Mass spectrometry-based imaging
Published May 2021
•
Copyright © IOP Publishing Ltd 2021
Pages I.9.g-1 to I.9.g-10
You need an eReader or compatible software to experience the benefits of the ePub3 file format.
Download complete PDF book, the ePub book or the Kindle book
Abstract
Mass spectrometry imaging (MSI) is a powerful imaging technique for visualizing the spatial distribution of chemical components in samples, from inorganic and organic materials to biological tissues and single cells. Contrary to other imaging techniques such as immunohistochemical imaging, chemical staining, and fluorescence microscopy, MSI is a label-free technique and enables monitoring thousands of compounds in a single experiment. Owing to its unique characteristics, especially the non-targeted detection, high chemical specificity, and high accuracy for structural elucidation, MSI has been rapidly and continuously developed to meet the expectations and needs of various research areas.
Mass spectrometry imaging (MSI) is a powerful imaging technique for visualising the spatial distribution of chemical components in samples, from inorganic and organic materials to biological tissues and single cells. Contrary to other imaging techniques such as immunohistochemical imaging, chemical staining, and fluorescence microscopy, MSI is a label-free technique and enables monitoring thousands of compounds in a single experiment. Owing to its unique characteristics, especially the non-targeted detection, high chemical specificity, and high accuracy for structural elucidation, MSI has been rapidly and continuously developed to meet the expectations and needs of various research areas.
1. Introduction
In MSI, a sample is scanned in pre-defined x and y coordinates in order to generate charged species that are analysed by a mass spectrometer. For each position, a mass spectrum is created, reporting the intensities of thousands of mass-to-charge ratios (m/z), each of them related to a specific molecular or elemental species.
An MSI experiment can achieve nano- to micrometer spatial resolution depending primarily on the type of ionisation technique and the sample preparation workflow. Among all ionisation techniques, matrix assisted laser desorption/ionisation (MALDI) [1] desorption electrospray ionisation (DESI) [2] and secondary ion mass spectrometry (SIMS) [3] are most commonly used for molecular imaging and laser-ablation combined with an inductively coupled plasma (LA-ICP) [4] for elemental imaging. MSI can be applied to many different surfaces, but this chapter will focus on the application for biological tissues.
Differences on ionisation techniques, mass analysers, sample preparation and data interpretations will be presented.
2. Principles and setups
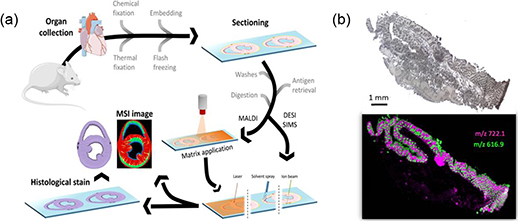
In MSI, elements, molecules or molecular fragments are measured on a surface by targeting an energy-rich primary beam (light, atoms, clusters) or a solvent spray towards a surface. During this step analytes are desorbed and ionised (D/I) and a secondary ion beam is formed. The ions are electrically guided to a mass analyser which separates them according to their mass-to-charge ratio (m/z). Subsequently a data cube is available consisting of x/y coordinates and m/z information that is correlated to ion intensities (figure 1).
Figure 1. (a) General workflow of an MSI experiment using biological tissue. Reproduced from [5] with permission of Springer. (b) Lipid distribution (m/z 722.1 and 616.9) in a 10 μm snap-frozen colon section (light microscopic image) as representative molecules for the submucosa (magenta) and the crypts (green) [6].
Download figure:
Standard image High-resolution image2.1. Physical principles [7]
2.1.1. Desorption/ionisation
The first MSI experiments were performed in the 1960s using a surface sampling and ionisation technique called SIMS. In SIMS, the surface is bombarded with a primary ion beam which causes neutral species and secondary ions to sputter from the surface. For biological imaging, SIMS instruments have to be operated in static mode where the primary ion dose remains below ∼1012 ions cm−2. Cluster ion beams (e.g. C60) have also led to softer ionisation providing information on molecules and elements from the uppermost layers of the samples (∼2 nm) in a molecular mass range of up to 1 kDa. The lateral resolution of SIMS is the highest to date with nm pixel sizes.
MALDI is a 'soft' ionisation technique which allows for the sensitive detection of large, non-volatile and labile molecules. MALDI is the most commonly used D/I technique, especially in the field of life sciences. For MALDI a matrix is deposited on top of the sample to co-crystallise with analytes. This matrix must be capable of strongly absorbing the photon energy emitted by the laser pulse to promote efficient D/I. Commercially available lasers include pulsed nitrogen (N2, λ = 337 nm) or frequency tripled solid state lasers (Nd:YAG, λ = 355 nm) with pulse repetition rates of up to 10 kHz guaranteeing low acquisition times. Today the spatial resolution is mostly limited by the laser beam diameter, but resolutions down to 2 μm can be achieved with special laser optics.
To ease sample preparation and avoid transfer of samples into vacuum, ambient D/I techniques are of interest. The most commonly used technique is DESI where a stream of charged solvent droplets is sprayed onto a sample surface and the liquid desorbs analytes prior to 'splashing' off the surface towards the analyser inlet. The solvent evaporates in a heated zone and charged analytes are formed. Spatial resolution depends mainly on the spray diameter and is in general between 150 and 200 μm.
LA-ICP MSI is undoubtedly powerful for the analysis of a large range of elements. Under ambient conditions a sample is continuously moved under a stationary laser beam which is ablating material. Solid state (Nd:YAG at 213 or 266 nm) or gas excimer lasers (ArF at 193 nm) are commonly used and modern systems offer spot sizes down to 1 μm. The ablated particles are transported by an inert gas flow to the secondary excitation source of the ICP instrument for digestion and ionisation. The excited ions in the plasma torch are then introduced to a mass analyser for elemental and isotopic analysis.
2.1.2. Mass analysers
Once ions have been generated, the mass analyser measures the physical property of the introduced ions based on their m/z. The most common types of mass analysers used for MSI are quadrupoles (Q), time-of-flight (TOF) and Fourier transform (FT) based analysers including orbitraps and ion cyclotron resonance instruments (ICR).
The Q consists of four cylindrical rods, set parallel to each other. Ions are separated based on the stability of their trajectories in oscillating electric fields that are applied to the rods. Such analysers have rather low resolution but excel at applications where particular ions of interest are being studied. One place where this is useful is LA ICP MSI where Qs serve as exceptionally high specificity detectors for single elements and isotopes.
Because of its simple use, high speed of analysis and comparable low costs, most laser-based MSI work is performed on TOF instruments. The TOF mass analyser in its simplest form comprises an evacuated flight tube with a pulsed ion source and a detector. The m/z of an ion is determined by accelerating it in an electric field towards a field-free drift region and measuring the time it takes to traverse the distance from the source to the detector. Since all ions are accelerated with the same kinetic energy, their m/z ratio can be easily calculated. Besides being the fastest mass analysers and thus facilitating larger areas to be ablated, TOF analysers provide an exceptional high mass range which makes them well suited not only for small but also large biomolecule MSI (up to 100 kDa [8]). However, inherent to the uneven surface of biological samples and energy distribution introduced by some D/I techniques (e.g. MALDI), different starting locations and energy distributions of desorbed ions at the point of acceleration lead to variations in drift times. The integration of an ion reflector (RTOF) partially compensates for this leading to enhanced mass resolution and accuracy. In axial TOF instruments, the source forms part of the flight tube, whereas in most other designs, the link between ionisation source and mass analyser is indirect, making it easier to achieve real high resolution and accuracy. The most successful type of such instruments is a hybrid instrument coupling a Q analyser with a TOF instrument. For such systems high mass resolution (RFWHM > 10.000) and accuracy (2–5 ppm) comes at the cost of mass range (0–3 kDa). Therefore, mainly small molecule analysis (drugs, metabolites) is performed on TOFs.
High performance instruments, such as FT-ICR and FT-orbitrap offer significantly higher mass resolution (RFWHM at m/z 512 up to 2 M on FT-ICR instruments) and mass accuracy (0.5–1 ppm). In FT-ICR instruments the ions are trapped in a magnetic field combined with an electric field perpendicular to each other where the ions are excited to perform a cyclotron motion. The cyclotron frequency depends on the m/z ratio and strength of the magnetic field. In FT-orbitrap instruments ions are trapped in an orbital motion around an inner spindle-like electrode which is surrounded by an outer barrel-like electrode. However, long acquisition times restrict the applications of such instruments in MSI to direct profiling or limited ablation areas. Yet, today the benefit of high confidence identification and separation of ions at the same nominal mass is well recognised so that the number of FTMS instruments is steadily increasing in the field despite their high costs.
2.2. Setup and state-of-the-art
Instrumentation for MSI is at the moment dominated by MALDI applications as it gives access to a wide range of different molecules and has excellent sensitivity to measure very low abundant analyte concentrations. The majority of experiments are carried out at 5–20 μm, although lower resolution can be achieved using special laser optics. Mass analysers used for MSI are more diverse. While TOF instruments have low resolution, their benefit of giving access to the full mass range and the rather low purchasing costs make this instrument excellently suited for many labs. Yet, high mass resolution instruments (FT-orbitraps, FT-ICR) got more important during the last years because sum formulas can be directly assigned to measured m/z values allowing for direct identification of molecules, a benefit well used in lipid, metabolite and drug analysis. But these instruments are the most expensive ones on the market. Ambient ionisation conditions like DESI are an excellent fit for vacuum labile samples and LA-ICP MSI is the only method reliably visualising elements (e.g Pt in cytostatic drugs) in tissue. Innovative ambient ionisation principles combine electrospray ionisation with a secondary laser ionisation (LESI) for higher D/I efficiencies and show their strength for certain biological tissues.
3. Biomedical relevance
3.1. Application range and relevance
MSI enables visualisation of the spatial distribution and sometimes even quantities of endogenous and exogenous molecules in tissue sections without labelling, which can significantly impact translational research or pharmaceutical developments as the molecular makeup of a biological sample describes its structure and often unveils functional aspects. Relevant fields of application are the visualisation of the biochemical micro-environment in relation to the detection of cancer margins for supporting intraoperative diagnostics, metabolic disorders like diabetes, cardiovascular diseases, orthopedics, neurological disorders, ophthalmology or nephrology among other pathologies in the search of region-specific biomarkers. It is also widely used in pharmaceutical industry since, compared to autoradiography and LC-MS from tissue homogenates, MSI can monitor the drug distribution in a whole animal or organ without labels, while at the same time detecting the products of its metabolisation.
3.2. Sample preparation
Sample preparation is a fundamental step in any analytical technique. In a regular MSI experiment, it concerns the surgical removal of a specimen, sectioning and placement on microscope glass slides. Depending on the ionisation technique and the analyte of interest subsequent specific sample treatment steps need to be followed.
3.2.1. Specimen removal and preservation
Sample preparation protocols for MSI should maintain the molecular and topographical integrity of the tissue section. The sample preparation protocol needs to be adjusted based on, among other factors, tissue storage conditions (snap-frozen, alcohol preserved or formaldehyde fixed and paraffin embedded (FFPE)).
The preferred specimen preservation for MSI experiments is snap freezing in liquid nitrogen or in cold isopentane followed by storage at −80 °C. Snap-frozen material can be pretreated by heat stabilisation right after sample collection (heat and pressure under vacuum, which prevents sample deformation while thermally inactivating the enzymes).
Small tissue specimens or with different tissue densities need to be embedded in media that does not interfere with the ionisation of the analytes of interest for sample handling and sectioning (e.g. OCT, gelatin, sucrose, agarose, carboxymethylcellulose) [9].
Most pathology laboratories and large biobanks follow FFPE workflows. The fixation in alcohol-based solutions and embedding in paraffin is ideal for the long term (decades), however, the detection of certain lipids and metabolites is usually compromised due to the sample preparation protocols required for MSI analysis of these types of samples.
3.2.2. Sectioning
Cryosectioning for snap frozen material is performed in a cryo-stat at different temperatures depending on the tissue and thicknesses ranging from 4 to 20 μm, the latter depending on sample density properties. FFPE material is sectioned at room temperature using a microtome. Conventional TOF instruments require the use of special conductive indium tin oxide (ITO) slides in order to ensure sufficient D/I which can also be Poly-lysin coated.
3.2.3. Washing steps
Washing steps are useful to remove salts or interfering molecules, and thus improve the extraction and ionisation of analytes.
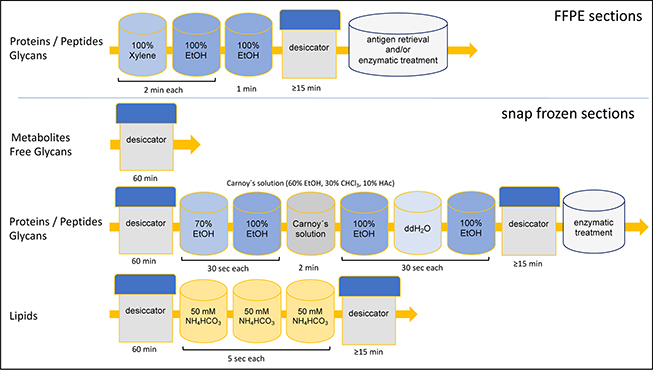
For proteins or peptides, different ethanol and chloroform washing series are employed to remove lipids and other molecules interfering with their ionisation, while for lipid analysis especially alkali ions have to be removed and metabolite analysis is done directly after drying of the tissue (figure 2). In the case of FFPE material, paraffin is usually removed by xylene and/or ethanol washing steps prior to further sample handling.
Figure 2. Overview of washing procedures for tissue sections targeting different analyte types (ethanol EtOH, double distilled water ddH2O, ammonium bicarbonate NH4HCO3).
Download figure:
Standard image High-resolution image3.2.4. On tissue chemistry
Enzymatic digestion: formalin fixation has the drawback of creating chemically cross-linked proteins. Classical antigen retrieval protocols followed by protein digestion with trypsin allows the detection of peptide fragments from chemically cross-linked FFPE tissues and generates peptides of 400–3500 Da (higher instrumental sensitivity than for intact proteins).
Derivatisation: selective derivatisation by introducing a fixed-charge quaternary nitrogen group to the analyte increase its MALDI ionisation efficiency. On-line derivatisation methods such as reactive DESI can help ameliorate potential analyte de-localisation.
3.2.5. Matrix application
DESI and SIMS do not require matrix application. For MALDI, the selection of the matrix, solvent and method deposition are critical to achieve an optimal spatial resolution while maintaining a good analyte extraction and ionisation.
The most commonly used methods for matrix application are sublimation/recrystallisation and spotting and spraying, which can both be used for matrix and enzyme deposition.
Sublimation and Recrystallisation: matrix sublimation minimises the compound delocalisation (dry deposition). A glass slide with the tissue section is placed in a vacuum chamber where a matrix layer is deposited on the tissue. To incorporate larger molecules in the matrix crystals a short recrystallisation step in an acidified vapour has to be added.
Spotting: automated acoustic or piezoelectric spotting systems generate high quality spectra, but higher resolution molecular images can be achieved with pneumatic and vibrational spray systems.
Spraying: a spray generator produces small matrix droplet sizes by 'atomising' the matrix solution with a spray nozzle using compressed air. Some systems allow users to heat the matrix to improve analyte embedding in the matrix.
Several matrices have found popularity for their universal use in MALDI MSI. Some examples are: sinapic acid, used for the analysis of large molecules (proteins), alpha-cyano-4-hydroxycinnamic acid for lower molecular weight compounds (peptides, metabolites), 2,5-dihydroxybenzoic acid is well suited for small molecules (drugs, carbohydrates, lipids), and 1,5-diaminonaphtalene and 9-aminoacridine for small compounds. Also, ionic liquid matrices have been investigated for MSI.
4. Parameters of image quality
4.1. Spectral quality
The overall quality of an MSI image depends in the quality of each single mass spectrum influenced by sample preparation and instrument parameters like mass resolution and accuracy, signal intensity, and its noise level. Specificity of a result is given by mass resolution and mass accuracy and therefore a proper calibration of the system is necessary. Every spectrum also registers electronic and chemical noise, which limits its sensitivity. To enhance true molecular signals, per pixel a number of n spectra are averaged, leading to a noise reduction with factor of sqrt(n), where n usually spans two to three orders of magnitude. Peaks have to surpass a minimum signal-to-noise ratio (IUPAC standard: minimum 3) to be recognised as actual signals of molecules and therefore eligible for visualisation and/or statistical analysis.
4.2. Image quality
MSI is a multiplex technique and therefore data sets consist of as many molecular images as there are valid peaks in the data set, usually in the range two to three orders of magnitude, depending on the mass resolving power of the instrumentation. One parameter of image quality that is shared by all MSI images within one experiment is the spatial resolution depending on the ionisation method. A higher spatial resolution, however, puts higher demands on the sensitivity of the instrument since the material ablated at a pixel is reduced in square with increasing spatial resolution. At higher spatial resolutions also delocalisation or leakage of analytes during sample preparation can become visible, manifesting themselves as blurriness in the image or signals outside of the tissue, respectively. In the end, the gold standard for the evaluation of quality remains the correlation of the tissue's underlying histomorphological structures in the MSI images. Based on that assumption, spatial chaos measures have been proposed to assess image quality [10].
5. Data processing
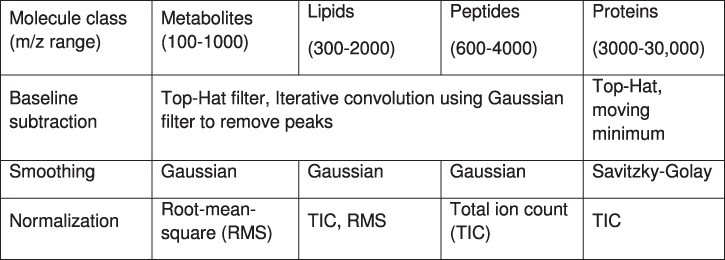
As mentioned, mass spectra contain contamination by electronic and chemical noise with high- and low-frequency components. Due to inhomogeneities in matrix or chemical microenvironments in the tissue, the chemical noise can differ significantly within one MSI experiment. To correct for that and restore a pixel-to-pixel comparability, spectral smoothing, baseline subtraction, and normalisation are performed on every spectrum of the data set. The type and parameterisation of the methods should be chosen carefully depending on the instrumentation and matrix. As MALDI TOF is the most versatile, and hence the most common method, it also encompasses the widest set of processing methods (table 1).
Table 1. Data processing strategies in MSI.
 |
Crucial is the choice of the pixel-to-pixel normalisation method for both intra- as well inter-sample comparisons where a spectrum (pixel) is divided by one of its characteristics, such as its total ion count (TIC), its median intensity, or the intensity of single or combined mass channels. Other transformations such as log-transformations of intensities also have proven useful to stabilise variance and reduce multiplicative detrimental effects within and between experiments, such as detector gain.
Most data points in a mass spectrum describe noise and real signals of molecules are sparse. The computational detection of those signals is usually based on local noise level estimations followed by detection of peaks that lie above a certain signal-to-noise ratio. Many peak picking routines for MS and MSI are available (Bioinformatics toolbox in MATLAB, MALDIquant, Cardinal in R, mMass, etc), of which advanced peak picking software offers isotope and peak width detection (e.g. SNAP algorithm for peptides). As MSI data sets comprise thousands of spectra, peak picking is due to efficiency usually performed on a representative spectrum of the data set. Calculating an average spectrum across all spectra has the advantage of reduced noise but suffers from the chance of missing spatially very localised signals, which might be lost in the average spectrum. The maximum intensity spectrum overcomes this problem by taking only the highest intensity for every mass channel across all spectra, thereby retaining localised signals, but also amplifying noise.
Once a global peak list for an MSI data set has been defined, an MSI image can be created by showing the peak intensity as a function of each spectrum's position. The calculation of the peak intensity is usually done by taking either the maximum value within the peak's interval or the peak's area. The latter is usually interesting for data where signals from the same molecule are not resolved, such as isotopes or lightweight post-translational modifications of proteins.
Spectral smoothing can be combined with spatial smoothing of the resulting MSI image. While this is biologically sensible, since nearby pixels are usually chemically more similar, there is no common standard on the type and size of the filter, and it is therefore left to the eye of the beholder. Another effective way to increase contrast in the image for visual interpretation is the removal of hotspots, usually the top 1% in the higher intensity range. Reduction of noise, and therefore increase of contrast, can also be achieved through the use of principal component analysis (PCA) since noise is usually characterised by smaller variance levels.
Since MSI delivers multivariate data, other dimensionality reduction methods (PCA, NNMF, pLSA, tSNE, SOM) are frequently used to reduce, inspect, and visualise MSI images for further data analysis.
6. Conclusions
6.1. Strength and limitations
MSI has the advantages of allowing following the label-free biodistribution of a compound of interest (about 2000 molecules at the same time), with a molecular specificity, while preserving the histological integrity, while being quantitative. However, some molecules cannot be detected with this technique, a problem that could be circumvented by derivatisation reactions, matrix optimisation, or tissue washing. However, many MSI approaches have limited spatial resolution and sub-micron features cannot be targeted.
6.2. Future developments
The understanding of the spatial distribution of biomolecules contains extremely valuable information for biomarker discovery. However different orthogonal imaging and Omics-technologies will need to be combined to map subtle molecular changes and to understand the complexity of biological processes. The integration of other imaging modalities to new correlated imaging modalities will allow one to gain structural, functional, dynamical and chemical information about a single sample for a well-defined time point or even time lapse series across all relevant length scales and levels of biological organisation. The combination of high-resolution, but also time-resolved low-resolution techniques with MSI will in the end enable a more comprehensive characterisation of in situ tissue pathology.
References
- [1]Chaurand P 2012 J. Proteom. 75 4883–92
- [2]Perez C J, Bagga A K, Prova S S, Taemeh M Y and Ifa D R 2019 Rapid Commun. Mass Spectrom. 33 27–53
- [3]McDonnell L and Heeren R 2007 Mass. Spec. Rev. 26 606–810
- [4]Becker J S, Matusch A and Wu B 2014 Anal. Chim. Acta 835 1–18
- [5]Mezger S T P, Mingels A M A, Bekers O, Cillero-Pastor B and Heeren R M A 2019 Anal. Bioanal. Chem. 411 3709–20
- [6]Holzlechner M, Strasser K, Zareva E, Steinhäuser L, Birnleitner H, Beer A, Bergmann M, Oehler R and Marchetti-Deschmann M 2017 J. Proteome. Res. 16 65–76
- [7]de Hoffmann E and Stroobant V 2007 Mass Spectrometry: Principles and Applications 3rd edn (Chichester: Wiley-Interscience)
- [8]Fröhlich S, Ellenberg M, Svirkova A, Grasl C, Liska R, Bergmeister H and Marchetti-Deschmann M 2015 Analyst 140 6089–99
- [9]Nelson K A, Daniels G J, Fournie J W and Hammer M J 2013 J. Biomol. Tech. 24 119–27
- [10]Palmer A et al 2017 Nat. Methods 14 57–60