (Bazzaz and Ackerly, 1992) was estimated using biomass as a representative currency for carbon unit. Allocation was also estimated using nitrogen as currency, and nitrogen concentration at maturity was estimated for each plant organ in six plants per treatment (selected for a wide range of plant weights) by the Kjeldahl method (Allen et al., 1974).
When plants in their first year reached physiological maturity, all reproductive fractions (seeds, fruits and supports) were removed in selected LM2 plants, in order to ensure that the reproductive biomass harvested the following year was entirely produced during this second season. Allocations for LM2 plants were calculated in terms of the dry mass produced during the second year. Thus, mean vegetative shoot and root biomass were estimated by the difference between the mean biomass harvested at the end of the second year (LM2) and the mean biomass from plants harvested at the end of the first year (LM1).
Biomass allometric relationships between each biomass fraction and total plant weight (LF and LM1) were assessed through linear regression models, following Coleman et al. (1994). Departure from linearity was tested by the Runs test (Bradley, 1968). As the x-intercepts did not significantly differ from zero, regressions were forced through the origin. Partitioning coefficients were assessed as the slope of the linear regressions. Slopes and means were compared using two-way ANOVA (species × resource availability treatments). When significant interactions were detected, one-way ANOVA and Tukey's test were performed in order to detect significant differences between factor combinations. Arcsine √x transformations were performed for N concentration measurements.
RESULTS
Leaf water potential
For both species, differences in leaf water potential between water treatments were detected at 103 and 98 DAS in expts 1 and 2, respectively (P < 0·05; Fig. 1), close to the initiation of the flowering period. In expt 1, significant differences in ψl between treatments were maintained during the whole period of water stress, reaching values as low as −3 MPa in the stressed plant, just before re-watering at 134 DAS (Fig. 1A). In expt 2, the duration of water stress prior to the transitory irrigation was similar to that of expt 1, although ψl was close to −5 MPa at 130 DAS in stressed plants (Fig. 1B). After the transitory interruption of drought at 132 DAS, significant differences in ψl were also detected at 163 DAS, just before the end of the water-stress treatment. No significant differences in leaf-water potential were found between species under the same water treatment in either experiment.
Biomass allocation
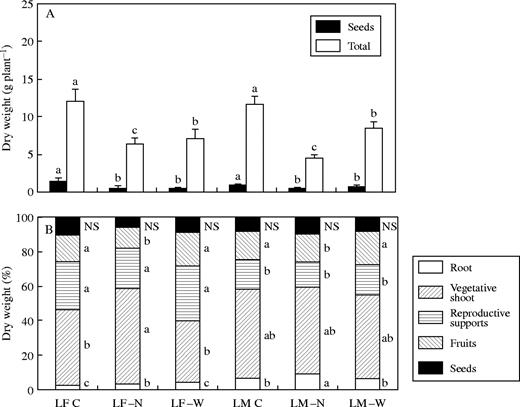
Similar seed and total biomass per plant were found within treatments for both species in expt 1, while seed and fruit allocation did not differ between species in this experiment, with 20 % of the total biomass (Fig. 2). A greater allocation to root, however (though counterbalanced by a lower allocation to reproductive support structures), was found for the perennial than for the annual. Averaging across species, total biomass was reduced, compared with controls, by 50 % and 35 % in −N and −W treatments, respectively (P < 0·05), while seed biomass was 65 % lower in both −N and −W than in control plants (P < 0·05). Seed allocation, however, was not modified by either low water or low nitrogen availability. While no effects on biomass allocation were produced by water stress in this experiment, low nitrogen availability increased root allocation in both species, and this response was associated with a lower allocation to fruits. Vegetative shoot allocation was increased by nitrogen stress in the annual, while no changes were observed for this variable in the perennial.
Total and seed biomass (A) and allocation to the different fractions (B) for L. fendleri (LF) and L. mendocina (LM) control plants (C) and subjected to nitrogen (−N) and water (−W) stress. Different letters indicate significant differences between treatments for each fraction (P < 0·05). n = 9–16. NS = not significant.
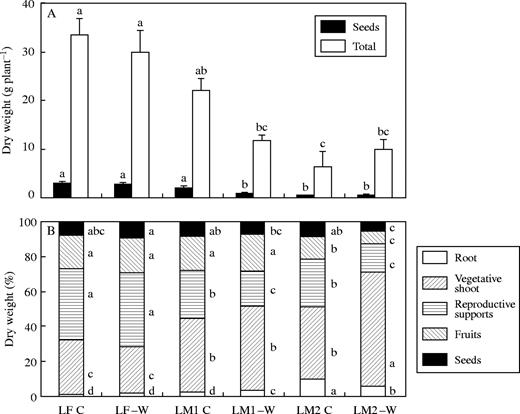
In expt 2, seed and total biomass of controls was also similar for both species at the end of the first year (LF C vs. LM1 C; Fig. 3). No significant reductions in total dry mass were found in −W treatments for both species, compared with that of control plants, although significant differences were detected between LF −W and LM1 −W plants (P < 0·05). Seed biomass was only reduced in LM1 plants by water stress. Seed allocation, however, was similar for both species in the controls and water treatment did not change seed allocation in either species. Both root and vegetative shoot allocations were higher in L. mendocina than in the annual, and this pattern was inversely associated with that of the allocation to reproductive support structures. Water stress increased root allocation only in the perennial, at the expense of a lower allocation to reproductive support structures.
Total and seed biomass (A) and allocation to the different fractions (B) for L. fendleri (LF) and L. mendocina plants harvested after its first (LM1) and second (LM2) flowering cycle, for control (C) and water-stressed (−W) treatments. In LM2 plant allocations and biomass are only for those produced during the second season. Different letters indicate significant differences between treatments for each fraction (P < 0·05). n = 33–40.
Total dry biomass produced during the second cycle was similar between control and water-stressed LM2 plants, although significant differences were found between LM1 C and LM2 C plants. Seed biomass harvested from LM2 C and −W plants, in contrast, was close to 75 % lower than for the controls harvested the previous year (P < 0·05). However, the analysis of allocation for the second year reveals that only fruit allocation decreased with age in both C and −W plants (counterbalanced with a greater allocation to vegetative shoot and root), while no significant changes associated with age were found for seed allocation.
Allometric analyses
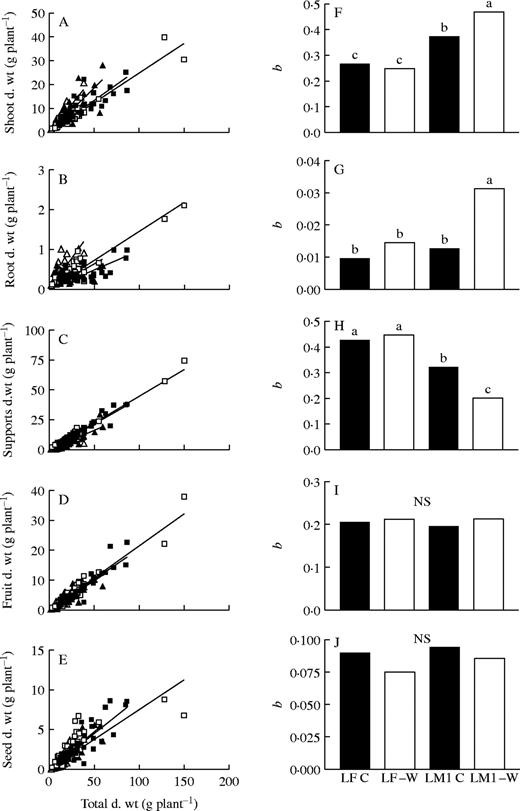
No departures from linearity were found for any allometric relationships (P = 0·05, data not shown), revealing that allocation increased linearly with plant size. The allometric relationship between vegetative shoot biomass and total biomass showed a greater allocation for vegetative shoots in the perennial than in the annual species (Fig. 4A), and the partitioning coefficient (assessed as the slope of the relationship; Fig. 4F) increased with water stress in L. mendocina. A similar pattern was observed for roots, although differences were non-significant between LF C and LM1 C plants (Fig. 4B and G). The higher partition coefficients observed in the perennial for vegetative organs were also produced at the expense of a lower partitioning to reproductive support structures (Fig. 4C and H). No significant differences between L. fendleri and L. mendocina were observed, however, for fruit (Fig. 4D, I) and seed partitioning coefficients (Fig. 4E and J).
Allometric relationships between organ biomass fractions and total plant biomass (left) and regression coefficients from linear fitting slopes (right, b) for vegetative shoots (A and F), roots (B and G), reproductive supports (C and H), fruits (D and I) and seeds (E and J). Lesquerella fendleri (squares, LF) and L. mendocina (triangles, LM1) irrigated (full symbols, C) and water-stressed (empty symbols, −W) plants, expt 2. Different letters indicate significant differences between slopes for each biomass fraction (P < 0·05). n = 33–40. NS = not significant.
N concentration and allocation
Allocation and allometry patterns for nitrogen did not differ from those for biomass (data not shown). In the perennial L. mendocina, however, N concentration was clearly greater in vegetative fractions (vegetative shoots and roots) than those for L. fendleri (Table 1). Higher values (close to those found in the seed) were found in LM1 C andLM1 −W plants for roots (both experiments) and vegetative shoots (expt 2 only) compared with those for L. fendleri, while intermediate values were found for LM2 plants. However, no differences between species were found for reproductive support structures and fruits, while higher N concentrations were found for control L. mendocina seeds in expt 1 compared with the same treatment in L. fendleri. In both experiments, the greatest N concentrations were found in the seeds.
Nitrogen concentrations (% of biomass)
. | . | . | Vegetative shoot . | Root . | Reproductive supports . | Fruits . | Seeds . |
|---|---|---|---|---|---|---|---|
| Expt 1 | L. fendleri | C | 1·88a | 1·45b | 2·44a | 2·28a | 2·77b |
| −N | 0·90b | 1·58b | 0·98b | 1·30b | 2·66b | ||
| −W | 2·09a | 1·80b | 2·18a | 2·25a | 3·41a | ||
| L. mendocina | C | 2·08a | 3·35a | 1·62a | 2·18a | 3·42a | |
| −N | 1·66a | 1·84b | 0·94b | 1·30b | 3·15ab | ||
| −W | 2·00a | 2·53a | 1·95a | 2·23a | 2·59b | ||
| Expt 2 | L. fendleri | * | 1·48b | 1·26c | 1·84 n.s. | 2·14 n.s. | 3·90 n.s. |
| L. mendocina (LM1) | * | 2·41a | 3·36a | 2·05 n.s. | 2·12 n.s. | 3·87 n.s. | |
| L. mendocina (LM2) | * | 1·91ab | 2·12b | 1·60 n.s. | 2·06 n.s. | 3·73 n.s. |
. | . | . | Vegetative shoot . | Root . | Reproductive supports . | Fruits . | Seeds . |
|---|---|---|---|---|---|---|---|
| Expt 1 | L. fendleri | C | 1·88a | 1·45b | 2·44a | 2·28a | 2·77b |
| −N | 0·90b | 1·58b | 0·98b | 1·30b | 2·66b | ||
| −W | 2·09a | 1·80b | 2·18a | 2·25a | 3·41a | ||
| L. mendocina | C | 2·08a | 3·35a | 1·62a | 2·18a | 3·42a | |
| −N | 1·66a | 1·84b | 0·94b | 1·30b | 3·15ab | ||
| −W | 2·00a | 2·53a | 1·95a | 2·23a | 2·59b | ||
| Expt 2 | L. fendleri | * | 1·48b | 1·26c | 1·84 n.s. | 2·14 n.s. | 3·90 n.s. |
| L. mendocina (LM1) | * | 2·41a | 3·36a | 2·05 n.s. | 2·12 n.s. | 3·87 n.s. | |
| L. mendocina (LM2) | * | 1·91ab | 2·12b | 1·60 n.s. | 2·06 n.s. | 3·73 n.s. |
Different letters indicate significant differences among treatments for each organ and experiment (P < 0·05); n.s., not significant (P < 0·05).
Average of C and –W plants.
Nitrogen concentrations (% of biomass)
. | . | . | Vegetative shoot . | Root . | Reproductive supports . | Fruits . | Seeds . |
|---|---|---|---|---|---|---|---|
| Expt 1 | L. fendleri | C | 1·88a | 1·45b | 2·44a | 2·28a | 2·77b |
| −N | 0·90b | 1·58b | 0·98b | 1·30b | 2·66b | ||
| −W | 2·09a | 1·80b | 2·18a | 2·25a | 3·41a | ||
| L. mendocina | C | 2·08a | 3·35a | 1·62a | 2·18a | 3·42a | |
| −N | 1·66a | 1·84b | 0·94b | 1·30b | 3·15ab | ||
| −W | 2·00a | 2·53a | 1·95a | 2·23a | 2·59b | ||
| Expt 2 | L. fendleri | * | 1·48b | 1·26c | 1·84 n.s. | 2·14 n.s. | 3·90 n.s. |
| L. mendocina (LM1) | * | 2·41a | 3·36a | 2·05 n.s. | 2·12 n.s. | 3·87 n.s. | |
| L. mendocina (LM2) | * | 1·91ab | 2·12b | 1·60 n.s. | 2·06 n.s. | 3·73 n.s. |
. | . | . | Vegetative shoot . | Root . | Reproductive supports . | Fruits . | Seeds . |
|---|---|---|---|---|---|---|---|
| Expt 1 | L. fendleri | C | 1·88a | 1·45b | 2·44a | 2·28a | 2·77b |
| −N | 0·90b | 1·58b | 0·98b | 1·30b | 2·66b | ||
| −W | 2·09a | 1·80b | 2·18a | 2·25a | 3·41a | ||
| L. mendocina | C | 2·08a | 3·35a | 1·62a | 2·18a | 3·42a | |
| −N | 1·66a | 1·84b | 0·94b | 1·30b | 3·15ab | ||
| −W | 2·00a | 2·53a | 1·95a | 2·23a | 2·59b | ||
| Expt 2 | L. fendleri | * | 1·48b | 1·26c | 1·84 n.s. | 2·14 n.s. | 3·90 n.s. |
| L. mendocina (LM1) | * | 2·41a | 3·36a | 2·05 n.s. | 2·12 n.s. | 3·87 n.s. | |
| L. mendocina (LM2) | * | 1·91ab | 2·12b | 1·60 n.s. | 2·06 n.s. | 3·73 n.s. |
Different letters indicate significant differences among treatments for each organ and experiment (P < 0·05); n.s., not significant (P < 0·05).
Average of C and –W plants.
Drought did not affect any fraction, with the exception of the contrasting effects found in expt 1 for the seeds of both species, compared with that observed for C plants. In expt 1, N concentration in fruits and reproductive support structures was drastically reduced by nitrogen stress in both species. No changes, however, were found for seeds in −N plants. In contrast, different responses to low nitrogen availability were found in both species for N concentration in vegetative biomass. Lower concentrations were found for roots in the perennial for −N plants, compared with those grown in the other treatments, while no changes were produced in root N concentration by resource availability in the annual L. fendleri. However, the lower concentrations found in roots for −N plants in L. mendocina were close to those found for L. fendleri under the three resource-availability treatments. On the other hand, N concentration in the vegetative shoots was only affected in the annual species by low nitrogen availability.
DISCUSSION
It was found that both the annual Lesquerella fendleri and the perennial L. mendocina showed similar seed allocation patterns, and seed-yield did not differ between control treatments harvested after a 1-year cycle. This is in agreement with the hypothesis that allocation to perpetuation organs in the perennial would not be directly related to a lower allocation to reproduction compared with L. fendleri. Seed allocation reported here was lower than that found for major grain crops (Lopez Pereira et al., 2000; Vega et al., 2000), possibly reflecting the lack of selection on Lesquerella species used in the present experiments. Also, no changes in seed allocation were found when resources were restricted, in contrast with the known reductions reported for most annual crops (Passioura, 1977; Ludlow and Muchow, 1990; Baigorri et al., 1999).
On the other hand, in spite of the similar allocation to seeds, higher vegetative shoot and root allocations were found for the perennial L. mendocina, compared with the annual L. fendleri. These changes were produced at the expense of a lower allocation to the reproductive support structures in the former. This effect increased with plant age and suggests that the survival strategy of the perennial L. mendocina is to establish an equitable trade-off between the perpetuation (root and rosette) and reproductive organs.
Allocation patterns for nitrogen did not differ from those for biomass. However, the analysis of nitrogen concentrations (Table 1) provided two additional contributions to the understanding of allocation between these two species. First, the higher nitrogen concentrations in L. mendocina vegetative shoots and roots strengthen the idea that vegetative organs are higher hierarchical sinks for both nitrogen and carbon in the perennial L. mendocina than in the annual L. fendleri. Secondly, nitrogen concentrations remained stable in seeds of both species independently of resource availability, in agreement with the idea that seed hierarchy did not differ between species. In contrast, Benech Arnold et al. (1992) found that seed nutrient concentrations remained constant only in the annual Sorghum bicolor, while concentration decreased significantly with decreasing nutrient supply in the perennial S. halepense. They also found that allocation to seeds in S. bicolor was similar to that to rhizomes in S. halepense, but clearly higher than that to seeds in the perennial.
The discussion above reveals that comparative allocation patterns between annuals and perennials cannot be summarized in universal responses. Ehrlen and Lehtila (2002) maintain that while information on life span is mainly available in terms of discrete categories (like annuals, biennials or perennials) it actually entails a continuous variation in longevity associated with morphological and demographic traits. Although little information is available about the life span of herbaceous perennials, it is presumed that it would be longer in a rhizomatous plant like S. halepense than the less aggressive L. mendocina, and that the former should be classified as more perennial than the latter under a continuous gradient. Consequently, the behavior found in L. mendocina would be expected to be intermediate between L. fendleri and S. halepense.
The comparison of results between L. fendleri and L. mendocina after a 1-year cycle clearly shows that a higher allocation to vegetative shoot and root does not necessarily reduce yield, since seed biomass did not differ between control treatments of the two species harvested after this period (Figs 2A and 3A). However, lower total biomass and seed biomass were found in control treatments in expt 1 compared with those of expt 2; the causes of these differences are not clear. The extremely rainy conditions during a great part of the grain-filling period in expt 1, combined with the fact that the critical period for yield determination in L. fendleri has been found to be around this period (Puppala et al., 2005), is a possible explanation for such differences. Further research is needed to determine precisely the critical period for yield in L. mendocina.
Seed allocation in the perennial during the second year was similar to that for the first year, although seed yield was clearly lower during the second year (Fig. 3). These lower individual yields are in agreement with that reported for other perennial grain crops (Suneson et al., 1963; Tsitsin, 1965; Weik et al., 2002), although the reasons are not clear. A possible explanation of the lower yields could be related to a potential limitation of the meristems available for the production of new reproductive structures, as observed in other herbaceous perennials (Watson, 1984; Geber, 1990; Bazzaz and Ackerly, 1992). This means that sink strength might be dramatically reduced, during the second year of growth, by lack of or poor functioning of developmental processes responsible of the production of primordia of reproductive sinks. Further research is needed to understand the physiological bases associated with the differences in yield between years in L. mendocina and other perennial crops.
In addition, the allometric responses found in the present work (Fig. 4) confirm that the partition to vegetative shoot and root is greater in the perennial and does not necessarily mean a reduction in seed allocation, in agreement with the mean allocations shown in Figs 2 and 3. Allometric analyses also revealed that differences in allocation between the annual and the perennial species increase linearly with plant size. No significant x-intercepts were found in this work for seed allocation as a function of total plant size, although several reports suggest that a threshold plant mass is a condition for reproduction (Gardner and Gardner, 1983; Thompson et al., 1991; Moot et al., 1997; Vega et al., 2000). In fact, reproduction of L. mendocina was not related to a higher threshold plant biomass, presumably associated with the maintenance of the vegetative shoot and root as perpetuation organs, compared with that of the annual.
In summary, the present work shows that both species produced similar seed allocation patterns. The allocation to vegetative organs in this perennial Lesquerella was achieved at the expense of a lower allocation to additional reproductive structures (instead of reductions in seed allocation), and this response increased with plant age. Although the patterns of nitrogen allocation were similar to those of biomass, differences in nitrogen concentrations strengthen the conclusion that vegetative organs are higher hierarchical sinks for both carbon and nitrogen in L. mendocina than in L. fendleri, but without reductions in the hierarchical importance of the seeds.
This work was supported in part by the Agencia Nacional de Promoción Científica y Tecnológica, under contract BID 802 OC/AR PID 9, and by Universidad de Buenos Aires grant AG-026. Administración Nacional de Parques Nacionales allowed us to collect L. mendocina seeds from natural populations. R. Golluscio provided useful comments about the manuscript. We also thank A. Alvarez Prado, A. Grimoldi, G. Striker and P. Insausti for their assistance in the field.
LITERATURE CITED
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C.
Baigorri H, Antolin MC, SanchezDiaz M.
Barclay AS, Gentry HS, Jones Q.
Bazzaz FA, Ackerly D.
Benech Arnold RL, Fenner M, Edwards PJ.
Ceccarelli S, Grando S.
Coleman JS, McConnaughay KDM, Ackerly DD.
Correa MN.
Cox TS, Bender M, Picone C, VanTassel DL, Holland JB, Brummer EC, et al.
DeHaan LR, Ehlke NJ, Sheaffer CC, Muehlbauer GJ, Wyse DL.
Dierig DA, Thompson AE, Nakayama FS.
Dierig DA, Thompson AE, Rebman JP, Kleiman R, Phillips BS.
Gardner WR, Gardner HR.
Geber MA.
Gentry HS, Barclay HJ.
Glover J.
Lopez Pereira M, Trapani N, Sadras VO.
Ludlow MM, Muchow RC.
Moffat AS.
Moot DJ, Wilson DR, McNeil DL.
Passioura JB.
Pearsall WH.
Penning de Vries FWT, Brunsting AHM, van Laar HH.
Piper JK.
Piper JK, Kulakow PA.
Ploschuk EL, Alvarez Prado AF, Cerdeiras G, Ravetta DA.
Ploschuk EL, Windauer L, Ravetta DA.
Ploschuk EL, Cerdeiras G, Windauer L, Dierig DA, Ravetta DA.
Priestley C, Taylor R.
Prihar SS, Stewart BA.
Puppala N, Fowler JL, Jones TL, Gutschick VP, Murray L.
Ravetta DA, Soriano A.
Roetheli JC, Carlson KD, Kleiman R, Thompson AE, Dierig DA, Glaser LK, et al.
Rollins RC, Shaw EA.
Roseberg RJ.
Sacks EJ, Roxas JP, Cruz MTS.
Sadras VO, Bange MP, Milroy SP.
Samson DA, Werk KS.
Scheinost P, Lammer DL, Cai X, Murray TD, Jones SS.
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA.
Sinclair TR, Bennett JM, Muchow RC.
Srinivasan G, Brewbaker JL.
Suneson CA, El Sharkawy A, Hall WE.
Thompson BK, Weiner J, Warwick SI.
Tsitsin NV.
Vega CRC, Sadras VO, Andrade FH, Uhart SA.
Watson MA.
Weik L, Kaul HP, Kubler E, Aufhammer W.
Windauer L.
Author notes
1Departamento de Producción Vegetal and 2IFEVA, Facultad de Agronomía (UBA), Av. San Martín 4453, (1417) Buenos Aires, Argentina and 3Department of Crop Production and Forestry, University of Lleida, Centre UdL-IRTA, Av. Rovira Roure 191, E-25198 Lleida, Spain