An introduction to nanoparticles and nanotechnology
Published April 2015
•
Copyright © 2015 Morgan & Claypool Publishers
Pages 1-1 to 1-14
You need an eReader or compatible software to experience the benefits of the ePub3 file format.
Download complete PDF book or the ePub book
Abstract
Nanoparticles (NPs; 1–100 nm in size) have a special place in nanoscience and nanotechnology, not only because of their particular properties resulting from their reduced dimensions, but also because they are promising building blocks for more complex nanostructures. This chapter gives an overview of NPs and their presence in our daily lives. It provides examples of the use of NPs in nanotechnology to obtain different end-products in different sectors of economic activity. In addition, a classification of NPs based on their dimensions, morphology and chemical composition is presented. NP uniformity and agglomerations, with a special focus on superparamagnetic NPs and their nanocomposites, are discussed.
1.1. An overview of nanoparticles and nanotechnologies
'Nano' is a prefix used to describe 'one billionth', or 10−9, of something. The concept of nanotechnology was introduced by physics Nobel laureate Richard P Feynman in his famous lecture entitled 'There's plenty of room at the bottom' at the December 1959 meeting of the American Physical Society [1]. Since then, there have been many revolutionary developments in physics, chemistry and biology that have demonstrated Feynman's ideas of manipulating matter at the atomic scale. In 1974, Norio Taniguchi (a professor at the Tokyo University of Science) invented the term 'nanotechnology' to describe extra-high precision and ultra-fine dimensions. He introduced the 'top-down approach' by predicting improvements and miniaturization in integrated circuits, optoelectronic devices, mechanical devices and computer memory devices. Approximately ten years later, K Eric Drexler introduced the 'bottom-up approach' when he discussed the creation of larger objects from their atomic and molecular components as the future of nanotechnology [2].
Nanotechnologies are now widely considered to have the potential to bring benefits in areas as diverse as drug development, water decontamination, information and communication technologies, and the production of stronger and lighter materials. Nanotechnologies involve the creation and manipulation of materials at the nanometre scale, either by scaling up from single groups of atoms or by refining or reducing bulk materials [3].
While the development of nanotechnologies is a modern multidisciplinary science involving the fields of physics, chemistry, biology and engineering, the production of nanoparticles (NPs), both in nature and by humans, dates from the pre-Christian era. For example, the Romans introduced metals with nanometric dimensions in glass-making; the famous Lycurgus cup (currently exhibited at the British Museum), which displays a different colour depending on whether it is illuminated externally (green) or internally (red), contains NPs of silver and gold [4]. In 1857, Faraday reported the synthesis of colloidal gold (and other metals such as Cu, Zn, Fe and Sn) and its interaction with light [5]. For an overview and chronological table of nanotechnologies, see [6].
Another example of interest is the case of magnetic NPs. Krishnan [7], illustrated the role that magnetic materials play in biology and medicine. In the field of magnetic NPs, a noteworthy pioneering work was published by Blakemore in 1975 [8], where biochemically precipitated magnetite (Fe3O4) was found in the tissues of various organisms including bacteria, algae, insects, birds and mammals. Many of these organisms use biogenic magnetite to sense the Earth's magnetic field for orientation and navigation. For more details on the development of magnetic NP synthesis and its presence in biomedicine and biotechnology see [7].
Throughout the last century, the field of colloid science has developed enormously and has been used to produce many materials, including metals, oxides and organic products [9, 10]. One of the first and most easily prepared magnetic colloidal systems was developed by Stephen Papell of the National Aeronautics and Space Administration in the early 1960s [11]. Papell's colloid consisted of finely divided particles of magnetite suspended in paraffin. To prevent particle–particle agglomeration or sedimentation, Papell added oleic acid as a dispersing agent. Subsequently, similar magnetic suspensions have also been synthesized with different nanometre sized particles of pure elements, such as iron, nickel and cobalt, in a wide range of carrier liquids [12, 13].
Ordinary materials, when reduced to the nanoscale, often exhibit novel and unpredictable characteristics such as extraordinary strength, chemical reactivity, electrical conductivity, superparamagnetic behaviour and other characteristics that the same material does not possess at the micro- or macroscale. A huge range of nanomaterials is currently being produced at an industrial scale, while others are being produced at smaller scales as they are still under research and development (table 1.1).
Table 1.1. A non-exhaustive list of nanomaterials, either used in industry or under investigation [14].
| aluminium | dendrimers | platinum |
| aluminium oxide | dimethyl siloxide | polyethylene |
| aluminium hydroxide | dysprosium oxide | polystyrene |
| antimony oxide | fullerenes | praseodymium oxide |
| antimony pentoxide | germanium oxide | rhodium |
| barium carbonate | indium oxide | samarium oxide |
| bismuth oxide | iron and iron oxides | silanamine |
| boron oxide | lanthanum oxide | silicon dioxide |
| calcium oxide | lithium titanate | silver |
| carbon black | manganese oxide | carbon nanotubes |
| cerium oxide | molybdenum oxide | tantalum |
| chromium oxide | nanoclays | terbium oxide |
| cluster diamonds | neodymium oxide | titanium dioxide |
| cobalt and cobalt oxide | nickel | tungsten |
| colloidal gold | niobium | yttrium oxide |
| copper (II) oxide | palladium | zinc oxide |
In summary, a number of examples of NPs with new magnetic, catalytic, magneto-optical or optical properties, among others, that differ from those of the bulk materials have been reported in the scientific literature. Size reduction has been found to be the reason behind many of these novel physical and chemical properties, which allow a wide range of applications with economic benefits. In 2010, more than 1000 products containing NPs became commercially available (table 1.2.) [15, 16].
Table 1.2. General classification and potential applications of NPs [14].
| Product areas with end-products containing NPs | Sectors where nanotechnology is expected to have a considerable impact |
|---|---|
| • Cosmetics and personal care products | • Medical and pharmaceutical sector |
| • Paints and coatings | • Bio-nanotechnology, bio-sensors |
| • Household products | • Energy sector, including fuel cells, batteries and photovoltaics |
| • Catalysts and lubricants | • Environment sector including water remediation |
| • Sports products and textiles | • Automotive sector |
| • Medical and healthcare products | • Aeronautics sector |
| • Food and nutritional ingredients | • Construction sector, including reinforcement of materials |
| • Food packaging and agrochemicals | • Composite materials |
| • Veterinary medicines | • Electronics and optoelectronics, photonics |
| • Construction materials | |
| • Consumer electronics |
1.2. Classification of nanomaterials
Typically, NPs are defined as an agglomeration of atoms and molecules in the range of 1–100 nm. They can be composed of one or more species of atoms (or molecules) and can exhibit a wide range of size-dependent properties. Within this size range, NPs bridge the gap between small molecules and bulk materials in terms of energy states [17]. NPs are generally classified based on their dimensionality, morphology, composition, uniformity and agglomeration [18, 19].
1.2.1. Dimensionality
1D nanomaterials. Materials with one dimension in the nanometre scale are typically thin films or surface coatings. Thin films have been developed and used for decades in various fields including electronics, information storage systems, chemical and biological sensors, fibre-optic systems, and magneto-optic and optical devices. Thin films can be deposited by various methods and can be grown controllably at the atomic level (a monolayer) [20].
2D nanomaterials. 2D nanomaterials have two dimensions in the nanometre scale. These include for example, nanotubes, dendrimers, nanowires, fibres and fibrils. Free particles with a large aspect ratio with dimensions in the nanoscale range are also considered to be 2D nanomaterials. The properties of 2D systems are less well understood and their manufacturing capabilities are less advanced.
3D nanomaterials. Materials that are nanoscale in all three dimensions are considered to be 3D nanomaterials. These include quantum dots or nanocrystals, fullerenes, particles, precipitates and colloids. Some 3D systems, such as natural nanomaterials and combustion products, metallic oxides, carbon black, titanium oxide (TiO2) and zinc oxide (ZnO) are well known, while others such as fullerenes, dendrimers and quantum dots represent the greatest challenges in terms of production and understanding of properties.
Figure 1.1 shows examples of nanomaterials with different dimensions. All the samples were deposited on a Si (111) substrate using the magnetron-sputtering-based inert-gas-condensation (MS-IGC) method as described in figures 2.1 and 2.2. The materials shown in figures 1.1(a) and (b) can be classified as 1D nanomaterials, while the Cu NPs shown in figure 1.1(c) are classified as 3D nanomaterials. The iron nanorods shown in figure 1.1(d) can be classified as 2D nanomaterials.
Figure 1.1. Scanning electron microscopy (SEM) images showing (a) a film of Ti NPs of 80 nm thickness, (b) a near-percolating Au film, (c) monodispersed Cu NPs and (d) Fe nanorods.
Download figure:
Standard image High-resolution image1.2.2. The morphology of NPs and nanocomposites
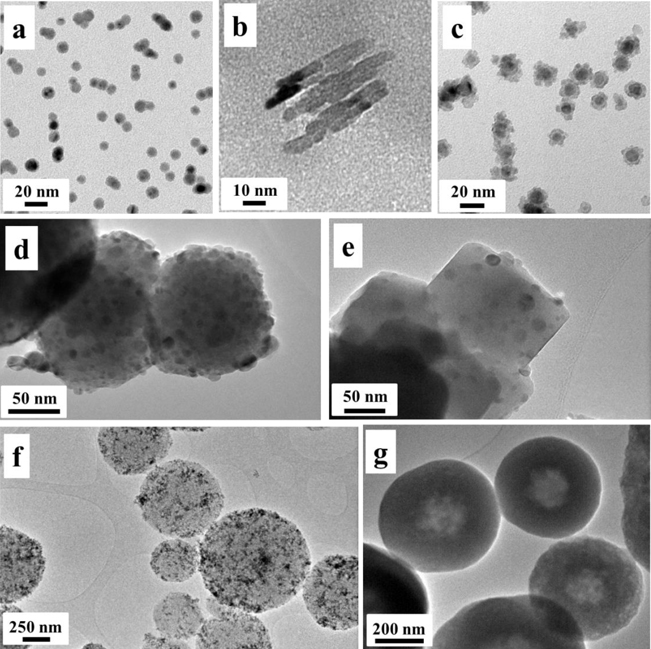
The morphological characteristics to be taken into account are the flatness, aspect ratio and spatial position of each element in the case of hybrid NPs (HNPs). A general classification exists between high and low aspect ratio particles. High aspect ratio NPs include nanotubes and nanowires. Small aspect ratio morphologies include spherical, oval, cubic, prism, helical and pillar shapes. Figure 1.2 shows examples of different morphologies of NPs and nanocomposites. Transmission electron microscopy (TEM) images of monodispersed Cu NPs, Fe nanorods and Cu core–Si shell NPs are shown in figures 1.2(a), (b) and (c), respectively. The details of the preparation methods for these NPs are presented in chapter 2. The TEM images in figures 1.2(d) and (e) show a porous magnetite NP and magnetite cubes decorated with Ni nanocrystals, respectively. These NPs were designed and synthesized using the hydrothermal process for purification of histidine-tagged proteins [21].
Figure 1.2. TEM images of examples of NPs with different morphologies and compositions. (a) Monodispersed Cu NPs, (b) Fe nanorods, (c) Cu–Si core–shell NPs, (d) porous Fe3O4 NPs, (e) Fe3O4 cubes decorated with Ni NPs, (f) porous silica spheres with γ-Fe2O3 NPs adsorbed on their surfaces and (g) γ-Fe2O3 NPs embedded in porous silica spheres. For more details about the preparation and characterization of these composites see [22, 23].
Download figure:
Standard image High-resolution imageWith regard to nanocomposites, substantial progress has been made in recent years in developing technologies in the fields of magnetic microspheres, magnetic nanospheres and ferrofluids. Nanospheres and microspheres containing a magnetic core embedded in a non-magnetic matrix are used in numerous biological applications [7]. They are used, for example, as carriers that can be targeted to a particular site by using an external magnetic field. In addition, the magnetic separation of organic compounds, proteins, nucleic acids and other biomolecules and cells from complex reaction mixtures is becoming the most suitable method for large scale production in bioindustrial purification and extraction processes. For in vivo applications, it is imperative that well-defined biocompatible coatings surround the magnetic particles to prevent any aggregation and also to enable efficient protection of the body from toxicity. However, for in vitro applications, biocompatible coatings are not essential; particles can be coated with non-toxic materials inert to chemical and biological media. The particles employed in all these applications are mainly superparamagnetic colloids with appropriate coatings, guaranteeing the stability and biocompatibility of the solutions.
Superparamagnetic NPs exhibit magnetizations of magnitudes similar to those of ferromagnetic materials, however, they have neither coercivity nor remanence. This behaviour, which is of quantum origin, is limited to nanocrystals with sizes below the critical size [24]. Conversely, most applications require superparamagnetic colloidal dispersions with large magnetic responses. Because the magnetization of a particle is proportional to its volume, the maximum magnetization that one can achieve is limited by the critical size of the superparamagnetic transition, which depends on the material [7]. A well-established strategy to create superparamagnetic particles with larger superparamagnetic responses is using nanocomposites (see, for example, figures 1.2(f) and (g)). These superparamagnetic composites are typically made by embedding superparamagnetic nanocrystals in a non-magnetic matrix such as polystyrene or nanoporous silica [25–27]. The resulting colloidal particles retain the superparamagnetic response of their constituent nanocrystals and show larger magnetization when an external magnetic field is applied. Furthermore, neither coercivity nor remanence are observed at the working temperature. However, in addition to the intrinsic superparamagnetic behaviour of the constituent NPs, one must consider the interactions between the NPs inside the skeleton matrix due to their proximity and surface effects due to the coating; these can lead to changes in the overall magnetic response of the colloidal particle.
1.2.3. NP chemical composition
NPs can be composed of a single constituent material or be a composite of several materials. The NPs found in nature are often agglomerations of materials with various compositions, while pure single-composition materials can be easily synthesized using a variety of methods (see chapter 2).
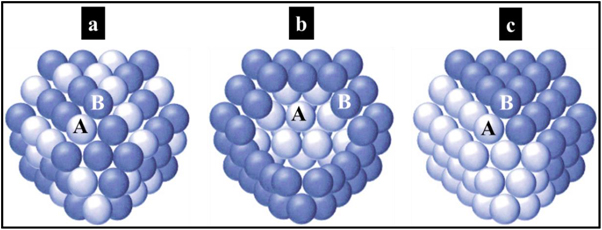
There are three main types of chemical ordering in HNPs (figure 1.3) that describe the way in which the atoms of the elements are arranged within the same NP [28, 29]:
- •Mixed NPs can be either random or ordered (figure 1.3(a)). Randomly mixed alloys correspond to solutions of solids, whereas ordered nanoalloys correspond to ordered arrangements of A and B atoms.
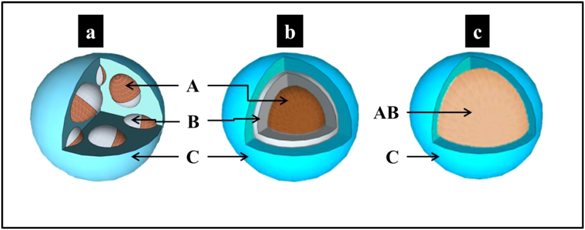
- •Core–shell NPs consist of a shell of one type of atom (B) surrounding a core of another type of atom (A) (figure 1.3(b)). This pattern is generally denoted by A@B and is common for a large class of NPs. Various thermodynamic considerations, discussed further in chapters 3 and 4, lead to the segregation of materials within the core or shell. A subset of the core–shell category consists of multishell (or 'onion-like') NPs. These NPs have alternating A–B–A shells, or A–B–C in the case of ternary NPs as depicted in figure 1.4(b). The formation of these latter structures is discussed in chapter 4, where it is demonstrated that Fe–Ag–Si multishell structures are obtained by adjusting the experimental conditions.
- •Layered NPs are commonly referred to in the literature as Janus (or 'dumbbell-like') NPs. They consist of two types of NPs (A and B) sharing a common interface (figure 1.3(c)). These types of NPs tend to minimize the number of bonds between elements A and B. This heterojunction structure facilitates phase separation.
Figure 1.3. Schematic images of binary NPs: a mixed structure (a), a core–shell structure (b) and a layered structure (c) of A and B elements.
Download figure:
Standard image High-resolution imageFigure 1.4. Schematic images of ternary NPs formed of elements A, B and C: (a) a multicore–shell morphology (the cores present a dumbbell-like morphology), (b) a core–multishell morphology and (c) an alloyed-core–shell morphology.
Download figure:
Standard image High-resolution imageBecause of the increasing need for multifunctional NPs, other complex structures of NPs such as multicore–shell structures in which the cores can present either 'dumbbell-like' or 'onion-like' structures have been reported in the literature [30–34]. This type of NP is presented and discussed in chapter 4. Another multifunctional subset of the core–shell arrangement is an alloyed single core NP encapsulated in an inert shell (figure 1.4(c)). The case of FeAl@Al2O3 NPs [35] is presented and discussed in section 3.2.
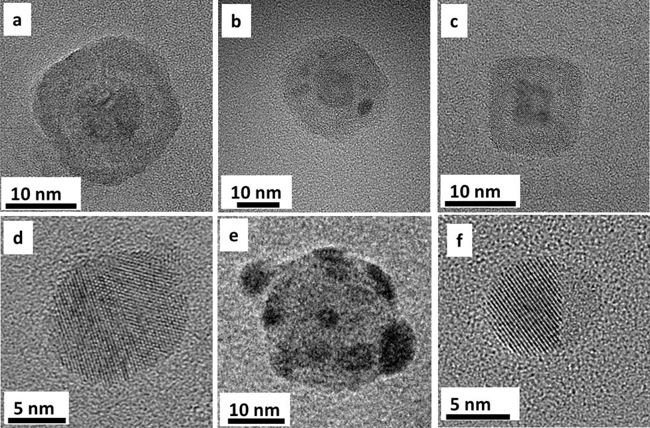
TEM images of NPs with various compositions and morphologies are shown in figure 1.5. These NPs were prepared using the MS-IGC method. Control over the composition and morphology of these NPs was achieved by adjusting the experimental conditions as explained in chapters 3 and 4. A detailed description of the deposition system is given in chapter 2.
Figure 1.5. (a) A Cu@Ag core–shell NP, (b) a Cu@Si multicore–shell NP, (c) a Fe@Fe2O3 core–shell NP, (d) a CuAg mixed NP, (e) a Si NP inoculated with Ag NPs resulting in a satellite morphology and (f) a FeAg dumbbell-like NP where the crystalline hemisphere corresponds to Ag.
Download figure:
Standard image High-resolution image1.3. NP uniformity and agglomeration
Based on their chemistry and electromagnetic properties, NPs can exist as dispersed aerosols, suspensions/colloids or in an agglomerate state. In fact, NPs tend to adhere to each other and to form agglomerates because of van der Waals forces that act over short distances, magnetic interactions, electrostatic forces present in the particles and adhesion forces related to the liquids adsorbed on their surfaces. Agglomeration due to Brownian motion is classified as 'coagulation'.
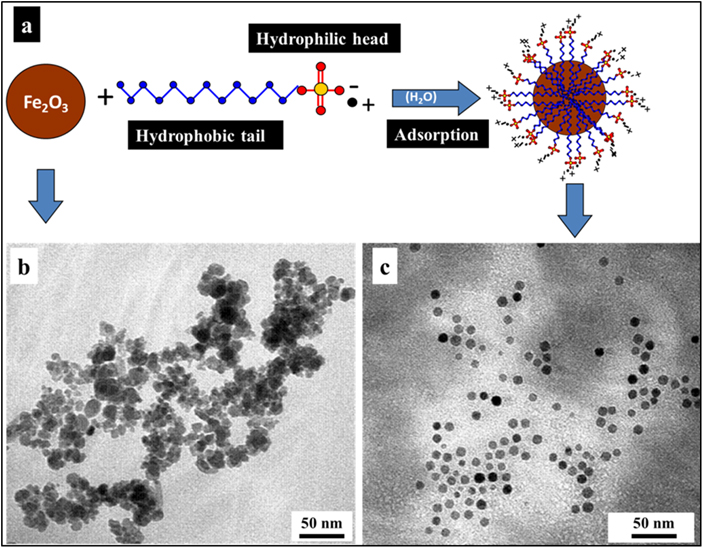
To avoid agglomeration, several processes include a post-synthesis stage to modify the particle surface by coating it with another organic or inorganic substance. In an agglomerate state, NPs may behave as larger particles, depending on the size of the agglomerate. For example, magnetic NPs tend to cluster, forming an agglomerate state, unless their surfaces are coated with a non-magnetic material. Figure 1.6 illustrates the typical process of stabilization of γ-Fe2O3 NPs in an aqueous suspension. The molecules of the anionic surfactant sodium dodecyl sulphate (SDS) are adsorbed onto the surfaces of the nanocrystals providing a negative charge in water. Therefore, the nanocrystals in the solution repel each other electrostatically resulting in a stable colloidal suspension. For more details on this procedure see [36].
Figure 1.6. (a) An iIllustration of the stabilization process applied to γ-Fe2O3 NPs using SDS surfactant. (b) A TEM image of the precipitated Fe2O3 NPs without SDS. (c) A TEM image of SDS-modified Fe2O3 NPs (adapted from [36] by permission of The Royal Society of Chemistry).
Download figure:
Standard image High-resolution imageIn the case of NPs deposited using vapour phase methods, the NPs are generally deposited on a solid substrate. The transfer of these NPs to a stable suspension is still under investigation. For example, attempts were made to co-deposit NPs from the vapour phase with a beam of water vapour, methanol, or isopropanol onto a nitrogen-cooled substrate [37, 38]. However, the stability of the resulting suspensions was not reported. Recently, a simple and environmentally friendly method for harvesting NPs was developed using polyvinylpyrrolidone (PVP) as a stabilizer [32]. PVP was selected as a non-toxic polymer with good wetting properties. Figure 1.7 shows the procedure used to harvest the NPs to a stable and homogeneous colloidal suspension.
Figure 1.7. (a) A schematic of the exfoliation procedure for the NPs. Step 1: multicore–shell NPs were deposited on a spin-coated PVP film on a glass substrate. Step 2: the NP/PVP/glass samples were immersed in methanol and sonicated for 15 minutes and then separated to remove excess PVP. Step 3: after washing the precipitated NPs with methanol, they were re-suspended in ultrapure water. (b) A dynamic light scattering histogram showing the size distribution of the HNPs. Reproduced from [32] by permission of The Royal Society of Chemistry.
Download figure:
Standard image High-resolution image1.4. NP characterization
Once NPs are synthesized, it is important to fully characterize and understand their structure. Over the years, many methods have been developed for this purpose. In this section, the focus will be on the main techniques with relevance to this book, namely TEM, scanning TEM (STEM), electron energy loss spectroscopy (EELS) and x-ray photoelectron spectroscopy (XPS).
TEM is a very powerful technique for the characterization of NP size, composition and crystalline structure. When an electron beam interacts with a sample, the electrons can be either transmitted, scattered, backscattered or diffracted [39, 40]. TEM uses the transmitted electron signal to form an image of the sample. The transmitted electron beam is dependent on the sample thickness; for thin samples (a few nanometres), the transmitted electrons pass through without significant energy loss. STEM differs from TEM by focusing the electron beam into a narrow spot that is scanned over the sample in a raster.
Because the attenuation of the electrons depends significantly on the density and thickness of the sample, the transmitted electron beam forms a 2D image of the sample. In hybrid samples, STEM imaging allows the identification of different components based on intensity variation. This intensity variation is related to the difference in the atomic numbers of each component (Z-contrast). In addition, the rastering of the beam across the sample makes it possible to couple STEM with other characterization methods such as EELS [41], allowing direct correlation of image and quantitative data thus obtain details regarding the chemical composition of NPs.
General TEM analysis does not have sufficient resolution to determine the crystallinity of a nanomaterial. However, high-resolution TEM (HRTEM) can be successfully employed for the characterization of the crystallinity of a sample with atomic resolution, as well as for providing information regarding electron diffraction analysis. This approach helps in gaining insight into the ordering of atoms in a NP. Figure 1.8 shows a photograph of the transmission electron microscope used in the characterization of the NPs presented in this book.
Figure 1.8. The transmission electron microscope used for the characterization of the NPs presented in this book. (Credit: OIST-Graduate University.)
Download figure:
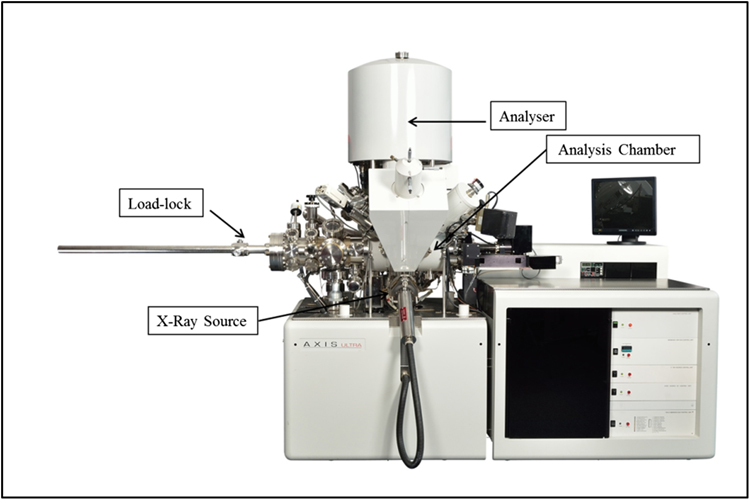
Standard image High-resolution imageXPS is a surface-sensitive quantitative spectroscopic technique that measures the elemental composition, chemical states and electronic states of the elements within the material. XPS spectra are obtained by irradiating a material with a beam of x-rays while simultaneously measuring the kinetic energy of the electrons that escape from the top 0 to 10 nm of the material being analysed. XPS requires high vacuum (P ∼ 10−8 mbar) or ultra-high vacuum (P < 10−9 mbar) conditions. However, when used to analyse NPs, the importance of the coverage of the NPs must be kept in mind: high coverage leads to high-quality spectra. On the other hand, to quantify the composition of the NPs, an inert transfer of the sample to the analysis chamber is necessary to avoid contamination and oxidation of the NP surface. The system used to analyse the NPs presented in this book is shown in figure 1.9.
Download figure:
Standard image High-resolution imageReferences
- [1]Feynman R P 1960 There's plenty of room at the bottom Engineering and Science 23 http://resolver.caltech.edu/CaltechES:23.5.1960Bottom 22–36
- [2]Drexler K E 1990 Engines of Creation: The Coming Era of Nanotechnology (Oxford: Oxford University Press)
- [3]National Nanotechnology Initiative www.nano.gov
- [4]The Lycurgus Cup www.britishmuseum.org/explore/highlights/highlight_objects/pe_mla/t/the_lycurgus_cup.aspx
- [5]Faraday M 1857 The Bakerian lecture: experimental relations of gold (and other metals) to light Phil. Trans. R. Soc. Lond. 147 145–81
- [6]Satoshi Horikoshi S and Serpone N (ed) 2013 Microwaves in Nanoparticle Synthesis 1st edn (Berlin: Wiley)
- [7]Krishnan K M 2010 Biomedical nanomagnetics: a spin through possibilities in imaging diagnostics and therapy IEEE Trans. Magn. 46 2523–58
- [8]Blakemore R 1975 Magnetotactic bacteria Science 190 377–9
- [9]Aitken R J, Creely K S and Tran C L 2004 Nanoparticles: an occupational hygiene review Health and Safety Executive Report www.hse.gov.uk/research/rrpdf/rr274.pdf
- [10]Ostiguy C, Lapointe G, Ménard L, Cloutier Y, Trottier M, Boutin M, Antoun M and Normand C 2006 Nanoparticles: actual knowledge about occupational health and safety risks and prevention measures IRSST Report www.irsst.qc.ca/files/documents/pubirsst/r-470.pdf
- [11]Papell S 1963 Low viscosity magnetic fluid obtained by the colloidal suspension of magnetic particles US Patent Specification 3215572A
- [12]Rosensweig R E 1982 Magnetic fluids Sci. Am. 10 136–45
- [13]Kaiser R and Miskolczy G 1970 Magnetic properties of stable dispersions of subdomain magnetite particles J. Appl. Phys. 41 1064–72
- [14]Lövestam G, Rauscher H, Roebben G, Sokull Klüttgen B, Gibson N, Putaud J-P and Stamm H 2010 Considerations on a definition of nanomaterial for regulatory purposes JRC Reference Report https://ec.europa.eu/jrc/sites/default/files/jrc_reference_report_201007_nanomaterials.pdf
- [15]Ostiguy C, Roberge B, Woods C and Soucy B 2010 Engineered nanoparticles: current knowledge about OHS risks and prevention measures IRSST Studies and Research Projects report 656, 2nd edn
- [16]Sebastian V, Arruebo M and Santamaria J 2014 Reaction engineering strategies for the production of inorganic nanomaterials Small 10 835–53
- [17]Johnston R L and Wilcoxon J P (ed) 2012 Frontiers of Nanoscience (Oxford: Elsevier)
- [18]Royal Academy of Engineering and Royal Society 2004 Nanoscience and Nanotechnologies: Opportunities and Uncertainties http://www.nanotec.org.uk/finalReport.htm
- [19]Buzea C, Pacheco-Blandino I and Robbie K 2007 Nanomaterials and nanoparticles: sources and toxicity Biointerphases 2 MR17–MR172
- [20]Seshan K (ed) 2002 Handbook of Thin-Film Deposition Processes and Techniques—Principles, Methods, Equipment and Applications (Norwich, NY: William Andrew/Noyes)
- [21]Benelmekki M, Xuriguera E, Caparros C, Corchero J L and Lanceros-Mendez S 2012 Nonionic surfactant assisted hydrothermal growth of porous iron oxide nano-spheres and its application for His-tagged proteins capturation, unpublished results
- [22]Benelmekki M, Xuriguera E, Caparros C, Rodriguez E, Mendoza R, Corchero J L, Lanceros-Mendez S and Martinez L M 2012 Design and characterization of Ni2+ and Co2+ decorated porous magnetic silica spheres synthesised by hydrothermal assisted modified-Stöber method for His-tagged proteins separation J. Colloid Interface Sci. 365 156–62
- [23]Caparros C, Benelmekki M, Martins P, Xuriguera E, Ribeiro C J, Martinez L M and Lanceros-Mendez S 2012 Hydrothermal assisted synthesis of iron oxide-based magnetic silica spheres and their performance in magnetophoretic water purification Mater. Chem. Phys. 135 510–7
- [24]Bean C P and Livingstone J D 1959 Superparamagnetism J. Appl. Phys. 30 120–9
- [25]Leun D and Sengupta A K 2000 Preparation and characterization of magnetically active polymeric particles (MAPPs) for complex environmental separations Environ. Sci. Technol. 34 3276–82
- [26]Jain T K, Richey J, Strand M, Leslie-Pelecky D L, Flask C A and Labhasetwar V 2008 Magnetic nanoparticles with dual functional properties: drug delivery and magnetic resonance imaging Biomaterials 29 4012–21
- [27]Behrens S 2011 Preparation of functional magnetic nanocomposites and hybrid materials: recent progress and future directions Nanoscale 3 877–92
- [28]Paz-Borbon L O 2011 Computational Analysis of Transition Metal Nanoalloys (Berlin: Springer)
- [29]Tiruvalam R C, Pritchard J C, Dimitratos N, Lopez-Sanchez J A, Edwards J K, Carley A F, Hutchins G J and Kiely C J 2011 Aberration corrected analytical electron microscopy studies of sol-immobilized Au + Pd, Au{Pd} and Pd{Au} catalysts used for benzyl alcohol oxidation and hydrogen peroxide production Faraday Discuss. 152 63–86
- [30]Shi W, Zeng H, Sahoo Y, Ohulchanskyy T Y, Ding Y, Wang Z L, Swihart M and Prasad P N 2006 A General Approach to Binary and Ternary Hybrid Nanocrystals Nano Lett. 6 875–81
- [31]Llamosa Perez D, Espinosa A, Martinez L, Roman E, Ballesteros C, Mayoral A, Garcia-Hernandez M and Huttel Y 2013 Thermal diffusion at nanoscale: from CoAu alloy nanoparticles to Co@Au core/shell structures J. Phys. Chem. C. 117 3101–8
- [32]Benelmekki M, Bohra M, Kim J H, Diaz R E, Vernieres J, Grammatikopoulos P and Sowwan M 2014 Facile Single-Step Synthesis of Ternary Multicore Magneto-Plasmonic Nanoparticles Nanoscale 6 3532–5
- [33]Benelmekki M, Vernieres J, Kim J H, Diaz R E, Grammatikopoulos P and Sowwan M 2015 On the Formation of Ternary Metallic–Dielectric Multicore–Shell Nanoparticles by Inert-Gas Condensation Method Mater. Chem. Phys. 151 275–81
- [34]Benelmekki M and Sowwan M 2015 Handbook of Nano-Ceramic and Nano-Composite Coatings and Materials , ed H Makhlouf and D Scharnweber (Amsterdam: Elsevier) at press, ch 1
- [35]Vernieres J, Benelmekki M, Kim J H, Grammatikopoulos P, Bobo J F, Diaz R E and Sowwan M 2014 Single-step gas phase synthesis of stable iron aluminide nanoparticles with soft magnetic properties APL MATERIALS 2 116105
- [36]Benelmekki M, Martinez L M, Andreu J S, Camacho J and Faraudo J 2012 Magnetophoresis of colloidal particles in a dispersion of superparamagnetic nanoparticles: Theory and Experiments Soft Matter 8 6039–47
- [37]Haeften K V, Binns C, Brewer A, Crisan O, Howes P B, Lowe M P, Sibbley-Allen C and Thornton S C 2009 A novel approach towards the production of luminescent silicon nanoparticles: sputtering, gas aggregation and co-deposition with H2O Eur. Phys. J. D 52 11–14
- [38]Galinis G, Yazdanfar H, Bayliss M, Watkins M and Haeften K V 2012 Towards biosensing via fluorescent surface site of nanoparticles J. Nanopart. Res. 14 1019
- [39]Williams D B and Carter B C 2009 Transmission Electron Microscopy: A Text Book for Material Science 2nd edn (Berlin: Springer)
- [40]Frenkel A I, Hills C W and Nuzzo R G 2001 A View from the Inside: Complexity in the Atomic Scale Ordering of Supported Metal Nanoparticles J. Phys. Chem. B 105 12689
- [41]Pearson D H, Ahn C C and Fultz B 1993 White lines and d-electron occupancies for the 3rd and 4th transition metals Phys. Rev. B 47 8471–8
- [42]Kratos Analytical www.kratos.com