Abstract
Zero-dimensional graphene quantum dots (GQDs) exhibit many different properties, such as strong fluorescence, nonzero bandgap and solubility in solvents, compared to two-dimensional graphene. GQDs are biocompatible and have low toxicity; hence, they are widely used in the biomedical field. The edge effect of GQDs is of particular interest because edge modification can regulate the performance of nanomaterials. In this review, various preparation methods for GQDs, which can be divided into three main categories, namely top-down, bottom-up and chemical methods, are discussed. The unique optical, electrical, thermal and magnetic properties of GQDs are reviewed. The functionalization of GQDs by doping with heteroatoms and forming composites with other materials is studied, and the characteristics of these GQDs are also discussed. The applications of these GQDs in the fields of optics, electricity, optoelectronics, biomedicine, energy, agriculture and other emerging interdisciplinary fields are reviewed to highlight the enormous potential of nanomaterials. This review reports on the recent advancement in GQD research and suggests future directions for the development of GQDs.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
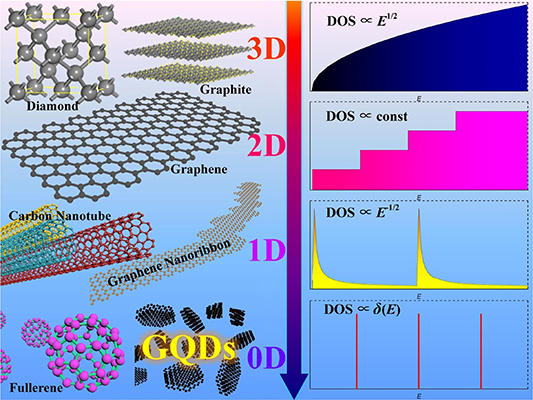
The discovery of new materials enables the realization of new physical and chemical phenomena that could result in the development of novel technologies and applications. For example, the discovery of graphene [1] in the 21st century has enabled the understanding of many excellent physical and chemical properties of two-dimensional materials [2–8], which led to the development of many exciting applications. Carbon materials have been of great scientific interest since the 1950s, particularly in the discovery of fullerene materials [9]. The fascinating properties of fullerenes have attracted the attention of researchers from around the world. Diamond [10], which is the oldest carbon material, has attracted scientists [11–20] from different fields because of its physical and chemical properties, such as hardness [21–24] and low dielectric constant [25–30]. Later, scientists from Japan [31] discovered a new one-dimensional carbon material, known as carbon nanotube, using the arc-discharge method. The high electron mobility in the one-dimensional material [32–36], chiral dependence [37–41] and other unique phenomena [42–46] have attracted enormous research interest. In 2004, the scientific community was filled with excitement upon the discovery of graphene [1], as its electrons exhibited the Dirac cone property of having no static mass [47]. Since then, carbon materials have been categorized according to different dimensions, such as three-dimensional bulk materials (e.g. graphite [48–50] and diamonds), two-dimensional nanosheets (e.g. graphene [51–53]), one-dimensional nanowires (e.g. carbon nanotube [54–56] and graphene nanoribbons [57–60]), and zero-dimensional dots (e.g. fullerenes [61–65] and graphene quantum dots (GQDs) [66–70]). These different dimensions of carbon materials can exhibit different electronic, physical and chemical properties. The electron densities of states (DOS) for the different dimensions of carbon materials are illustrated in figure 1. The electron DOS of a three-dimensional material is proportional to the 1/2 power of the energy. The DOS of a two-dimensional material is constant, whereas that of a one-dimensional material is negative 1/2 power relations. The DOS is quantized for a zero-dimensional material.
Figure 1. Different dimensions of carbon materials (left) and their related DOS against energy plots (right).
Download figure:
Standard image High-resolution imageAmong these carbon materials, graphene has attracted tremendous attention due to its excellent physical and chemical properties [71–78], which led to the development of many novel applications, such as magic-angle graphene superconductivity [79–81], ultrahigh-performance photodetector [82–84] and biomedical applications [85–90]. Although graphene has many excellent properties and applications, it has some limitations, such as a zero-bandgap structure [91], high preparation cost [92, 93] and difficulty in preparing large single crystals [73, 94, 95]. In 2008, Geim et al [96], who discovered graphene, used an electron beam etching technique to prepare zero-dimensional GQDs from graphene. GQDs, which are the newest members of the family of carbon materials, have received much attention because they inherit the excellent properties of graphene materials, such as high specific surface area, high carrier mobility, high inertia, high stability, nontoxicity and high light-to-heat conversion efficiency [91]. Due to the zero-dimensional properties of GQDs, these materials also exhibit quantum confinement in all three spatial directions and edge effects. Figure 2 depicts the characteristics of GQDs discussed in this review. Many excellent studies on GQDs have been reported [97–100] since the first demonstration of the nanomaterials. For example, Tang et al [100] reported a bottom-up synthesis technique (often known as the Tang–Lau method) that can effectively control the size of the GQDs, and hence their energy gap, which is an important parameter for many optoelectronic applications. Unlike carbon nanodots, GQDs exhibit crystalline properties with significant quantum confinement effects. The distinction between carbon dots and GQDs was discussed in a previous review [101]. The properties and potential applications of GQDs have not been fully realized because these nanomaterials are relatively new members of the carbon material family. In recent years, the physical and chemical properties of GQDs have been studied extensively, and their applications have been demonstrated. Although there already existed several excellent reviews [102–109] on GQDs, recent advancements in nanomaterials over the past two to three years have not been reviewed. Therefore, this review will provide an insight into recently reported remarkable studies on GQDs that are of significant interest to researchers working on similar nanomaterials, which offer many exciting and novel applications.
Figure 2. Schematic of the different properties of GQDs.
Download figure:
Standard image High-resolution imageGQDs exhibit a strong fluorescence effect due to the quantum confinement of carriers in the nanomaterials. The excellent fluorescence properties of GQDs have drawn significant attention in the biomedical field, particularly in applications such as fluorescent probes [110], monitoring [98] and cancer treatment [111]. The edge effect of GQDs allows effective and simple functionalization of nanomaterials via doping of impurity atoms at the edge [112–116], thereby regulating the fluorescence wavelength of the GQDs. Such an edge effect also facilitates the formation of GQD-based composite materials by hybridizing with other substances [117–122], paving the way for many novel applications. Previous studies on GQDs have demonstrated many potential applications of these nanomaterials in a wide range of fields, such as energy environment [123], agriculture [124], biomedicine [125], photoelectric detection [126] and gas sensing [127], as depicted in figure 3. In this review, many new and exciting applications of GQDs over the past two to three years are introduced in detail. Research into GQDs continues to gain momentum, as many of their properties have not yet been fully understood [101]. This review examines recent advancements in the preparation, functionalization and application of GQDs.
Figure 3. Different applications of functionalized GQDs.
Download figure:
Standard image High-resolution image2. Preparation methods
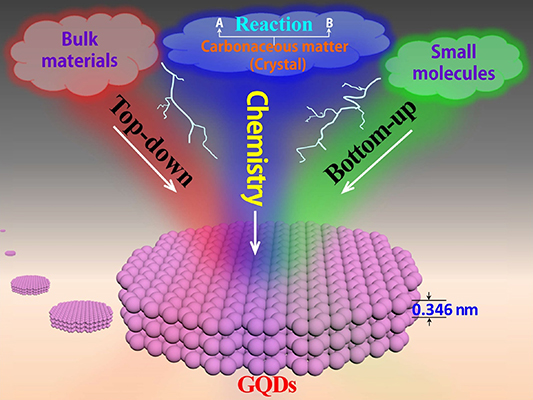
The preparation methodology of GQDs can significantly affect their widespread application, as it can influence the yield, cost and properties of the material. Most studies [108, 128–130] have divided the preparation methodology of GQDs into two categories: bottom-up and top-down methods. However, with the rapid development of new GQD preparation techniques, the two categories must be expanded to include other preparation methodologies. In this review, an additional category, which is a chemical method, is introduced. Chemistry is the science of reactions that produce changes in substances. Through the chemical reaction between two substances, an intermediate product or a precursor of GQDs is synthesized and subsequently converted into GQDs. This preparation methodology for GQDs is categorized as a chemical method. The bottom-up approach [131] for the preparation of GQDs is based on the polycondensation reaction of small molecular substances, whereas the top-down approach [132] is primarily based on the pyrolysis of bulk carbon materials. The main difference between these two methods and the chemical method is the formation of intermediates of GQDs in the latter method. The three categories that comprehensively classify the different preparation methodologies of GQDs are illustrated in figure 4. In the following sections, novel preparation methodologies of GQDs in all three categories developed over the last two years are introduced.
Figure 4. Schematic of the three categories of GQD preparation methods: top-down, bottom-up and chemical methods.
Download figure:
Standard image High-resolution image2.1. Top-down method
The top-down approach mainly involves the physical reduction of carbon materials, such as graphite [133], graphene [134], graphene oxide (GO) [135], carbon nanotubes [136] and fullerenes [137], into GQDs with sizes ranging from several nanometers to tens of nanometers by various means, as depicted in figure 5(a).
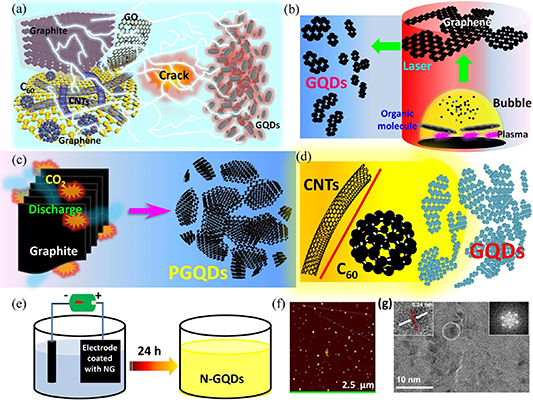
Figure 5. Top-down approach for the preparation of GQDs. (a) Schematic illustrating the preparation of GQDs, which involves the physical reduction of carbon materials. (b) Schematic depicting the use of laser ablation technique in the preparation of nitrogen-doped GQDs from graphite flakes. Reprinted from [138], © 2019 Elsevier B.V. All rights reserved. (c) Schematic of the exfoliation and cutting mechanism of graphite into GQDs using a shear mixer in supercritical CO2. Reprinted from [139], © 2018 Elsevier B.V. All rights reserved. (d) Schematic depicting the synthesis of GQDs from carbon nanotubes or fullerene via a thiol-ene reaction or solvothermal treatment. (e) Schematic of nitrogen-doped GQD preparation process by electrolysis. Reproduced from [140], with permission from Springer Nature. (f) Atomic force microscope image of GQDs prepared by solvothermal method. Reprinted with permission from [141]. Copyright (2020) American Chemical Society. (g) High-resolution transmission electron microscope images of GQDs prepared by pulsed laser ablation. Reprinted from [138], © 2019 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution imageLaser ablation is a commonly used technique for preparing nanoparticles. It is simple, environmentally friendly, highly tunable and favored by many researchers for the preparation of nanomaterials. For example, Kang et al [138, 142] recently prepared GQDs using a technique involving the use of inexpensive graphene flakes as carbon source. Nitrogen-doped GQDs with improved optical properties were prepared in solution using the technique of laser ablation. Figure 5(b) shows the preparation method. Although this method is relatively simple and effective, the controllability of the GQD size is unfavorable. Transmission electron microscopy (TEM) images of the GQDs revealed good crystallinity of the materials but with nonuniformity in their sizes, as shown in figure 5(g); hence, GQDs prepared using the laser ablation method might not exhibit an obvious quantum confinement effect. Recently, some interesting techniques similar to the explosive method used for the preparation of diamond film [143–146] have been reported for the preparation of GQDs. Alidad et al [139] used graphite powder as carbon source, which underwent instantaneous explosive reaction with carbon dioxide to transform the graphite powder into GQDs. This preparation method, illustrated in figure 5(c), produced high-purity GQDs with a relatively uniform size distribution over a short period of time; however, it was difficult to control the size of the GQDs. He et al [147] prepared GQDs from carbon nanotubes. As depicted in figure 5(d), during the preparation process, the GQDs were functionalized with thiomalic acid (TA), which enhanced the fluorescent properties of the nanomaterials. Fullerene was also used to prepare GQDs, as demonstrated for the first time by Loh et al [137]. Recently, Chen et al [141] prepared GQDs using fullerenes as a carbon source in a solvent thermal method (illustrated in figure 5(d)), which resulted in a high yield of GQDs at a relatively low cost; however, the nanomaterials were nonuniform in size, as shown in the atomic force microscope image in figure 5(f). The quantum yield of the GQDs prepared by this method was as high as 52.4% in the orange band (e.g. 617 nm), which is important for the future development of red-emission GQDs. The preparation of GQDs by electrolysis using carbon-based materials as electrodes has been reported [148, 149]; however, this process is time-consuming. Recently, Yang et al [140] developed a novel carbon cloth electrode coated with nitrogen-doped nanomesh graphene, which significantly improved the efficiency of GQD preparation and the carbon source utilization rate. The method, illustrated in figure 5(e), can produce a relatively high concentration of nitrogen-doped GQDs.
In summary, the top-down method allows rapid, low-cost mass production of GQDs [150–152], but the use of external energy to click carbon materials into GQDs results in poor controllability and nonuniformity of the GQD size. Therefore, this method is unsuitable for producing GQDs with well-controlled size, which is an essential requirement for optical applications.
2.2. Bottom-up method
The preparation of GQDs using bottom-up methods mainly involves the energy of small molecules with external energy. The formation of GQDs through condensation provides precise control of their sizes and morphologies, thus exhibiting an excellent quantum size effect. However, this method often requires the use of a ligand, similar to other quantum dot preparations [153–159], resulting in GQDs with a large number of ligands.
The size and morphology of GQDs can be controlled by selecting appropriate carbon sources and preparation conditions. Recently, Park et al [160] reported the preparation of the hexagonal structure of GQDs using D-glucose as a carbon source in a chemical liquid-phase catalytic condensation polymerization technique. The size of the GQDs was in the tens of nanometers with a uniform distribution. As shown in figures 6(a)–(d), the GQDs exhibited a hexagonal structure with an obvious grain boundary.
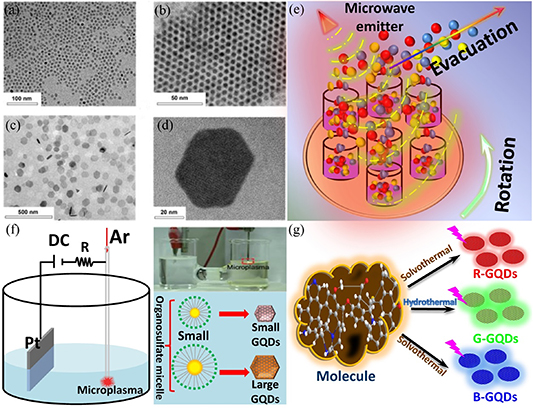
Figure 6. Bottom-up approach for the preparation of GQDs. (a) TEM image of 5 nm GQDs dispersed in methanol. (b) Self-assembled array of 5 nm GQDs upon solvent transfer to hexane. (c) Low-magnification and (d) high-resolution images of 70 nm GQDs in methanol. Reprinted with permission from [160]. Copyright (2019) American Chemical Society. (e) Schematic of the SPMA process used for the preparation of 2D nanostructures. Reproduced from [161] with permission from the Royal Society of Chemistry. (f) Synthesis of diameter-controlled colloidal GQDs using microplasmas. Schematic of the experimental setup for the microplasma-assisted electrochemical synthesis of colloidal GQDs (left). Photograph showing the experimental setup for microplasma-assisted electrochemical synthesis of GQDs (top right). Schematic of the microplasma-assisted electrochemical synthesis of diameter-controlled colloidal GQDs using organosulfate micelles (bottom right). Reproduced from [162] with permission from the Royal Society of Chemistry. (g) Schematic of the preparation of red, green and blue GQDs. Reprinted from [163], © 2020 Elsevier Inc. All rights reserved.
Download figure:
Standard image High-resolution imageThe use of microwaves in the synthesis of GQDs was first demonstrated by Tang et al [164–166], who also demonstrated the tunability of the optical bandgap of GQDs in their work. Recently, Gu et al [161] applied the microwave technique to prepare nitrogen-doped GQDs, as illustrated in figure 6(e). By regulating the proportion of the precursors, they reported the synthesis of C3N4 and graphite acetylene quantum dots, which are considered relatively new carbon materials. Using the microwave technique, Lee et al [167] prepared GQDs with functional groups passivated at their surfaces, and the GQDs were used in green-emitting lasers. The electrochemical method is another effective way to synthesize small molecules into GQDs, as shown in figure 6(f). Yang et al [162] used a microplasma-assisted electrochemical method to prepare GQDs with different sizes, which were dependent on the size of precursor molecules. Zhao et al [163] revealed that the size of GQDs can be determined by precursor types. For example, GQDs prepared using a similar synthesis method but different precursors produced GQDs of different sizes and therefore producing GQDs that can generate blue, green and red fluorescent bands, as shown in figure 6(g). Extensive research activities [164–166] have shown that the bottom-up method is an effective technique for controlling the size and morphology of GQDs. This allows the tuning of the energy band structure of GQDs, which is invaluable for many applications.
2.3. Chemical method
In contrast to the first two methods, the chemical method mainly involves chemical changes of substances during the formation of GQDs. First, carbon-containing compounds are converted into the precursors of GQDs by a chemical method. Next, the precursors are converted into GQDs by external energy or other means. Many methods [168–172] reported previously on the preparation of GQDs fall into this category, but earlier papers often attribute their preparation methods to either top-down or bottom-up method, according to the precursors of GQDs. In this review, the preparation method of GQDs is classified as a chemical method if the starting materials undergo chemical changes. This review discusses recent advancements in chemical methods for preparing GQDs.
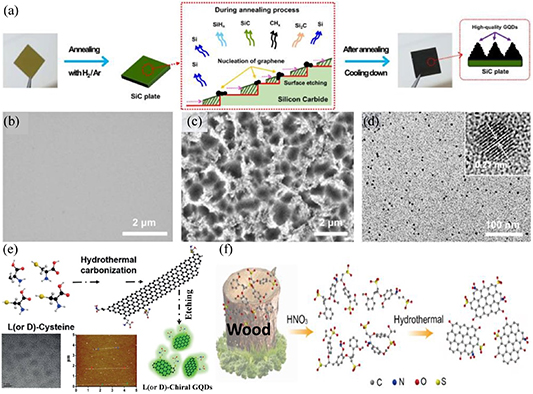
Inspired by the preparation of graphene from silicon carbide (SiC) epitaxy, Cho et al [173] prepared high-crystallinity and high-purity GQDs by hydrogen-assisted pyrolysis of SiC. The preparation process is illustrated in figure 7(a). GQDs were formed on the SiC surface by controlling the process conditions, such as annealing temperature and vacuum pressure. The morphology of the GQDs was characterized, as shown in figures 7(b)–(d), and they showed good uniformity in size. Another frequently used chemical method for the preparation of GQDs involves the use of small molecules that contain carbon to prepare graphene through hydrothermal synthesis, followed by etching or other techniques to prepare GQDs. Recently, Nie et al [174] reported the preparation of graphene by a hydrothermal method using l- or d-cysteine as a precursor; the graphene was subsequently etched into GQDs. Figure 7(e) shows the preparation process and morphology of the GQDs. The fluorescence quantum efficiency of the GQDs prepared using this method was as high as 41.26%. Because of the etching process, the GQDs exhibited relatively poor crystallinity but good uniformity in size. Kapoor et al [175] demonstrated the synthesis of graphene nanosheets from graphite electrodes via electrochemical exfoliation method and subsequently performed hydrothermal cutting of the graphene into GQDs with controllable size and morphology. Natural materials are often used as source [176–180] for the preparation of GQDs. These materials would undergo chemical reactions to produce precursors necessary for the synthesis of GQDs. Therefore, it is possible to introduce dopants into GQDs using a chemical method, which can improve the doping efficiency. For example, Xu et al [176] used lignosulfonates as the source material, which underwent chemical reaction to produce precursors for hydrothermal synthesis of GQDs. During the synthesis, small molecules were condensed into GQDs doped with sulfur, as illustrated in figure 7(f). Recent advances in chemical methods have demonstrated many advantages, such as the large volume production of GQDs at a relatively low cost and good controllability.
Figure 7. Preparation of GQDs using chemical methods. (a) Schematic layout of the synthesis of high-quality GQDs via hydrogen-assisted pyrolysis of SiC. Field-emission scanning electron microscope images of (b) pristine SiC plate and (c) GQDs on the SiC plate after annealing at 1500 °C in hydrogen etching gas. (d) Transmission electron microscope (TEM) image of the detached GQDs (inset: high-resolution transmission electron microscope image of the GQDs with their lattice spacing). Reproduced from [173]. CC BY 4.0. (e) Schematic (top) depicting the synthesis of chiral CDs by hydrothermal treatment of chiral cysteines. TEM (left) and atomic force microscope (middle) images of the GQDs. [174] John Wiley & Sons.© 2018 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Two-step synthesis method of GQDs from lignosulfonates. Reprinted from [176], © 2019 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution imageIn summary, the three methods of preparing GQDs have advantages and disadvantages, which are summarized in table 1. It can be seen from table 1 that the fluorescence properties of GQDs can be significantly affected by the preparation method, which can influence the morphology of the GQDs and therefore their essential properties [181–183], such as size (e.g. affecting quantum confinement), uniformity (e.g. affecting fluorescence peak width) and edge effect (e.g. affecting functionalization). Next, this review describes the recent work performed to study the new properties of GQDs to provide ideas for developing novel applications based on the use of GQDs.
Table 1. Related property of GQDs prepared by top-down, bottom-up and chemical methods.
| Methods | FQE (%) | Precursors | FL (ns) | PL (nm) | Size (nm) | DE | References | Pros | Cons | RM |
|---|---|---|---|---|---|---|---|---|---|---|
| Top-down | 0.7/1.8 | Graphite | 0.45/2.95 | 328 | 20.7/49.8 | — | [133] | Easy mass production, low cost, good crystallization, high purity and short period | Environmentally hazardous, poor uniformity, poor controllability and difficulty in functionalization | Graphite, carbon nanotubes or fullerenes are used as precursor materials, which are converted into GQDs through a series of cracking techniques, such as laser ablation, electrochemical electrolysis, explosion and electron beam etching, resulting in the change of size or dimension |
| 8.9 | GO | 3.16 | Yellow | 4.8 | — | [135] | ||||
| 7.12/6.53 | GF/MWCNTs | — | 423 | 20 | — | [136] | ||||
| 9.1 | GF | 9.8 | 450 | 6 | N | [138] | ||||
| 8.6 | Graphite | — | 431 | 2.5–6 | — | [139] | ||||
| 52.4 | C60 | 7.04 | 617 | 3.5 | DAN | [141] | ||||
| 10 ± 3 | Nitrogen-doped nanomesh graphene | — | 458 | 3.18 ± 0.2 | N | [140] | ||||
| 5 | TBAP | — | 450 | 8.6 ± 1.0 | N | [149] | ||||
| Bottom-up | 10.4 | d-glucose | 4.86 | 440 | 70 | N | [160] | Good controllability, excellent performance, good uniformity, easy functionalization and eco-friendly | High cost, long period, low yield rate, aggregation and high power consumption | Using small organic molecules containing carbon rings, such as glucose, sucrose and pyridine, as precursor materials. GQDs are formed by polymerization using techniques, such as hydrothermal, microwave, solvothermal and plasma methods. This involves polymerization of materials to form GQDs with controllable size and morphology. |
| 38.7 | CA + urea | — | 400–525 | 3.5 | N | [161] | ||||
| 24.2 | p-phenylenediamine + DMF | — | Red | 4.1 | N | [163] | ||||
| 19.7 | 1,3,6-trinitropyrene + NaOH | — | Green | 3.0 | N | [163] | ||||
| 20.2 | 1,3,6-trinitropyrene + triethylamine | — | Blue | 3.1 | N | [163] | ||||
| 7.5 | Glucose | 1.61 | 590 | 4.1 | N | [164] | ||||
| 7.1 | Fructose + H2SO4 | 0.65–0.88 | 446 | 5.2 | S | [165] | ||||
| 57.44 | CA + MPA | — | 460 | 4 | S | [184] | ||||
| 60 | Glucosamine | — | Near infrared | 3.9 | N + S | [185] | ||||
| 77 | FA + Tris | — | 380 | 1.95 | — | [186] | ||||
| 31.43 | C16H10 | — | Blue | 2.93 | — | [187] | ||||
| 80 | o-phenylenediamine | 1.3 | 585 | 3.8 | N | [188] | ||||
| 75 | CA + ethylenediamine | 12.86 | Blue | 1.04–4.81 | B + N | [189] | ||||
| 68.1 | CA + urea | — | Blue | 4.2 | N | [190] | ||||
| 18.9 | d-glucose | 6.4 | 550 | 5.4 | — | [191] | ||||
| 1.5 | Organosulfate | 448 | 4.9 | — | [192] | |||||
| 22.2 | CA + urea | — | 5–10 | N | [193] | |||||
| 81 | Salicylic acid | 2.2 | 460 | 3.0 | — | [194] | ||||
| Chemistry | 17.5 | p-Benzoquinone | 4.37 | Yellow | 20 ± 8 | — | [172] | Easy to control, easy functionalization, low cost, high performance and wide selection of source materials | Complex reaction, long period, high power consumption and hostile environment | Carbon-based substances, such as methane, wood and organic macromolecules, are used as precursor sources to form precursor of GQDs through chemical reactions. GQDs are formed using top-down or bottom-up techniques, which involve changing the chemical of raw materials used as precursor. |
| 41.26 | l- or d-oysteine | 7.56 | 510 | 5–7 | N + S | [174] | ||||
| 1.4 | C90H30 | — | Brown | 3.14 | — | [178] | ||||
| 11.76 | Graphite | — | 550 | 6.5 | — | [179] | ||||
| 17.4 | Graphite | — | 475 | 3.78 ± 0.83 | — | [195] | ||||
| 64 | Graphite | — | 475 | 19 ± 2.9 | N | [196] | ||||
| 23.1 | Graphite | — | Green | — | N | [197] | ||||
| 46 | Graphite | 7.2 | Blue | 1.84 ± 0.28 | N | [198] | ||||
| 65 | SCNWTs | 8.1 | 400 | 4.8 ± 1.4 | — | [199] |
Note: GO, graphene oxide; GF, graphite flakes; TBAP, tetrabutylammonium perchlorate; DAN, 2,3-diaminoaphthalene; CA, citric acid; DMF, dimethylformamide; MPA-3, mercaptopropionic acid; FA, folic acid; tris, tris(hydroxymethylamino methane); SCNWTs, single-walled carbon nanotubes; FQE, fluorescence quantum efficiency; FL, fluorescence lifetime; DE, doping element; RM, reaction mechanism.
3. Properties of GQDs
GQDs exhibit many unique properties because of the characteristics of graphene, which is confined to all three directions. The quantum confinement and edge effect of GQDs manifest extraordinary optical, electrical, thermal and magnetic properties. There has been a growing number of studies [200–208] exploring new properties of GQDs that could potentially lead to the development of novel applications. The optical and thermal properties of GQDs have been studied extensively because of their excellent fluorescent properties and high thermal efficiency. Other properties of GQDs have also received much attention over the past years. In this review, the optical, thermal, electrical and magnetic properties of GQDs are discussed in detail.
3.1. Optical property
The optical properties of GQDs are completely different from those of graphene. This is because of the quantum confinement effect of GQDs, which leads to the opening of the energy bandgap in graphene. The optical absorption of GQDs can be in the UV-visible and near-infrared (NIR) range by controlling the size and functionalization of GQDs. In addition, GQDs also demonstrate excellent fluorescence properties because of their quantum confinement and edge effects. The optical properties of GQDs are focused on the modulation of the optical absorption band as well as the fluorescence band of GQDs and the improvement in their fluorescence efficiency. It often requires the understanding of the up-conversion luminescence mechanism, which involves the absorption of low-energy excitation light and the emission of high-energy ultraviolet or visible light [209–212].
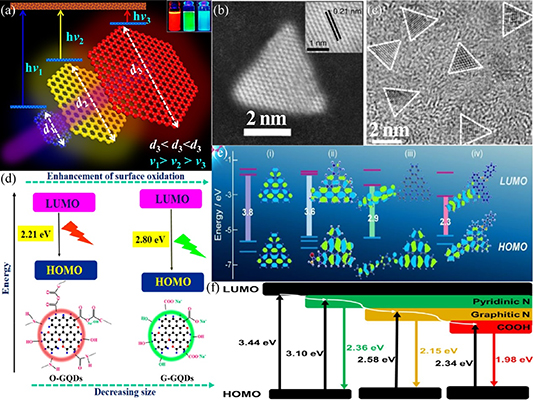
The optical properties of GQDs have been studied extensively [213–216]. The size, functionalization (e.g. doping) and morphology of GQDs have a significant impact on their optical properties. For example, there is a relationship between the size of GQDs and their optical bandgap [216]. As shown in figure 8(a), the optical bandgap decreased as the transverse size of the GQDs increased. Sahu et al [217] demonstrated this remarkable fluorescence properties of GQDs, which showed different fluorescence wavelengths for the GQD solutions with varying nanomaterial sizes (e.g. from blue to red for GQDs with reduced size, as shown in the inset of figure 8(a)). The morphology of GQDs can also affect their optical properties. Several theoretical studies [218, 219] have reported the influence of the morphology of GQDs on their optical properties. Recently, Yang et al [220] reported an experimental study on the optical properties of triangular GQDs. Figures 8(b)–(c) show the TEM images of the triangular GQDs. In addition to the modulation of the fluorescence spectrum by controlling the size of the triangular GQDs, they found that the triangular GQDs had a significant influence on the fluorescence color purity. The optical properties of GQDs can also be affected by their edge effects. For example, different functional groups at the edges of GQDs can result in different optical properties. Theoretical studies reported by Geethalakshmi et al [221] suggested that it is unfavorable to obtain infrared fluorescence by increasing the size of GQDs to reduce their energy bandgap. Instead, the fluorescence of GQDs in the infrared band can be obtained through the action of the edge functional groups. This was demonstrated experimentally by Sahu et al [217] and Xiong et al [222]. They reported the influence of oxygen functional groups at the edges of GQDs on the fluorescence properties of GQDs. The fluorescence spectral range of GQDs can be influenced by their size and surface functional groups, as illustrated in the left diagram of figure 8(d). GQDs with different functional groups in solutions and films would result in a shift in their fluorescence peaks, as observed by Wang et al [223] and illustrated in the right diagram of figure 8(d). Figure 8(e) shows schematics illustrating the modulation of the optical energy bandgap of the GQDs functionalized with different functional groups. The energy bandgap of GQDs functionalized with H, NH2, pMR and DAN functional groups can vary from 3.8 to 2.3 eV [224]. The degree of graphitization can influence the optical properties of GQDs, as reported by Wei et al [225]. They observed a reduction in the optical bandgap of GQDs (i.e. leading to a fluorescence redshift) as the degree of graphitization of GQDs increased. The optical properties of GQDs can also be affected by the introduction of heterogeneous atoms, such as elements from Group V and/or VI, into the GQDs [226–230]. The introduction of sulfur atoms into GQDs is commonly studied to determine their influence on the optical properties of GQDs. Recently, Fan et al [231] introduced two different elements, namely nitrogen and sulfur atoms, into GQDs and observed a change in their fluorescence properties, which were attributed to the impurity level produced by the heterogeneous atoms, as illustrated in figure 8(f). Other optical properties of GQDs have been studied recently, such as the stress of GQDs for optical modulation at infrared wavelengths [232], intrinsic GQDs exhibiting a single-photon emission phenomenon [233] and nonlinear optical properties of GQDs [234, 235]. An enhanced understanding of the optical properties of GQDs allows further exploration and development of GQD applications, particularly in integrated photonic devices [236].
Figure 8. Optical properties of GQDs. (a) Schematic of the change of optical bandgap with different sizes of GQDs (inset: fluorescence of GQDs having different sizes). Reprinted with permission from [217]. Copyright (2019) American Chemical Society. (b) Synthesis route of the NBE-T-CQDs by solvothermal treatment of PG triangulogen. Typical aberration-corrected HAADF-STEM image of R-NBE-T-CQDs (inset: corresponding high-resolution image). (c) Wide-area TEM image of G-NBE-T-CQDs. The triangular projections are highlighted by white contour lines. Reproduced from [220]. CC BY 4.0. (d) Reasonable mechanism behind blue shift of O-CDs at pH 13. Reprinted with permission from [217] Copyright (2019) American Chemical Society. (e) Predicted energy level diagrams for graphene with different functional groups: i, H-GQDs; ii, NH2-GQDs; iii, pMR-GQDs; iv, DAN-GQDs. The schematics show the chemical structures used for the theoretical calculations. The isosurface presents the HOMO and LUMO [224]. John Wiley & Sons.© 2020 The Chemical Society of Japan & Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Schematic of the possible energy levels of the PL-tunable GQDs. Reprinted from [231], © 2020 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution image3.2. Thermal property
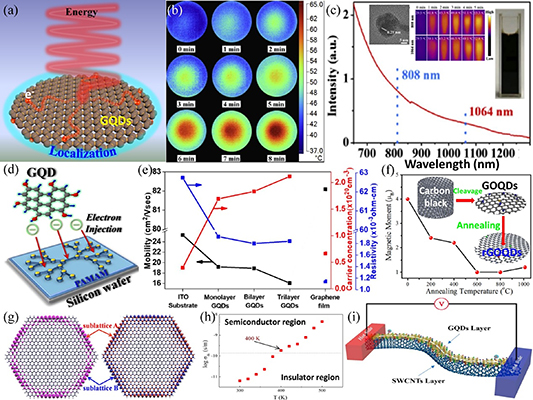
The transformation between field and heat conduction can be observed in GQDs due to the quantum confinement of the nanomaterials. When energy is incident on the GQDs, electrons are confined within the GQDs, with their edges as boundaries. Because of the localized electrons and high electron mobility of the GQDs, the edges of the GQDs become charge aggregation regions that ease the transfer of charge in GQD-hybrid materials [237]. Li et al [238] found that GQDs can convert infrared light into heat energy. Figure 9(a) shows the photothermal conversion of the GQDs. The temperature of the GQD solution irradiated by the NIR laser was reported to change drastically within 8 min, as shown in figure 9(b). Shen et al [239] reported that GQDs with nitrogen-containing groups exhibited greater absorption effect to NIR band of 808 and 1064 nm, as shown in figure 9(c). When the nitrogen content of the GQDs was increased from 1.68 at. % to 4.3 at. %, the photothermal conversion efficiency increased from 43.6% to 81.3% under laser irradiation of 808 and 1064 nm. The temperature of the GQD solution increased rapidly in less than 5 min, as shown in figure 9(c). The morphology and solution of the GQDs are shown in figure 9(c). In addition, the coupling of GQDs with carbon nanotubes can improve the thermoelectric properties of carbon nanotubes, as demonstrated by Du et al [240]. They reported an improvement in the power factor and a reduction in the thermal conductivity of carbon nanotubes upon coupling of GQDs with carbon nanotubes, as shown in figure 9(i). The thermal conductivity of GQDs has attracted significant interest [208, 241, 242]. Studies include functionalization of GQDs and the preparation of soluble GQDs to enhance heat dissipation and thermal control in a solution.
Figure 9. Other properties of GQDs. (a) Schematic of the photothermal conversion of GQDs. (b) Infrared thermal images of GQDs (100 μg ml−1) under NIR irradiation (808 nm; 2 W cm−2). Reprinted from [238], © 2020 Elsevier B.V. All rights reserved. (c) Optical absorption spectrum of GQDs in the NIR region from 650 to 1300 nm (inset: TEM image of GQDs (left), infrared thermographic images of GQD solutions filled in centrifuge tubes under continuous 808 and 1064 nm laser irradiation from 0 to 5 min (middle) and photograph of the solution (right)). Reprinted from [239], © 2020 Elsevier Ltd. All rights reserved. (d) Schematic of electron injection from GQDs into organic materials. Reprinted from [243], with permission from AIP Publishing. (e) Carrier mobility, concentration and resistivity for the ITO substrate, GQDs with different wet transfer numbers and graphene film. Reprinted from [244], © 2019 Elsevier B.V. All rights reserved. (f) Dependence of magnetic moment of GQDs on annealing temperature (inset: synthetic scheme of GQD samples). Reprinted from [245], © 2019 Elsevier B.V. All rights reserved. (g) Spin density isosurfaces of GQDs in antiferromagnetic (left) and ferromagnetic (right) couplings at the inter-edges. The red and blue isosurfaces represent the spin-up and spin-down states, respectively. Reproduced from [246]. CC BY 4.0. (h) Variation in dc conductivity of GQDs with temperature. Reprinted from [247] with permission from AIP Publishing. (i) Schemes of GQDs/SWCNTs. Reprinted with permission from [240]. Copyright (2020) American Chemical Society.
Download figure:
Standard image High-resolution image3.3. Electronic property
To date, only a few experimental studies have been performed to study the electrical properties of GQDs because of the small size of the nanomaterials, ranging from a few nanometers to tens of nanometers. Recently, Lin et al [243] reported the effective injection of electrons from GQDs into organic compounds, as illustrated in figure 9(d). They performed steady-state and time-resolved photoluminescence techniques in their studies. To understand the electrical properties of materials, it is often necessary to study their electrical parameters, such as carrier mobility and resistivity. Fu and Lin [244] studied the carrier mobility, resistivity and carrier concentration of GQDs with different numbers of layers. They found that the electrical properties of GQDs were significantly influenced by the number of layers; for example, GQDs with an increased number of layers would lead to a decline in their carrier mobility, which can be attributed to the interlayer coupling that influences the ability to transfer charge. Figure 9(e) shows a plot of several electrical parameters against the number of GQD layers. Interestingly, GQDs can be converted from insulators to semiconductors at a certain critical temperature, as discovered by Sinha et al [247]. They recorded a large change in the electrical conductivity of the GQDs when the temperature was approximately 400 K, as shown in figure 9(h). More recently, superconductivity has been reported in a new carbon material [248], which is of great interest to the scientific community. Although there are only a few studies on the electrical properties of GQDs [249, 250], this topic continues to attract the attention of many researchers, as it has significant implications for the use of GQDs in nanoelectronic devices.
3.4. Magnetic property
Owing to the high edge-to-area ratio of GQDs, a large number of spin-polarized edge states may exist that could theoretically generate attractive magnetic properties [251]. The magnetic properties of GQDs can be modulated by their morphology [252], size [253] and other external factors [254] due to the effect of localized electrons at the edge states. Sun et al [245] found that the magnetic properties of GQDs with oxygen-containing functional groups were significantly modulated by annealing temperature. For example, the magnetic moment of the GQDs decreased significantly when the annealing temperature increased and the oxygen content decreased, as shown in figure 9(f). The discovery of this phenomenon will enable the development of novel applications of GQDs in spin devices. By coupling antiferromagnetic and ferromagnetic with GQDs, Yang et al [246] found that the spin states at the edges of the GQDs experienced significant changes, suggesting that the magnetic properties of the GQDs were determined by the local electronic states at their edges, as illustrated in figure 9(g). Much of the research performed [255, 256] on the magnetic properties of GQDs is intended for the potential use of carbon materials in spin devices, which would benefit from the quantum confinement and edge effects of GQDs.
4. Functionalization
The many unique properties described in the preceding section are attributed to the quantum confinement and edge effects of the GQDs. To explore other functionalities of GQDs for novel applications or to improve their unique properties, the nanomaterials can be modified by means of functionalization, which either takes the form of doping or composite formation. Similar to their parent material (i.e. graphene), intrinsic GQDs have limited chemically active sites [257, 258] that restrict the performance of the nanomaterials, resulting in low fluorescence quantum efficiency and chemical catalytic activity. Doping is highly effective for GQDs due to their edge effect and can significantly improve the chemical activity of the nanomaterials. The large specific surface area of intrinsic GQDs makes them attractive for forming composites with other materials. GQDs can form composites with organic [259–261] and inorganic [262–266] materials. In this section, progress in the functionalization of GQDs over the past two years is discussed in detail.
4.1. Doping
Doping is an effective method to improve the properties of materials. Single-element [267–269] and multielement [270–272] doping of GQDs has been studied extensively. Nitrogen-doped GQDs [273–276] have been studied extensively and have three different carbon–nitrogen atomic combinations: graphitic nitrogen, pyridinic nitrogen and pyrrolic nitrogen. Interestingly, graphitic nitrogen had the greatest influence on the performance of the GQDs. Hence, the properties and performance of GQDs can be enhanced by doping them with heterogeneous atoms, as illustrated in figure 10(a). Controlled doping of GQDs and the ability to control their properties is of great importance. Kim et al [277] demonstrated the use of laser ablation technique in liquid to produce nitrogen-doped GQDs from carbon nano-onions in a mixed solution containing nitrogen. The nitrogen content in GQDs can be regulated by controlling the laser parameters and nitrogen concentration in the solution. This technique is illustrated in figure 10(b). Zhang et al [278] reported a simple, environmentally friendly, one-step method for preparing nitrogen-doped GQDs. This involved the opening of fullerenes using the microwave-activated nitrogen plasma technique, as illustrated in figure 10(c). The method produced crystalline nitrogen-doped GQDs, which exhibited blue fluorescence in a solution. Furthermore, the fluorescence intensity decreases when the concentration of iron ions in the solution increases; hence, the nitrogen-doped GQDs can be used in biosensing applications.
Figure 10. Doping of GQDs by heteroatoms. (a) Schematic of doping of GQDs with heteroatoms for enhancing their performance. (b) Schematic of controlled nitrogen doping of GQDs through laser ablation in aqueous solutions. Reprinted with permission from [277]. Copyright (2019) American Chemical Society. (c) Illustration of the nitrogen-doped GQD fabrication process. Reprinted with permission from [278]. Copyright (2018) American Chemical Society. (d) Schematic of the synthesis of nitrogen-doped GQDs by chemical vapor deposition. Reprinted with permission from [279]. Copyright (2018) American Chemical Society. (e) Schematic depicting nitrogen-and-iron-co-doped GQDs for the detection of ferric ions in biological fluids and cellular imaging. Reproduced from [280] with permission from the Royal Society of Chemistry. (f) Jablonski diagram (top) representing the energy levels of nitrogen-doped GQDs along with associated absorption, PLE and PL spectra (bottom) and optical image of nitrogen-doped GQD solution. Reprinted with permission from [281]. Copyright (2018) American Chemical Society. (g) Synthesis scheme of GQDs with different doping elements via solvothermal method. Reprinted from [282], © 2020 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution imageChemical vapor deposition (CVD) is often used to prepare graphene films. However, Kumar et al [279] demonstrated the use of CVD to produce nitrogen-doped GQDs using chitosan as a carbon and nitrogen source. Chitosan was decomposed at high temperature into carbon compounds containing nitrogen, which were absorbed at the surface of copper foil. The nucleation of the compounds subsequently led to the formation of nitrogen-doped GQDs, as illustrated in figure 10(f). Doping of GQDs with nitrogen can effectively improve their optical properties, as demonstrated by Khan and Kim [281]. They found that the absorption spectra of nitrogen-doped GQDs would extend into the low-energy photon range, as shown in figure 10(f). Moreover, doping GQDs with nitrogen introduced more chemically active sites and enhanced the fluorescence quantum efficiency to 99%. Kuo et al [283] found that the fluorescence of nitrogen-doped GQDs could cover a wide spectrum range (e.g. from ultraviolet to NIR) when the size of the doped GQDs was regulated, hence indicating that the size-dependent fluorescence of GQDs is still significant. The doping of metallic elements in GQDs has recently received much attention. As shown in figure 10(g), isopropyl alcohol containing aluminum, gallium salt and nonmetallic boric acid were used to synthesize aluminum-, gallium- and boron-doped GQDs, respectively [282]. These metal-doped GQDs demonstrated a significant improvement in their fluorescence quantum efficiency, which was due to the chemical bonds of carbon–nitrogen–metal–oxygen. Gao et al [280] reported on the synthesis of nitrogen-and-iron-co-doped GQDs using hydrothermal method. These GQDs were used for the detection of iron ions in biological samples and for cellular imaging, as shown in figure 10(e).
There has been a significant amount of research conducted on the subject of doping GQDs, which includes single-element doping with elements, such as sulfur [184, 284, 285], phosphorus [286], nitrogen [287], fluorine [288], boron [289] and silicon [290], as well as multielement doping with combinations, such as nitrogen–sulfur [291–296], nitrogen–phosphorus [297, 298], nitrogen–boron [299–301] and nitrogen–oxygen [302, 303]. To achieve better performance, multielement doping consisting of four elements has also been reported [304]. Recently, much progress has been made in the development of metal-doped GQDs. For example, the doping of GQDs with magnesium [305] and rare metal elements [306] has been studied for biological sensing and imaging applications. Furthermore, GQDs doped with selenium [307] have been explored for therapeutic applications, such as in the treatment of acute kidney injury. In summary, doping of GQDs [308–316] has been demonstrated to be an effective way to improve the performance of GQDs, which are beneficial for many applications.
4.2. Composite
GQDs can form composites with other materials, which can be inorganic [317–321] or organic [322–331], either to develop a novel material system with new properties or to enhance the properties of the secondary material, as illustrated in figure 11(a). For example, the edge effect of GQDs would facilitate charge transfer between two materials, leading to an enhancement in the performance of GQD composite materials. This section of the review provides a detailed description of the GQD composite materials.
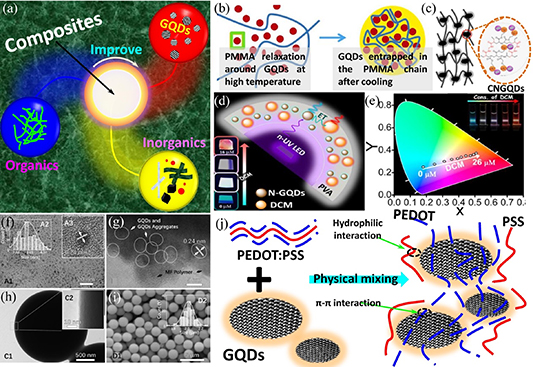
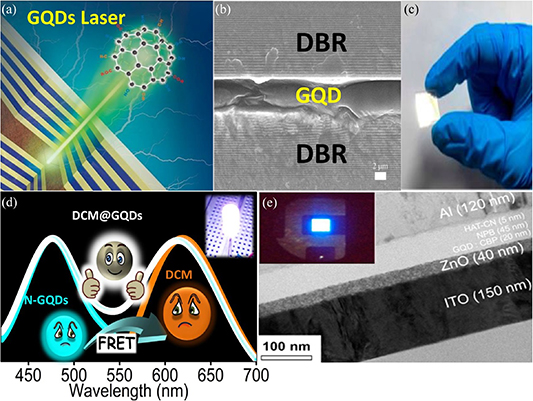
Figure 11. Composite of GQDs with other functional materials. (a) Schematic of the GQD composite with organic or inorganic materials to improve performance. (b) Illustrative representation of the formation and entrapment of CQDs within the PMMA structure. Reprinted from [332], © 2020 Elsevier Ltd. All rights reserved. (c) Chemical structure of the GQD: aerogel. Reprinted with permission from [333]. Copyright (2018) American Chemical Society. (d) Strategy for designing of F-WLED using DCM@N-GQDs0.7 LD as the light emitter and n-UV light-emitting diode as the pumping source (inset: digital photograph of DCM@NGQDs0.7 LD in the PVA matrix under UV radiation). (e) Variation of CIE color coordinates of the FRET-based LD system with different DCM concentrations. Reprinted from [334], © 2020 Elsevier Inc. All rights reserved. (f) High-resolution transmission electron microscope (HRTEM) images of GQDs (left inset: related size distribution). (g) Pulverized GQD–MF microspheres and (h) HRTEM images of the GQD–MF microspheres. (i) SEM image of the GQD–MF microspheres (inset: related size distribution). Reproduced from [335]. CC BY 4.0. (j) Scheme of assembly process of GQDs and PEDOT:PSS. Reproduced from [336]. CC BY 4.0.
Download figure:
Standard image High-resolution image4.2.1. Composite with organic materials.
The formation of GQD composites with organic materials has several advantages. For example, GQDs can be embedded in organic materials, allowing for effective charge transfer. Moreover, the functional groups at the edge of the GQDs can form a strong and effective bond with the organic materials, thus easing the preparation of the GQD composites. Arthisree and Madhuri [337] prepared a composite film consisting of GQDs, polypropylene nitrile and polyaniline. The composite film was used to prepare a supercapacitor, which exhibited excellent performance of several orders of magnitude better than a film without the GQDs. The implementation of GQDs in polymer compounds can also improve the optical properties of the polymer, as demonstrated by Arthisree et al [338]. They prepared a composite of GQDs and polyvinyl butyral and reported an enhancement in the fluorescence spectrum of the GQD/polymer composite. This is due to the bonding of the GQD edge with hydroxyl and carboxyl groups in the polymer, leading to an increase in the number of chemical activity sites, which are favorable to the fluorescence performance of the composite. Using the bottom-up synthesis method, a composite of GQDs and polymer can be prepared using a one-step method. As shown in figure 11(b), Sarno et al [332] prepared a composite of GQDs and poly (methyl methacrylate) using oleic and citric acids via a one-step method. Traces of GQDs, acting as lubricant additives were found in the composite, which resulted in a remarkable improvement in the lubrication characteristics of the polymer. Such a GQD/polymer composite can therefore offer a new type of lubricant. He et al [335] reported the preparation of a white light-emitting device using a composite film of GQDs and melamine formaldehyde (MF). The GQDs were aggregated by encapsulation in the MF microsphere, and the concentration of the GQDs can effectively alter the fluorescence properties of the composite film. The TEM images of the GQD–MF microsphere are shown in figures 11(f)–(i).
In addition, polymers containing GQDs exhibit excellent thermoelectric properties. Du et al [336] reported on the synthesis of GQDs/PEDOT:PSS composite that significantly improved the thermoelectric properties of PEDOT:PSS and demonstrated 550% increase in power consumption factor compared to pure PEDOT:PSS. The assembly process of the GQDs and PEDOT:PSS is illustrated in figure 11(j). The stability of GQDs can be enhanced by developing a strong chemical bond with organic compounds. Martín–Pacheco et al [333] reported on the synthesis of GQD composite based on cationic covalent network, as depicted in figure 11(c). The polymeric network containing GQDs exhibited remarkable physical and optical stabilities, which are crucial for biosensing applications. The properties of the GQD composite could be influenced by both GQDs and organic compounds, as demonstrated by Pramanik et al [334]. They reported the preparation of white light-emitting luminescent composite films by tuning the concentrations of GQDs and DCM dye, as illustrated in figures 11(d)–(e).
4.2.2. Composite with inorganic materials.
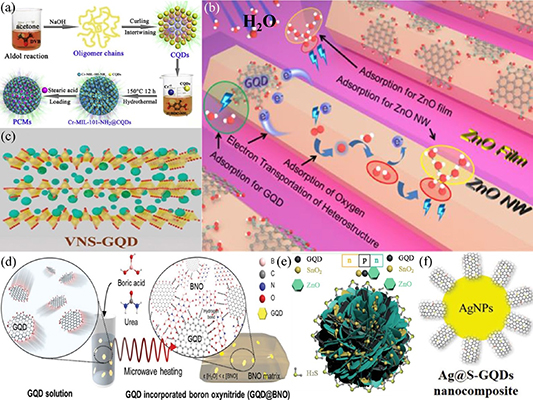
Much research has been reported on the synthesis of GQD-based composites with different inorganic materials, which can enhance the properties of inorganic materials for specific applications. For example, there are reports on the preparation of GQD-based composites with inorganic materials in the form of flakes [339], layers [340], rods [341], nanowires [342], networks [343] and other shapes [344–347], resulting in a remarkable improvement in the properties of inorganic materials. Chen et al [348] demonstrated the integration of GQDs in a metal–organic framework to form a fluorescence-functionalized phase change material (PCM), which was excellent in thermal energy and fluorescence harvesting, as illustrated in figure 12(a). In addition, composites of GQDs and zinc oxide have been widely studied for applications in solar cells, photodetectors, photocatalysis and other fields [349–351]. Recently, Wu et al [352] developed a flexible wearable humidity sensor based on GQDs and zinc oxide nanowire composites. The formation of a p-n junction between the GQDs and zinc oxide nanowires and the large specific surface areas of the nanomaterials contributed to the ultrahigh sensitivity of the device, as illustrated in figure 12(b). In addition, Shao et al [353] prepared a highly selective and responsive gas sensor based on GQD-modified metal oxide materials, consisting of porous and layered structures of tin dioxide and zinc oxide nanomaterials forming n-p-n heterojunctions with p-type GQDs, as shown in figure 12(e). Furthermore, Ahmadi et al [354] prepared electrochemical and photoelectrochemical sensors based on nanocomposites of GQDs, titania and ceria for the detection of dopamine. Compared with the electrochemical sensor, the photoelectrochemical sensor exhibited a lower limit of detection, better sensitivity and a wider detection range. The use of GQD-based composites with inorganic materials in capacitors for energy storage applications has also attracted considerable attention [355–359]. GQDs have significant advantages for improving the performance of capacitors because of their large specific surface area and good electrical conductivity. Yun et al [360] prepared a 3D composite aerogel, comprising of GQDs, reduced graphene oxide (rGO) and porous iron oxide. The composite was used as an anode material for alkaline aqueous batteries, which demonstrated an ultrahigh specific capacity and excellent cycle performance, partly due to the good electrical conductivity of the GQDs. Furthermore, GQDs play an important role in the future development of all solid-state capacitors, thereby circumventing the need for electrolytes in capacitors. Recently, Ganganboina et al [361] introduced GQDs between layers of vanadium oxide nanosheets to form nanocomposites of GQDs and vanadium oxide for use as anode electrodes in energy storage, as shown in figure 12(c). A small amount of GQDs can significantly improve the performance of energy storage batteries. Their work demonstrated the novel use of GQDs in developing nanocomposites with multilayers of two-dimensional materials for energy storage applications. The enhancement of the optical properties of GQD-based composites with inorganic materials has been reported by several groups [362–369]. Park et al [370] synthesized GQDs and boron oxynitride composite using a one-step microwave heating process, as illustrated in figure 12(d). The composite exhibited a high photoluminescence quantum yield (PL-QY) of up to 36.4%, which was eightfold higher than that of pristine GQD in water. Charge transfer between GQDs and titanium dioxide nanoplates in nanocomposites was studied by Murali et al [371]. The large specific surface area and edge effect of the GQDs would promote charge transfer between the two materials, which was used in gas sensing of nitric oxide. Cobalt/nickel-based capacitors can be found in many commercial applications; hence, improvement in their performance is of great commercial interest. Luo et al [372] reported on the synthesis of composites consisting of tremella-like NiCo2O4 coated with GQDs. The composite exhibited an excellent specific capacitance and energy density due to the abundant edge sites of the GQDs. GQD-based composites can also be used for antibacterial applications, as demonstrated by Kadian et al [373]. They prepared nanocomposites consisting of silver nanoparticles decorated with sulfur-doped GQDs, as shown in figure 12(f). The nanocomposites demonstrated good dispersion and stability with a significant improvement in antibacterial activity. Cyclic stability and thermal safety are of paramount importance in supercapacitors. Sun et al [374] coated the surface of cobalt–lithium nanoparticles with GQDs, thus forming a stable conductive layer at the surface of the nanoparticles. The thermal safety and cycling performance of the cobalt–lithium capacitors improved remarkably due to the excellent conductivity and stability and the large specific surface area of GQDs. Yuan et al [375] reported the synthesis of nanocomposite consisting of graphitic carbon nitride nanorods decorated with GQDs using a hydrothermal method, which allowed the formation of closely contacted nanorods and GQD interface. An improvement in the photocatalytic activity of the nanocomposite for the removal of antibiotics was observed compared to that of the pristine nanorods. GQDs decorated on the surface of multiwall carbon nanotubes were studied by Arumugasamy et al [376]. The nanocomposite was used in electrochemical sensor for the detection of dopamine. The incorporation of GQDs enhanced the electrocatalytic activity, sensitivity, selectivity and reproducibility of the sensor. The properties of inorganic materials have been shown to improve significantly upon incorporation of GQDs [377–390]. The excellent properties of the GQD-based composite materials could lead to many novel applications.
Figure 12. Composite of GQDs with various inorganic substances. (a) Schematic of the photoluminescence-functionalized composite PCMs. Reprinted from [348], © 2018 Published by Elsevier B.V. (b) Schematic of the adsorption mechanism of H2O molecules on composite sensing layer. Reprinted with permission from [352]. Copyright (2020) American Chemical Society. (c) Synthesis scheme of interlayer-embedded GQDs endows V2O5 with the hydrothermal method. Reproduced from [361] with permission from the Royal Society of Chemistry. (d) Schematic of the fabrication of GQD@boron oxynitride by microwave heating [370]. John Wiley & Sons.© 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Schematic of the band configuration at the interface of the GQD−SnO2/ZnO nanostructure in different atmospheres. Reprinted with permission from [353]. Copyright (2020) American Chemical Society. (f) Schematic of the structure of Ag@S-GQDs nanocomposite. Reproduced from [373] with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution image5. Applications
GQDs have many exciting applications in various fields due to their excellent properties and facile preparation techniques. The size dependence of GQDs on their optical and electronic properties has enabled the application of nanomaterials in the field of photoelectronics, such as broadband photodetectors [391], solar cells [273], white light-emitting diodes (LEDs) [141], fluorescent probes [110], lasers [167] and integrated optics [224]. As GQDs are members of the carbon family and have similar biological compatibility, especially GQDs in the nanometer regime, the nanomaterials have been explored for use in various biological applications, such as biomedicine [307], biological markers [98] and cancer treatment [111]. The ease of functionalization of GQDs has enabled them to find important applications in the field of agriculture for the removal of contaminants and detection of hazardous analytes, as well as agricultural nitrogen engineering using GQD-based nanocomposites [392]. The large specific surface area of GQDs has led to their utilization in various applications, such as in anticorrosion [393] and gas sensor [394]. In addition, Janus micromotors have been developed using modified GQDs to provide ultrafast detection of bacterial endotoxins [395]. In recent years, the characteristics and properties of GQDs have been studied extensively, which has brought benefits to many different fields of application. This section provides an overview of the different applications of GQDs, ranging from biomedical to energy applications.
5.1. Biomedical applications
GQDs have attracted considerable attention from researchers in the field of biomedicine due to their nanometer-scale size and biocompatibility [125, 396–404]. The nanometer-scale size of GQDs allows them to penetrate cells for diagnostic and therapeutic applications. Furthermore, the ease of functionalization of the edges of GQDs has led to the use of nanomaterials for drug delivery into cells, thereby improving the therapeutic effects of drugs. The remarkable thermal and electrical properties of GQDs allow the effective transformation of light energy into heat energy under NIR light irradiation, making them suitable for use in photothermal and photodynamic therapies. In addition, GQDs have been found to exhibit special properties that act as peroxidase or oxidase via electron transportation to convert certain biomolecules from normal species (e.g. H2O2 and 3O2) to cytotoxic reactive oxygen species (ROS) (e.g. ˙OH and 1O2) upon light irradiation [237], thus promoting wound healing. The strong and broad fluorescence properties of GQDs have benefitted applications, such as bio-imaging [405, 406] and metal ion detection [407–412] in a variety of fields. Indeed, GQDs have many important applications in the biomedical field, ranging from diagnostics to treatment, as illustrated in figure 13(a).
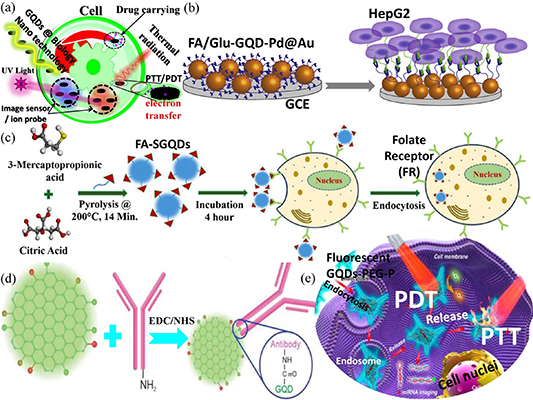
Figure 13. Biological applications of GQDs. (a) Bionanotechnology applications of GQDs, ranging from drug carriers and biotherapy to bioimaging and probe applications. (b) Schematic of the fabrication of an electrochemical sensing platform for cancer cells. Reprinted from [413], © 2020 Elsevier B.V. All rights reserved. (c) Schematic of the synthesis process of FA-SGQDs and their application in targeted bioimaging of FR overexpressed cancer cells. Reproduced from [414], with permission from Springer Nature. (d) Schematic of attaching anti-PSMA antibody on GQDs. Reproduced from [415] with permission from the Royal Society of Chemistry. (e) Theranostic platform for intracellular miRNA detection and combined photothermal therapy (PTT)/photodynamic therapy (PDT) [416]. John Wiley & Sons.© 2020 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
Standard image High-resolution image5.1.1. Cancer cell and tumor therapy.
Recently, Ruiyi et al [413] reported the use of GQDs functionalized with folic acid and glutamic acid in gold-coated palladium nanoparticles (acting as redox probes) for electrochemical detection of circulating cancer cells in human blood. Figure 13(b) shows the preparation process of the hybrid nanomaterials. The folic acid- and glutamic acid-functionalized GQDs would offer strong binding to cancer cells and provide reversible redox reactions that produce electrochemical signals upon binding. Such a hybrid significantly enhances the electrocatalytic activity and redox characteristics, leading to a low detection limit of two cells per milliliter. Kadian et al [414] prepared sulfur-doped GQDs functionalized with folic acid and used them as a fluorescent probe, which exhibited high quantum efficiency of 78%. The functionalized GQDs were capable of identifying folate receptor (FR)-positive and FR-negative cancer cells, as depicted in figure 13(c). The edge of the GQDs can be modified with antibodies specific to cancer-derived exosomes for medical diagnosis, as demonstrated by Barati et al [415]. Figure 13(d) shows a schematic of the GQDs immobilized with antibodies that can be used for the detection of exosomes. The ability to immobilize antibodies in GQDs will allow the future development of GQD-based immunosensors for the rapid detection of diseases. GQDs exhibit a high photothermal conversion efficiency under NIR light irradiation. This characteristic has enabled the development of photochromic nanoparticles for photoacoustic imaging-guided photothermal chemotherapy [416]. Once the GQDs enter cells, such as viral or cancer cells, the temperature of the GQDs increases upon irradiation with NIR light, resulting in selective cell death due to the elevated temperature, hence leading to biological therapy. This is the basic principle of photothermal therapy (PTT) using GQDs. In addition, functionalized GQDs, which exhibit high singlet oxygen generation, are suitable for use in photodynamic therapy (PDT). The production of singlet oxygen promotes the redox reaction in cells, thus causing rapid decay of cells. The mechanism of these two therapeutic strategies using GQDs for cancer treatment related to irradiation with light energy is shown in figure 13(e).
The use of GQDs to improve disease diagnosis and treatment has been demonstrated by several research groups [417–421]. Composites based on GQDs have also been studied by many researchers to further enhance their efficiency in biomedical applications. Recently, Zheng et al [422] prepared porous copper sulfide nanoparticles decorated with GQDs for controlled intracellular drug release. Anti-cancer drugs, such as doxorubicin, were embedded in the porous copper sulfide nanoparticles. Upon irradiation with NIR light, the drug was released due to an increase in temperature experienced by the nanocomposite of doxorubicin, GQDs and copper sulfide nanoparticles. Therefore, the nanocomposite provided a combination of PTT and chemotherapy for the treatment of cancer, as illustrated in figure 14(a). Figures 14(c)–(f) show confocal images of cancer cells after different treatments. Sung et al [423] reported on the preparation of GQDs and docetaxel composite supported with red blood cell membrane. The nanocomposite served as a stealth agent and photolytic carrier, which could deliver drugs deep into the tumor tissue via the bloodstream. A combination of chemotherapy and photolytic effects from the GQD-based nanocomposite upon irradiation with NIR light effectively damaged and inhibited the tumor cells, as illustrated in figure 14(b). GQDs are often used as markers for biological imaging. Interestingly, the fluorescence properties of GQDs are not only dependent on their size and functional groups but also on their temperature, as discovered by Gao et al [424]. Figures 14(g)–(l) show the confocal images of HeLa cells with GQDs as fluorescent labels at different temperatures. Their work suggests that GQDs are suitable for use as biological thermoprobes and selective temperature detectors, hence adding new functionalities to GQD fluorescent probes. Early detection of cancer is important for the successful treatment of the disease. Much effort has been made to study the use of GQDs for cancer diagnosis. Zhang et al [425] designed and prepared nanocomposites of GQDs and single-molecule DNA as diagnostic probes to detect cellular apurinic/apyrimidinic endonuclease 1 (APE1), which has been identified as a predictive cancer biomarker. A large accumulative fluorescent signal in living cells can be generated by a small quantity of cellular APE1 through repeated enzyme catalytic circulation, as depicted in figure 14(m). The nanocomposite can also be used for the highly sensitive and specific detection of other APE1-dysregulated diseases. GQDs are attractive nanomaterials for application in the field of biotherapy due to their excellent biocompatibility and nontoxicity. The toxicity of GQDs and GO was studied by Hashemi et al [426]. They found that GQDs exhibited lower toxicity than GO, as the latter had a greater influence on the basal level of genes and mitochondrial membrane potential (MMP). Figures 14(n)–(s) show the fluorescence microscopy images of MCF-7 cells with nontoxic doses of GO and GQDs.
Figure 14. (a) Schematic representation of multifunctional DOX-CuS@GQDs NPs: fabrication process and illustration of controlled intracellular release and combined photothermal chemotherapy. (b) Schematic representation of targeted RBC-membrane-enveloped nanosponge-mediated tumor accumulation and drug/GQD penetration. Reprinted with permission from [423]. Copyright (2018) American Chemical Society. (c)–(f) Confocal images of MDA-MB-231 cells after different treatments and co-staining with calcein AM and PI; the green and red areas represent the regions of living and dead cells, respectively. Reproduced from [422], with permission from Springer Nature. (g)–(l) Confocal cell imaging (488 nm laser excitation) of HeLa cells with R-GQDs at different temperatures: 32 °C (g), (j), 37 °C (h), (k), and 42 °C (i), (l). (g), (h) and (i) are the fluorescence images of HeLa cells; (j), (k) and (l) are the merged (dark field merged with bright field) pictures. Reprinted with permission from [424]. Copyright (2020) American Chemical Society. (m) Schematic display of GQD-based nanocomposites for diagnosing cancer biomarker APE1 in living cells. Reprinted with permission from [425]. Copyright (2020) American Chemical Society. (n)–(s) Effects of nontoxic doses of GO-100 and GQDs-50 (15 μg ml−1) on MMP. The cells were stained with rhodamine 123. Fluorescence microscopy images of MCF-7. Reprinted from [426], © 2020 Elsevier Ltd. All rights reserved. (t) Laser confocal scanning microscopy (LCSM) images of merged images of breast CSCs incubated with CSCNP-R-CQDs (200 μl 1 mg ml−1) for 12 h and breast CSCs stained with DAPI (inset: photographs of the CSCNP-R-CQD aqueous solution under UV light (365 nm)). Reprinted with permission from [427]. Copyright (2020) American Chemical Society.
Download figure:
Standard image High-resolution imageBy coating GQDs with anticancer drugs at their surface, nanomaterials can be used to deliver drugs to cancer cells to treat the disease. Recently, Fan et al [427] reported the preparation of GQDs loaded with doxorubicin (an anticancer drug) and found that the drug-loaded GQDs could penetrate cancer cells and cancer stem cells. The doxorubicin-loaded GQDs demonstrated remarkable therapeutic effects by killing cancer stem cells. Figure 14(t) shows a laser confocal scanning microscopy image of the drug-loaded GQDs in cancer stem cells. Over the last couple of years, there have been an increasing number of reports on the use of GQDs to treat cancer with some intriguing experimental results, thus bringing the possibility of curing cancer closer to reality.
5.1.2. Therapy for other diseases.
GQDs have demonstrated a wide range of applications in the biological field due to their ease of functionalization and biocompatibility. Recently, Gong et al [428] designed and prepared an artificial enzyme consisting of histidine-functionalized GQDs and a hemin (His-GQDs/hemin) complex. Figures 15(a) and (b) show the TEM images and structure of the artificial enzyme, respectively. The His-GQDs/hemin complex, which can detect hydrogen peroxide and blood glucose, exhibited a relatively high catalytic performance and excellent acid resistance and can operate over a wide temperature range. The use of GQDs in the design of artificial enzymes provides an effective platform for practical applications. The functional groups of GQDs can significantly influence their optical and electrical properties. Landry et al [429] found that the degree of oxidation of GQDs has a remarkable effect on the adsorption of biopolymers, such as single-strand DNA (ssDNA). For example, the adsorption of ssDNA was weak at low-oxidized GQDs, whereas strong adsorption of ssDNA was observed in nonoxidized GQDs, as illustrated in figure 15(c). The intrinsic fluorescence of the GQDs was reduced dramatically when ssDNA was absorbed onto low-oxidized GQDs, suggesting that the GQD properties can be regulated by the polymer sequence and type. GQDs have also been shown to be effective in treating diseases, such as Alzheimer's disease [430], diabetes [431] and mitochondrial dysfunction [432]. For example, aggregation and transmission of α-synuclein (α-syn) in the midbrain may be related to the pathogenesis of Parkinson's disease. Kwon et al [430] found that GQDs could inhibit fibrillization of α-syn and interact directly with mature fibrils to trigger disaggregation, as depicted in figure 15(d). Hence, GQDs can be potentially used to treat Parkinson's disease. GQDs have been studied for use as drug carriers due to their biocompatibility. Namazi et al [433] reported on the use of GQDs as cross-linker for carboxymethyl cellulose. The nanocomposite hydrogel was biocompatible and exhibited pH-sensitive swelling and degradation properties. It can be loaded with drugs, and the release of drugs can be triggered by the pH. Using doxorubicin as an example, the researchers investigated the drug delivery properties of the nanocomposite hydrogel, as shown in figure 15(e). Recently, GQDs were used as contrast agents in magnetic resonance imaging (MRI), as reported by Li et al [434]. They prepared Gd3+-loaded polyethylene glycol-modified GQDs as contrast agent for MRI. They found that changing the localized superacid microenvironment of the nanocomposite can significantly improve its magnetic relaxivity, which was much higher than that of commercially available contrast agents, thus enhancing the performance of MRI, as shown in figure 15(f). Furthermore, the nanocomposite modified with folic acid was suitable for MRI-fluorescent dual-mode targeted tumor imaging with low biotoxicity, both in vitro and in vivo. The GQD-based contrast agent is of great interest in its applications in MRI, as it provides accurate monitoring and diagnosis of diseases. The use of GQDs as metal ion probes in biosensing applications has attracted much research interest [435–438]. Wang et al [439] prepared nitrogen-doped GQDs exhibiting yellow emission with high quantum yield. The doped GQDs were used to detect iron ions in natural water and potentially for intracellular iron ion detection. Complexation between iron ions and nitrogen-doped GQDs can significantly quench the fluorescence intensity of the doped GQDs, which is highly selective for iron ions, as illustrated in figure 15(g). Another example of the use of GQDs as metal ion probes was demonstrated by Fan et al [440]. They modified a solution-gated graphene transistor with GQDs, as shown in figure 15(h). A change in the electrical double-layer capacitance at the gate due to the interaction between copper ions and GQDs results in a change in the channel current. They found that copper ions exhibited excellent binding characteristics with GQDs, making the sensor highly sensitive and selective to copper ions. GQDs have significant therapeutic effects on tumors. In addition to the combined PTT, PDT and drug delivery therapy, GQDs also play an important role in tumor radiotherapy. Tung et al [441] reported on the use of GQDs grafted with 2-deoxy-d-glucose as radiosensitizer to treat osteosarcoma, which showed improvement in the therapeutic efficacy, as shown in figure 15(i). The improved therapeutic effect is due to a significant increase in oxidative stress response and DNA damage in osteosarcoma cells caused by the GQD complex, which selectively targets tumor cells. Therefore, the GQD complex has the potential to achieve low-dose high-precision radiotherapy treatment for osteosarcoma. In recent years, GQDs have been used in many applications in the biomedical field, such as biomedical imaging [442–446], immune probes [447], fluorescence probes [448–456], drug carriers [457, 458], sterilization [459–462], wound healing [463–465] and cancer treatment and diagnosis [421, 425, 433, 441]. The effectiveness of nanodrugs to different age groups has also been studied [466]. In summary, the excellent properties of GQDs will significantly impact the biomedical field in the near future, ranging from diagnosis to treatment of diseases.
Figure 15. (a) Morphology of His-GQD/hemin, as recorded by TEM. (b) Proposed structure of His-GQD/hemin containing hemin, histidine functional groups and the hydrophobic basal plane of GQD. Reproduced from [428], with permission from Springer Nature. (c) Molecular dynamics simulations confirmed the dependency of A30 ssDNA adsorption on the GQD oxidation level. The final configurations of A30 ssDNA with GQD-0%, GQD-2% and GQD-17% (from left to right) for a 100 ns simulation. Reproduced from [429]. CC BY 4.0. (d) TEM images of preformed α-syn fibrils at various time points (6 and 12 h, and 1, 3 and 7 d) in the absence (top) and presence (bottom) of the GQDs. Reproduced from [430], with permission from Springer Nature. (e) Photograph of the doxorubicin-loaded CMC/GQDs. Reprinted from [433], © 2019 Elsevier B.V. All rights reserved. (f) An in vivo MRI of a nude mouse is shown (left) pre-injection, and then (right) 30 min post-injection with GP6G-3 via the tail vein. The mouse was injected subcutaneously and imaged using a 7.0 T animal MRI scanner; the portion in the carmine circle was a tumor. Reprinted from [434], © 2020 Elsevier Ltd. All rights reserved. (g) The zeta potentials of nitrogen-doped GQDs toward different Fe3+ concentrations in the system. Reprinted from [439], © 2020 Elsevier B.V. All rights reserved. (h) Schematic of the Cu2+ ion sensor based on solution-gated graphene transistors. Reprinted with permission from [440]. Copyright (2020) American Chemical Society. (i) The invasion capacity was examined by transwell chamber assays after incubation with 2DG-g-GQD for 24 h. Reproduced from [441] with permission from the Royal Society of Chemistry.
Download figure:
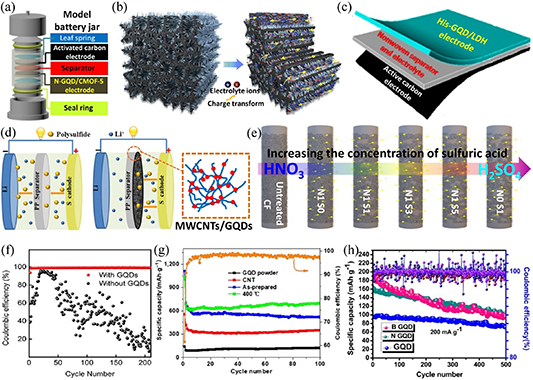
Standard image High-resolution image5.2. Energy applications
GQDs have wide-ranging applications in the field of energy, which covers energy generation to consumption (as illustrated in figure 16(a)), because of their excellent properties and low-cost facile preparation methods. In energy generation, GQDs have been explored for use in solar photovoltaic devices [467–472] and hydrogen production from photo-hydrolysis of water [473–477]. In energy storage, GQDs have been used in the preparation of electrodes for supercapacitors due to their large specific surface areas and good electrical properties [478–482]. In terms of energy consumption, GQDs have been used to enhance the brightness and tailor the color of light-emitting devices due to their excellent optical properties [483–486]. Therefore, this review provides a detailed description of the applications of GQDs in the three aspects of energy generation, storage and consumption, especially the important research achievements in recent years.
Figure 16. Energy applications of GQDs. (a) Schematic of the energy applications of GQDs from energy generation to energy storage to utilization. (b) Schematic of the engineered interfaces throughout the entire perovskite solar cell via the incorporation of N-and-S-co-doped GQDs. Reprinted with permission from [487]. Copyright (2020) American Chemical Society. (c) Schematic of the positive role of FGQDs in perovskite films. Reprinted with permission from [488]. Copyright (2020) American Chemical Society. (d) Schematic of nitrogen-doped GQDs for solar hydrogen production. Reprinted with permission from [489]. Copyright (2020) American Chemical Society. (e) Photographs of nitrogen-doped GQD solution under light irradiation at different times. Reprinted from [490], © 2020 Elsevier Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageMany efforts in the field of solar energy research have been directed toward achieving a high level of efficiency in the utilization of solar energy. In addition to achieving high solar energy conversion efficiency, researchers are investigating means to reduce the cost of solar energy utilization, store solar energy and improve the ability to capture solar energy. Recently, many research groups have studied the use of GQDs in solar cells [380, 491–505] and in solar hydrolytic hydrogen production [369, 506–514]. Both solar cells and solar hydrolytic hydrogen production provide clean sources of energy, and the former convert light energy directly into electricity; hence, it can be considered as an efficient means of solar energy utilization. GQDs can play a significant role in improving the performance of solar cells due to their remarkable electrical and optical properties. For example, Kim et al [281] found that GQDs exhibit a significant photon down-conversion effect, which is particularly significant when doped with nitrogen. The nitrogen-doped GQDs exhibited fluorescence quantum efficiency of 99% and large Stokes shift of 98 nm. When combining nitrogen-doped GQDs with copper indium gallium selenide (CIGS), the conversion efficiency of the thin-film solar cell reached 15.3%. The enhancement in performance was due to the photon down-conversion and light-trapping effect of the nitrogen-doped GQDs. Perovskite solar cells have attracted much research interest in recent years due to their many advantages, such as low cost and high conversion efficiency. Gan et al [276] used nitrogen-doped GQDs as functional semiconductor additives in perovskite thin films. The nitrogen active sites in the GQDs passivated the grain boundary trap states. The matching of the energy structure of the GQDs at the grain boundary with that of the perovskite enabled charge transport at the grain boundaries. Furthermore, the n-type behavior of the nitrogen-doped GQDs significantly improved the electronic properties of the perovskite thin film, resulting in an improvement in charge transport as well as a reduction in interface recombination. The conversion efficiency of the solar cells was reported to be as high as 19.8%. Moreover, the solar cell exhibited stable performance without encapsulation due to the protected grain boundaries and the hydrophobicity of the modified film with the addition of nitrogen-doped GQDs. Chen et al [487] also studied the effect of adding GQDs to perovskite solar cells and found that GQDs have several functions, such as promoting crystal growth of perovskite, easing extraction of charge at cathode and anode interfaces, inducing defect passivation and inhibiting charge recombination. Upon introducing GQDs into Fe2O3-based perovskite solar cells (as shown in figure 16(b)), the conversion efficiency of the solar cells increased from 14% to 19.2%. The solar cells also demonstrated a significant improvement in durability, including humidity, ultraviolet light and temperature stabilities. To date, although the performance of most flexible solar cells is not as good as that of rigid solar cells, the wearable and portable nature of flexible solar cells has attracted much research effort, as they offer many exciting applications. Yang et al [488] found that fluorine-doped GQDs can effectively reduce defect density at the perovskite thin film by passivating the grain boundaries and surface, thus increasing the conversion efficiency of the solar cell to 20.40%. The perovskite solar cell with fluorine-doped GQDs exhibited excellent thermal and environmental stabilities, as it could prevent the invasion of external water molecules and the spread of ions outside the perovskite, as depicted in figure 16(c). A solar cell converts light energy into electrical energy, whereas a photodetector converts light energy into electrical signals. Although these two devices yield different results, the design of the solar cell is similar to that of a photovoltaic detector. Shin et al [515] reported the use of GQDs as hole transport layers in perovskite solar cells. They found that GQDs improved the crystallinity of the perovskite film and increased the work function of the hole transport layer, resulting in enhanced solar cell performance. They also suggest that GQDs in perovskite films, whether as solar cells or photodetectors, would have the same effect. To understand the passivation of grain boundary in a perovskite film using GQDs, Ma et al [516] added GQDs containing hydroxyl and carbonyl functional groups to a perovskite solution. The addition of GQDs led to an improvement in the photoluminescence intensity of the film and carrier lifetime, thereby suggesting a reduction in nonradiative recombination due to the passivation of the grain boundary at the film. Furthermore, the addition of GQDs increased the thickness of the perovskite film, resulting in an improved conversion efficiency of up to 18.24% for this solar cell. Silicon is currently the most widely used material for commercially available solar cells. The performance of silicon solar cells has almost reached its maximum efficiency due to the maturity of the silicon technology. However, researchers are still making relentless efforts to reduce the cost and increase the conversion efficiency of silicon solar cells. Diao et al [517] reported the fabrication of heterojunction solar cells consisting of GQDs and silicon. The GQDs were used as the hole transport layer and electron-blocking layer. They separate the photogenerated electron–hole pairs and inhibit carrier recombination at the anode. These heterojunction solar cells could achieve a conversion efficiency of 12.35%. In recent years, much research effort has been devoted to studying the use of GQDs in solar cell applications. Table 2 provides a list of the GQD-based solar cells with their performance indicators.
Table 2. Properties of GQD-based solar cells.
| Device structure | Doped elements | Rigid/flexible | Short-circuit current (mA cm−2) | Open-circuit voltage (V) | Fill factor (%) | External quantum efficiency (%) | Power conversion efficiency (%) | References |
|---|---|---|---|---|---|---|---|---|
| Gr/Mo/CIGS/CdS/ZnO/NGQDs/Ni-Al | N | Rigid | 31.77 | 0.668 | 73.09 | 80 at 600 nm | 15.31 | [281] |
| N-GQDs PMMA/FTO/ZnO:Al/ZnO/CdS/CIGS/Au | N | Rigid | 36.13 | 0.6592 | 67.91 | — | 16.13 | [273] |
| NiOx /GN-GQDs + PVK/PCBM/BCP/Ag | N | Rigid | 23.4 | 1.06 | 80 | 95 at 500 nm | 19.8 | [276] |
| Glass/ITO/PANI-GQDs/Al | PANI | — | 25.11 | 0.34 | 0.10 | — | 0.86 | [323] |
| FTO/PEDOT:PSS/GQDs/PVK/PCBM/Ag | — | Flexible | 21.41 | 1.002 | 75.31 | — | 16.15 | [324] |
| ZnO/GQDs (DSSCs) | — | Rigid | 12.2 | 0.68 | 63 | — | 5.27 | [347] |
| TFSA-GR/MoS2/P3HT:PCBM:GQDs/Al | — | Flexible | 10.88 | 0.588 | 66.08 | 70 at 500 nm | 4.23 | [467] |
| FTO/TiO2/GQDs/N719/Iodolyte (DSSCs) | — | Rigid | 13.77 | 0.7 | 44 | — | 5.1 | [470] |
| PEDOT:GQDs/porous Si/n-Si/TiO2 | — | Rigid | 28.53 | 0.537 | 68.48 | 80 at 500 nm | 10.49 | [471] |
| Gr/GQDs/Si | — | Rigid | 29.33 | 0.51 | 66.59 | — | 9.97 | [472] |
| FTO/α-Fe2O3/N,S-GQDs/PVK/N,S-GQDs/Spiro-OMeTAD/Au | N,S | Rigid | 23.6 | 1.047 | 79 | — | 19.2 | [487] |
| Gr/GQDs/n-Si/In-Ga | — | Rigid | 30.74 | 0.58 | 63 | 85 at 500 nm | 12.35 | [517] |
| FTO/S,N-GQDs-sensitized C-ZnO (DSSCs) | S,N | Rigid | 1.84 | 0.36 | 45.28 | — | 0.293 | [380] |
| ITO/SnO2/SnO2:GQDs/PVK | — | Rigid | 24.4 | 1.11 | 78 | 90 at 550 nm | 21.1 | [491] |
| FTO/N-GQDs-N719/TiO2/Pt/FTO (DSSCs) | N | Rigid | 17.65 | 0.72 | 59 | — | 7.49 | [492] |
| FTO/TiO2-GQDs-N719/Pt/FTO (DSSCs) | — | Rigid | 20.03 | 0.73 | 61 | — | 8.92 | [493] |
| FTO/TiO2-GQDs-Ulva/Pt/FTO (DSSCs) | — | Rigid | 2.04 | 0.75 | 52 | — | 0.81 | [494] |
| FTO/TiO2/GQDs/PVK/Spiro-OMeTAD/Au | — | Rigid | 21.92 | 0.97 | 67 | 82 at 400 nm | 14.36 | [495] |
| ITO/NiOx /NiOx :AGQDs/PVK/PCBM/BCP/Ag | Amino | Flexible | 22.3 | 1.05 | 83.1 | — | 19.4 | [496] |
| FTO/SnO2:GQDs/PVK/Spiro-OMeTAD/Au | — | Flexible | 23.5 | 1.08 | 77 | 90 at 500 nm | 19.6 | [497] |
| ITO/NiO/NiO:GQDs/PVK/PCBM/Ag | — | Rigid | 20.22 | 1.08 | 77.15 | — | 16.97 | [498] |
| In-Ga/Si/CNTs/GQDs/PVP/Ag | — | Rigid | 35.58 | 0.598 | 70.22 | 90 at 700 nm | 14.94 | [499] |
| Glass/ITO/PEDOT:PSS/P3HT:PCBM:GQDs/Al | — | Rigid | 18.9 | 0.55 | 32 | — | 3.32 | [500] |
| FTO/ZnO:GQDs/PVK/Spiro-OMeTAD/Au | — | Rigid | 24.7 | 1.02 | 70 | — | 17.63 | [501] |
| ITO/TiO2:GQDs/N719/Pt (DSSCs) | — | Flexible | 14.32 | 0.68 | 53.2 | — | 5.18 | [502] |
| GQDs/PVK | N | Rigid | 24.7 | 0.81 | 43.8 | — | 8.77 | [504] |
| GQDs/Si | N | Rigid | 31.94 | 0.601 | 70 | — | 13.4 | [505] |
Note: PTAA, poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine]; PVK, perovskite; PANI, polyaniline; DSSCs, dye sensitized solar cells; CIGS, copper indium gallium selenide; TFSA-GR, bis-(trifluoromethanesulfonyl)-amide-doped graphene.
GQDs have also been used to produce hydrogen using solar energy. Hydrogen can be used as a green energy source, and recent research has focused on methodologies to produce hydrogen efficiently. The key to efficient hydrogen production is to improve the catalytic performance of the photocatalyst. Huang et al [518] prepared a ternary composite photocatalyst that comprises BiOCl, GQDs and rGO. The GQDs significantly improved the photocatalytic performance by 8.4 times compared to pure BiOCl due to an enhancement in charge separation and injection. Compared with rGO, the GQDs demonstrated much better performance. The study found that the GQDs did not significantly enhance the light absorption and showed that the improvement in photocatalytic performance was not necessary due to an enhancement in optical absorption; however, the electrical properties of the composite were also of particular importance. GQDs doped with nitrogen exhibited remarkable optical and electrical properties, which could affect their photocatalytic performance. Tsai et al [489] found that C–N bond induced visible light absorption when GQDs were doped with nitrogen, as shown in figure 16(d). Moreover, an increase in the carrier lifetime and concentration was reported upon increasing the nitrogen concentration in the GQDs. Compared to intrinsic GQDs, the nitrogen-doped GQDs demonstrated an increase in photocatalytic hydrogen production efficiency due to an enhancement in charge dynamics and reaction kinetics and increased carrier concentration. This finding suggests that doping of GQDs is an effective way to improve their photocatalytic performance. The use of GQD-based composites, consisting of other photocatalytic materials, is another effective way to improve the efficiency of photocatalytic hydrogen production. Chang et al [519] demonstrated the use of GQDs in CdSe-sensitized TiO2 nanorods to improve the photocatalytic efficiency for solar hydrogen production. It was found that the introduction of GQDs resulted in vectorial charge transfer and improved reaction kinetics. Importantly, the GQDs reduced the photoetching at CdSe, thereby ensuring the long-term stability of the electrode. In addition, Xue et al [520] prepared a CdS–GQD–titanate nanotube ternary nanocomposite for hydrogen production. The nanocomposite demonstrated remarkable photocatalytic performance due to its enhanced ability to capture visible light, longer lifetime of photogenerated carrier, faster interfacial charge transport rate and longer electron transport distance. In addition to improving the catalytic performance for the solar photolysis of water, GQDs can also perform photocatalytic hydrolysis of certain pollutants [521–524]. Dejpasand et al [490] prepared nitrogen-doped GQDs with a broad absorption spectrum and down-conversion effect. The photodegradation of methylene blue using nitrogen-doped GQDs under illumination was studied using energy states. Figure 16(e) shows the degradation of the methylene blue solution using nitrogen-doped GQDs under different illumination durations. Therefore, the aforementioned studies clearly indicate the important role of GQDs in improving the photocatalyic performance.
Energy storage is critical for the implementation of renewable energy. It is also important for mobile devices and electric vehicles. A battery is used to store electrical energy and has a device structure similar to that of a capacitor consisting of a dielectric material sandwiched between two electrodes to charge and discharge electric charges. Much research has been conducted on the development of environmentally friendly and low-cost capacitors that exhibit high energy densities and stabilities. Carbon was first used as an electrode material in lithium-ion batteries in the 1980s [525]. Subsequently, many groups have studied other forms of carbon materials, such as graphite, carbon nanotubes, graphene and GQDs, as electrode materials. Li et al [526] prepared nitrogen-doped GQDs onto a carbonized metal–organic framework (cMOF), which was used as an electrode material for supercapacitors. The nitrogen-doped GQDs played a vital role in improving the pseudocapacitive activity and surface wettability of the electrodes, leading to enhanced performance of the supercapacitor. Figure 17(a) shows the structure of the supercapacitor consisting of nitrogen-doped GQDs/cMOF as the anode and activated carbon as the cathode.
Figure 17. Applications of GQDs in energy storage batteries. (a) Schematic of asymmetric nitrogen-doped GQD/cMOF-5/AC supercapacitor. Reproduced from [526] with permission from the Royal Society of Chemistry. (b) Schematic of ions and charge transfer inside the PWC/MnO2/GQDs electrode. Reprinted from [527], © 2020 Elsevier Ltd. All rights reserved. (c) Illustration of the composition of a supercapacitor. Reprinted from [528], © 2020 Elsevier Inc. All rights reserved. (d) Schematic representation of lithium–sulfur (Li–S) batteries employing a commercial PP separator and the MWCNT/NCQD-coated separator [529]. John Wiley & Sons.© 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (e) Illustration of the generation of the hydrophilic group and GQDs on CF with different acid treatments. Reprinted with permission from [530]. Copyright (2020) American Chemical Society. (f) Cycling performances of the Li|Cu cells using electrolyte with and without GQDs at the current density of 1 mA cm−2 with 1 mAh cm−2 plating capacity in each cycle. Reprinted from [531], © 2019 Elsevier Ltd. All rights reserved. (g) Cycling performance at 100 mA g−1 for GQD powders, original CNTs, as-prepared and GQD@CNTs annealed at 400 °C. Reproduced from [532], Copyright © 2020, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature. (h) Cyclic stability curve of GQD, NGQD and BGQD. Reprinted from [533], © 2019 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution imageThe low-cost, facile preparation of supercapacitor electrodes is of great commercial interest. Zhang et al [527] prepared composite electrodes consisting of porous wood carbon (PWC), MnO2 and GQDs. After pyrolyzing natural wood to produce PWC, MnO2 and GQDs were decorated on PWC using a facile hydrothermal method. A schematic of the PWC/MnO2/GQDs composite electrode is shown in figure 17(b). The GQDs significantly promoted the transport of ions and protected MnO2 from falling off from the surface of the PWC, leading to an improvement in the electrochemical performance of the electrodes and demonstrating good rate capability and cycling stability. GQD-modified composite materials have attracted much research interest for the development of low-cost high-performance energy storage devices. Qiu et al [528] reported the synthesis of histidine-functionalized GQD/layered double hydroxide (His-GQDs/LDH) composite using a microwave method. The composite exhibited flower ball-like structures and was used as the anode material. The large specific surface area and electrical conductivity of the composite resulted in high specific capacitance and remarkable cycling stability. They also produced a supercapacitor exhibiting excellent energy storage performance and cycling stability using the His-GQDs/LDH composite and active carbon as the positive and negative electrodes, respectively, as shown in figure 17(c). In addition to being used as electrode materials, GQDs can form composites with other materials for use as separators in capacitors because of their large specific surface area and small size. Pang et al [529] developed a separator coated with a composite of multiwall carbon nanotubes and nitrogen-doped GQDs and used it in Li–S batteries. This provided a physical barrier against polysulfide movement and chemical adsorption of polysulfides by the composite, as illustrated in figure 17(d). The composite-coated separator significantly improved the cycle life and anti-self-discharge performance of Li–S batteries. This development is important for practical applications of lithium-ion batteries. The use of GQD heterostructure electrodes can effectively improve the energy density of aqueous supercapacitors by increasing their potential window, as reported by Jia et al [534]. They found that heterostructure electrodes consisting of GQDs and MnO2 provided good interfacial bonding via Mn–O–C bonds. Using the GQDs/MnO2 electrodes, the potential window can be extended to 1.3 V (more than the theoretical value) due to a potential drop in the built-in electric field of the heterostructure. Flexible capacitors have attracted much interest in recent years [535, 536]. One of the key components is a flexible electrode material, and carbon fiber is known to be an ideal candidate. Hsiao and Lin [530] treated carbon fiber with a mixture of nitric acid and sulfuric acid at different ratios. They found that GQDs and functional groups were formed on the surface of the carbon fiber after acid treatments, as depicted in figure 17(e). At the same time, the treatment roughened the surface of carbon fiber. Both the formation of GQDs and roughening of the carbon fiber led to a large specific surface area at the electrode, which significantly increased the energy storage capacity of the flexible capacitors. GQDs can also be added to the electrolyte to regulate the electrochemical interface, thereby improving the performance of the capacitor. Hu et al [531] found that the addition of GQDs into the electrolyte would prevent the growth of dendrite in Li–S batteries containing high sulfur loading. The GQDs acted as heterogeneous sites for uniform nucleation and provided continuous regulation for dendrite-free lithium deposition under the control of an electric field and ion flux. This improved the cycling stability of the Li–S batteries, as demonstrated in figure 17(f). This study provides a solution to the inherent problems of the Li–S battery anode. A composite of GQDs and carbon nanotubes was explored for use as an electrode material for capacitors. Zhao et al [532] prepared a coaxial structure of GQD-coated carbon nanotubes as electrode material for energy storage of lithium ions. The GQDs were grafted onto 3D carbon nanotube frameworks to avoid agglomeration of the GQDs. These GQDs with oxygen functional groups provided enormous storage sites for lithium ions and were therefore responsible for the enhanced performance of the lithium-ion battery anode. Figure 17(g) shows the remarkable cycling performance and stability of the annealed GQD-coated carbon nanotubes as a lithium-ion battery anode. The introduction of impurities in GQDs can create defect states that have a significant effect on the properties of GQDs. Vijaya Kumar Saroja et al [533] reported that the edge defects of both GQDs and doped GQDs contributed to the improvement in the energy storage performance of the capacitors. As shown in figure 17(h), the GQDs doped with either nitrogen or boron atoms showed an improvement in the energy storage capacity with good cycling stability. The GQDs can improve the performance of capacitors when applied as electrodes, electrolytes and separators [537–549]. These strategies provide ideas for the development of new and improved capacitors.
Efficient energy use is an important topic in the field of energy application. Because of their unique optical properties, GQDs have been found to improve the performance of light-emitting devices. This is mainly due to the formation of a composite membrane that can be coated on the surface of monochrome LEDs. The fluorescence effect of the GQDs changes the wavelength of the light emitted from the LED. In recent years, GQDs have been used to develop light emitters that utilize the quantum confinement effect of the nanomaterials. White LEDs have been of great interest in the field of luminescence because of their energy-saving characteristic. Pramanik et al [334] reported the generation of white light using a combination of Förster resonance energy transfer and rare-earth-free luminescent material duo, as illustrated in figure 18(d). The white LEDs were prepared using composite colloids comprising nitrogen-doped GQDs and DCM dye. The energy gap can be regulated by nitrogen doping in GQDs, thereby tuning and widening the luminescence range of the dye.
Figure 18. Applications of GQDs in light-emitting devices. (a) Schematic structure of the GQD-VCSEL, consisting of top and bottom dielectric Ta2O5/SiO2 DBRs with synthesized GQDs sandwiched between them. (b) Cross-sectional SEM image of the fabricated GQD-VCSEL. (c) Photograph of the fabricated DBR under room light. Reprinted with permission from [167]. Copyright (2019) American Chemical Society. (d) Schematic of Förster resonance energy transfer (FRET)-assisted generation of white light in doped GQD-DCM dye luminescent duo (LD) system for fabrication of light-emitting LEDs (WLEDs) (inset: picture of WLEDs). Reprinted from [334], © 2020 Elsevier Inc. All rights reserved. (e) Cross-sectional TEM image of GQD–LEDs (inset: photograph of characteristic deep blue light emitted from the GQDs–LEDs at an applied voltage of 4.75 V). Reprinted with permission from [160]. Copyright (2019) American Chemical Society.
Download figure:
Standard image High-resolution imageInstead of using rare-earth material, Wang et al [550] found that chloride-doped GQDs can emit white light directly when under the irradiation of UV light. The chloride-doped GQDs were embedded in a silicon resin to form a composite film, which exhibited high transparency, flexibility, excellent optical stability and thermal stability. The film was attached directly to the LEDs to produce a white light. As the chlorine-doped GQDs were dispersed homogeneously within the film, the resultant natural white light was uniform without defects. These rare-earth-free white-luminescent chlorine-doped GQDs not only prevent the shortcomings of multicolor phosphors, but they also provide a green alternative for producing light emitters. There are reports on the formation of other GQD-based composite films, whose optical properties can be regulated by adjusting the concentration of the doped GQDs. Wu et al [335] prepared GQDs encapsulated MF polymer microspheres. The emission wavelength was extended from blue to full visible range under UV irradiation, resulting in white-light luminescence. The luminescence properties were tunable by varying the doping concentration of the GQDs. A flexible thin film was formed by dispersing the GQD–MF microspheres in the polymer matrix and was used on LEDs to produce high-quality white-light emission and light diffusion. However, the aggregation of GQDs due to the interaction of π bonds often leads to photoluminescence quenching, thus limiting the performance of GQD-based light emitter devices. Recently, Park et al [370] incorporated GQDs with boron oxynitride (GQDs@BNO), which resulted in high PL-QY. The effective dispersion of GQDs in the BNO matrix significantly suppressed the aggregation of GQDs and therefore minimized photoluminescence quenching. An increase in the spontaneous emission rate of the GQDs was observed as the GQDs were surrounded by the BNO matrix having a high refractive index and enabled fluorescence energy transfer from the BNO donor with a larger bandgap to the GQD acceptor with a smaller bandgap, thus enhancing its electroluminescence activity. The optical properties of the GQDs are also influenced by the morphology of the nanomaterials. Lee et al [160] found that the edge states of GQDs have a significant role in their luminescence properties. They studied the relationship between the GQD crystalline size and their exciton lifetime by effectively controlling the morphology of the GQDs. Figure 18(e) shows the device structure of the GQD-based light-emitting device, which exhibited blue luminescence, as shown in the inset. Interestingly, the blue emission was not affected by the doping level of GQDs. In addition to LED, laser is also an important optoelectronic device, especially for optical communication. There are only a few reports on the use of GQDs in lasers because of the complexity of the structural design of lasers compared to that of LEDs. Lee et al [167] prepared a vertical optical cavity consisting of GQDs and distributed Bragg reflectors (DBRs). Figures 18(a) and (b) show the device structure of the GQD-based vertical-cavity surface-emitting laser. The design of the DBR provided a broad stopband that spectrally overlapped with the emitted GQDs and allowed high transmittance of light excitation in the UV region. An optical image of the fabricated DBR is shown in figure 18(c). The emission wavelength of the GQD-based laser was mainly concentrated in the green-light band. These results demonstrate that the GQDs can be used as optical gain materials.
In summary, GQDs can be used as fluorescent and light-emitting materials. Furthermore, the facile, low-cost and environmentally friendly preparation process for GQDs would allow widespread applications. GQDs have many important applications in the field of energy, ranging from energy generation to storage and utilization, because of their unique physical and chemical properties. The research and development of GQDs will continue to grow and find many novel applications in the field of energy.
5.3. Detector and sensor applications
The bandgap of GQDs can be modulated by means of size control and dopants, which can result in tunable spectral responses ranging from UV to NIR bands.
When GQDs form heterojunctions with other photoelectric materials, the quantum confinement effect of GQDs can reduce the recombination of electron–hole pairs, thereby increasing the exciton lifetime and resulting in a high gain. The photoelectric properties of GQDs are not affected by external compressive stress when coated on flexible substrates due to their small dimensions. Therefore, GQDs are suitable for the preparation of wideband, high detectivity and responsivity-flexible photodetectors. The large specific surface area and edge effect of GQDs make them attractive for use in gas sensors. In particular, GQDs with functional groups are highly selective to gas; thus, they are also suitable as the active layer in gas sensors. The excellent characteristics of GQDs could lead to high-performance photodetectors and gas sensors, in which the mechanism is based on the absorption of either incident photons or gases, causing a change in the electronic properties of the GQDs, as illustrated in figure 19(a). This section provides a review of the recent developments in GQD-based photodetectors and gas sensors.
Figure 19. Applications of GQDs in photodetectors. (a) Schematic of photodetector and gas sensor based on GQDs. (b) Schematic of ultrafast pump–probe measurements in reflection configuration. The 400 nm pulse pumps the GQD/MoS2 heterostructures, and the 650 nm probe arrives at the sample with a delay time of Δτ. Reprinted from [551],© 2019 Elsevier Ltd. All rights reserved. (c) Schematic of the hybrid architecture of the GQD/two-dimensional material heterojunction photodetector. (d) Photograph of S,N–GQDs–rGO–cotton photodetector during twisting. Reproduced from [552], with permission from Springer Nature. (e) SEM image of a typical GQD. Reproduced from [553] with permission from Springer Nature.
Download figure:
Standard image High-resolution imageThere have been reports on the application of GQDs in photodetectors. However, the mechanism of charge transport in heterojunctions formed between GQDs and other materials, especially those of van der Waals heterojunctions, is still unclear. Shan et al [551] applied femtosecond pump–probe spectroscopy, two-phase electron injection model and modified rate equations to quantitatively solve the charge transfer rate at the interface of a mixed van der Waals GQD/MoS2 heterostructure. A schematic of the device structure is shown in figure 19(b). Understanding the electron transfer and relaxation processes in the heterostructure would assist in developing methods to alter the optical performance of the device. They found that the cascaded relaxation of hot electrons in GQDs, due to the quantum confinement effect, can significantly influence the interfacial dynamics. This finding can be used to optimize the performance of photoelectric devices based on mixed-dimensional heterostructures. The use of graphene in photodetectors is limited because of its low optical absorption coefficient. Therefore, modifying graphene with materials having high absorption coefficients is an effective way to develop high-performance graphene-based photodetectors. Zhu et al [554] attached GQDs onto vertically oriented graphene (VOG), which was used to form a heterojunction with germanium. A photodetector consisting of GQDs/VOG/Ge with enhanced performance in the detection of NIR light was prepared. The improved properties of the photodetector were caused by the synergistic effect of the GQDs and VOG, which resulted in enhanced light absorption and increased electron transport. The modification of VOG with GQDs is an effective way to control the Fermi level of VOG, increase the internal electric field of the Schottky junction and promote the separation of photoinduced electron–hole pairs. Under 1550 nm light irradiation, the responsivity and detectivity of the prepared devices were 1.06 × 106 A W−1 and 2.11 × 1014 cm Hz1/2 W−1, respectively. The use of plasmonic nanostructures is another effective way to improve the performance of photodetectors, especially in extending the response range of the detector. Thakur et al [555] directly synthesized gold nanoparticle/GQD nanohybrid using micro-plasmas and used it in photodetectors. Due to the plasmonic absorption of gold nanoparticles, the spectral response range of the photodetector was extended. The synergistic effect was attributed to the strong fluorescence quenching in AuNPs@GQDs combined with 2D graphene layer in the device, resulting in ultrahigh responsivity and detectivity of 103 A W−1 and 1013 Jones (1 Jones is equal to 1 cm Hz1/2W−1), respectively. Flexible photoelectric devices, especially wearable photoelectric detectors, have attracted much interest recently. Luo et al [552] prepared a flexible photodetector by spraying S-and-N-co-doped GQDs on rGO-coated cotton substrate, as shown in figure 19(d). The photodetector demonstrated high responsivity and detectivity of 0.2–1.25 A W−1 and 3.86 × 1010 Jones, respectively, in the broad wavelength range between UV and NIR. This can be attributed to the charge transfer between S,N-doped GQDs and rGO, resulting in the separation of the photogenerated carriers. The strong optical absorption of GQDs and the good conductivity of rGO are believed to contribute to the excellent performance of the flexible photodetector. Jang et al [556] reported a flexible deep UV photodetector based on GQDs sandwiched between two graphene layers on a polyethylene terephthalate substrate. The photodetector exhibited responsivity and detectivity of 0.1 A W−1 and 1.1 × 1013 Jones, respectively, when irradiated at a wavelength of 254 nm. Figure 19(c) shows a schematic of the photodetector based on multidimensional graphene structures. Further study on the use of a hybrid multidimensional nanostructures for photodetectors was conducted by Nguyen et al [378]. They prepared a high-performance photodetector based on 2D tungsten diselenide (WSe2) coated with nitrogen-doped GQDs. The enhanced photoluminescence was due to the neutral exciton emission caused by the nitrogen-doped GQDs. In addition, the strong optical absorption of GQDs and effective charge transfer from the GQDs to 2D WSe2 resulted in a 480% increase in photoresponsivity compared to the pristine 2D WSe2 photodetector. It was found that the photogating effect has an important role in enhancing the performance of the multidimensional heterojunction photodetector. The luminescent downshifting effect of GQDs has been discovered for a long time. Kumbhakar et al [557] have taken the advantage of such an effect of graphene to improve the performance of photoconductive cells. More recently, Hasan et al [558] discovered that the optical properties of nitrogen-doped GQDs can change upon exposure to short- (254 nm), medium- (302 nm) and long-wave (365 nm) UV irradiation, resulting in a reduction in absorption from 200 to 320 nm and improvement beyond 320 nm. The UV treatment of the nitrogen-doped GQDs would lead to the quenching of blue and NIR fluorescence along with a substantial increase in green/yellow emission; hence, this phenomenon can be used as a potential UV sensing mechanism. The change in the optical properties was mainly attributed to the increase in the size of the nitrogen-doped GQDs driven by free radicals and the decrease in their functional groups. As the GQDs exhibit strong absorption and high sensitivity to UV radiation, they can find potential applications in UV photodetectors. In addition to their response to high-energy ultraviolet and visible bands, GQDs have also been explored for use in detecting the NIR band. El Fatimy et al [553] prepared GQD bolometers that demonstrated excellent performance at temperatures of up to 77 K. This is attributed to the quantum confinement of GQDs that resulted in a remarkably high variation in electrical resistance with temperature. This is also due to the intrinsic properties of graphene; for example, light absorption in graphene causes a large change in electron temperature, making graphene suitable for hot-electron bolometers in the terahertz frequency range, as shown in figure 19(e). Recently, there has been much research interest and activities in the development of GQD-based photodetectors [559–564], especially in areas, such as the use of GQD composite materials, array-type photodetectors [565], broadband detection and terahertz detectors [566]. Table 3 provides a list of the recently developed GQD-based photodetectors and their performance indicators.
Table 3. List of recent GQD-based photodetectors and their performance indicators.
| Device structure | Detected wavelength (nm) | Response time (rise/fall time) (ms) | Detectivity (cm Hz1/2 W−1) | Responsivity (A W−1) | Operating temperature (K) | Rigid/flexible | References |
|---|---|---|---|---|---|---|---|
| ZnO/GQDs/ZnO | 385 | 0.37 | 1.54 × 1014 | 89.3 | RT | Rigid | [351] |
| WSe2/N-GQDs | 405 | — | — | 2578 | RT | Rigid | [378] |
| GQDs/VOG/Ge | 1550 | 0.051/0.054 | 2.11 × 1014 | 1.06 × 106 | RT | Rigid | [554] |
| Au@GQDs/Gr | 325–808 | 65/53 | 5.1 × 1013 | 4535 | — | Rigid | [553] |
| S,N-GQDs/rGO | 300–808 | — | 3.86 × 1010 | 0.2–1.25 | — | Flexible | [555] |
| Gr/GQDs/Gr | 256 | 24/17 | 1.1 × 1013 | 0.11 | RT | Flexible | [556] |
| CdS/N-GQDs@PVA | 395 | — | 9 × 1013 | 3 | — | Rigid | [557] |
| N-GQDs | 254–365 | — | 1.03 × 1011 | 0.59 | — | Rigid | [558] |
| GQDs | THz | — | — | 1 × 1010 VW−1 | 6 | Rigid | [553] |
| N-GQDs | 1550 | 0.05/0.053 | 1.3 × 1010 | 0.058 | — | Rigid | [559] |
| GQDs/n-Si | 300–1100 | — | — | 3.5 | — | Rigid | [560] |
| ZnO/GQDs | 365 | — | 3.5 × 107 | 0.14 | RT | Rigid | [563] |
| GQDs | 1064 | — | — | 0.96 × 10−3 | 533 | Rigid | [565] |
| GQDs | THz | — | — | — | 0.17 | Rigid | [566] |
| ZnO/GQDs/Poly-TPD | 365 | 0.37 × 10−3/0.78 × 10−3 | 2 × 1011 | 0.56 | — | Rigid | [567] |
| GQDs/ZnO/GaN | 200–800 | 159/68.7 | 7 × 1011 | 3.2 × 103 | — | Rigid | [568] |
Note: PVA, poly(vinyl alcohol); poly-TPD, poly(NN'-bis-4-butylphenyl-N,N'-bisphenyl)benzidine.
GQDs have also been studied for use in gas sensors because of their zero-dimensional properties and large specific surface areas. Arunragsa et al [569] prepared a room-temperature ammonia gas sensor by functionalizing the edges of GQDs with hydroxyl (OH) and deposited the functionalized GQDs onto nickel interdigitated electrode, as shown in figure 20(a). The results obtained from both experimental and theoretical studies showed that the hydroxyl functional group was the main factor affecting the sensitivity and selectivity of the sensor in detecting ammonia gas, which suggests that edge functionalization of GQDs is an effective way to obtain high-performance gas sensors with excellent selectivity to target gas. Composite materials based on GQDs have been studied to improve the selectivity of gas sensors. Shao et al [353] loaded ZnO nanosheets with GQDs and SnO2 nanoparticles in the preparation of a highly selective gas sensor for the detection of H2S. The resultant gas sensor exhibited high response speed, quick response/recovery time and excellent selectivity toward H2S, as illustrated in figure 20(b). The heterojunction between p-type GQDs and n-type SnO2 and ZnO widened the resistance variation upon gas adsorption. Another example of using GQD-based composite materials to improve the selectivity of gas sensors was demonstrated by Purbia et al [570], who prepared a gas sensor based on a nitrogen-doped GQDs/SnO2 quantum dot heterostructure for the detection of NO2. The improved sensitivity and selectivity of the sensor can be attributed to the enhanced electron transfer between SnO2 and the nitrogen-doped GQDs, as well as the preferential absorption of NO2 on the GQDs. Furthermore, the 0D heterostructure provided a large specific surface area, more active sites and a better nanoscale interface, thereby improving the performance of the gas sensor. Increasing the specific surface area and electron transfer characteristics of sensing materials is a way to improve the performance of gas sensors. Lv et al [319] reported the modification of a three-dimensional ordered macroporous In2O3 with nitrogen-doped GQDs. Figures 20(c)–(f) show the SEM images of the GQD/In2O3 composites. The formation of a heterojunction between the three-dimensional ordered macroporous In2O3 and nitrogen-doped GQDs, as well as the nitrogen doping in GQDs, are believed to play vital roles in improving the sensitivity, selectivity, response/recovery time and stability of NO2 gas sensors. The mechanism of the gas sensor is illustrated in figure 20(g). As demonstrated, GQDs have shown great potential for the development of high-performance gas sensors due to their high specificity and ease of functionalization.
Figure 20. Applications of GQDs in gas sensors. (a) Schematic of the fabrication process of OH-GQD gas sensors. Reprinted from [569], © 2020 Elsevier B.V. All rights reserved. (b) Pattern recognition based on the principal component analysis method to show the selectivity of the GQD−SnO2/ZnO sensor. Reprinted with permission from [353]. Copyright (2020) American Chemical Society. (c–f) SEM images of (c) PS microspheres, (d), (e), (f) three-dimensional ordered macroporous In2O3 under different magnifications and (g) N-GQDs/In1. Reprinted with permission from [319]. Copyright (2020) American Chemical Society.
Download figure:
Standard image High-resolution image5.4. Other applications
Many novel applications of GQDs are still being explored and developed, as the nanomaterials are still in the early stages of research. For example, there are reports on the application of GQDs in corrosion resistance [571–574] because GQDs tend to form complexes with other substances. Jiang et al [575] prepared a composite coating of nitrogen-doped GQDS and polymethyltrimethoxysilane (PMTMS) on the surface of magnesium alloy. Because of the chemical bonding of nitrogen-doped GQDs with the Mg substrate and PMTMS, the corrosion resistance performance of the composite coating was enhanced remarkably, as shown in figure 21(a). Interestingly, GQDs also have potential applications in agriculture. Xu et al [576] found that GQDs can be used as catalysts for the absorption of water and nutrients, as they would significantly increase the specific surface area of epidermis cells at the root surface, hence promoting plant growth. They also discovered that the size of the GQDs has an effect on plant growth; for example, large GQDs neither promoted nor inhibited plant growth, whereas GQDs with a size of 10 nm promoted plant growth, as shown in figure 21(c). Based on both experimental and theoretical studies, the mechanism by which GQDs promote plant growth is illustrated in figure 21(b). GQDs have also been explored in the preparation of micro-motor, as demonstrated by Maria-Hormigos et al [577] and shown in figures 20(d)–(g). Other applications of GQDs are in fuel cells as previously reported [265]. Mohamad Nor et al [578] prepared a proton exchange membrane for fuel cell application using cross-linked highly sulfonated polyphenylsulfone (SPPSU) membrane that comprised of GQDs. Because of the cross-linking of GQDs and SPPSU after annealing at 180 °C, the proton conductivity of the cross-linked membrane was higher than that of the pristine SPPSU membrane. Furthermore, the cross-linked membrane also exhibited excellent dimensional stability. The schematic of the proton-conductive membrane is shown in figure 21(h). In addition, there are reports on the applications of GQDs as catalysts in a new type of fuels [579, 580], which can address challenges relating to energy shortage and environmental pollution. There are many other novel applications of GQDs [581], which are of great interest. Indeed, GQDs have many important and wide-ranging applications that warrant our attention.
Figure 21. Other applications of GQDs. (a) Surface morphologies of the samples with different coatings before and after immersion tests in 3.5 wt% NaCl. Bare Mg alloy (①), PMTMS (④), N-GQDs (⑦) and N-GQDs/PMTMS specimens (⑩); (②, ⑤, ⑧, ⑪) corresponding specimens of (①, ④, ⑦, ⑩) after immersing 8 h; bare Mg alloy (③), PMTMS (⑥) and N-GQD (⑨) specimens after immersing 26 h; N-GQD/PMTMS specimen after immersing 194 h (⑫). Reprinted from [575], © 2019 Elsevier Ltd. All rights reserved. (b) Mechanism of the influence of GQDs on the growth of Zephyranthes grandiflora with ions and GQDs attached to the surfaces of the epidermal cells. (c) Photographs of (top) shallots and (bottom) Zephyranthes. grandiflora treated with GQDs having dimensions of 5, 10, 20 and 30 nm. Reproduced from [576] with permission from the Royal Society of Chemistry. (d)–(g) Time-lapse images (taken from Video S3, ESI†) and the corresponding trajectories of GQD micromotors moving in 1% and 2% peroxide solutions. Reproduced from [577] with permission from the Royal Society of Chemistry. (h) Schematic of cross-linked GQDs with high SPPSU as proton exchange membranes for fuel cell applications. Reprinted from [578], © 2020 Hydrogen Energy Publications LLC. Published by Elsevier Ltd. All rights reserved.
Download figure:
Standard image High-resolution image6. Conclusions
The essential properties of GQDs include quantum confinement and edge effects. While GQDs inherit many properties of graphene, they also exhibit strong fluorescence characteristics, strong optical absorption and excellent solubility. As an emerging member of the carbon material family, GQDs have many advantages over other carbon materials, such as biocompatibility, low toxicity and environmental friendliness. In this review, the unique properties of zero-dimensional GQDs are described in detail and compared to those of different low-dimensional carbon materials. From the perspective of GQD preparation, there are many different preparation methods of GQDs, which can be divided into three main categories: top-down, bottom-up and chemical methods. These methods were compared according to the size, functionalization, cost of production and other aspects of GQDs. The electrical, optical, magnetic, thermal and other properties of GQDs were also discussed in detail. Some of these properties can be controlled by the functionalization of GQDs, which has attracted much attention and has therefore been an important part of this review. This includes the introduction of impurity atoms and the formation of composites with other substances to modify the properties of GQDs. The excellent properties of GQDs and their composite materials have led to numerous exciting and wide-ranging applications in many different fields, such as biomedicine, energy, optoelectronics, agriculture and other emerging areas. The widespread applications of GQDs demonstrate their great variety of functionalities. As researchers continue to discover new properties of GQDs, novel applications based on the nanomaterials will continue to emerge. This review on the recent development of GQDs provides an important insight into the future research directions and applications of GQDs. It also provides a summary of recent research achievements of GQDs.
7. Future perspectives
The rapid development of GQDs is mainly due to their many potential applications in a wide variety of fields, such as biomedicine, sensors, optoelectronics, agriculture, environmental protection and robotics. In this section, future research directions and applications of GQDs are highlighted based on their unique characteristics and functions. The preparation technique of GQDs is the key to their application. Unlike other quantum dots, GQDs are nontoxic; hence, they have great potential for important applications in biomedical and environmental protection. However, many preparation techniques for GQDs require the use of toxic chemical reagents. Therefore, further research on the preparation of GQDs using green and environmentally friendly techniques is crucial for the future application of GQDs. The application of GQDs in optoelectronics is still in its initial stage. At present, the application of GQDs in optoelectronics has two main problems: ability to prepare high-quality GQD films and ability to broaden the response wavelength of GQDs without losing their quantum confinement effect. To overcome the aforementioned problems, there is a need to perform in-depth research on the edge and quantum confinement effects of GQDs. In addition, only a few studies have investigated the electrical and magnetic properties of GQDs. Further research on GQDs can effectively develop more applications in emerging fields, such as the application of GQDs in solar cells and energy generation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 61106098, 51201150 and 11374250), Key Project of Applied Basic Research of Yunnan Province, China (Grant No. 2012FA003), PolyU Grant (1-ZVGH) and Research Grants Council of Hong Kong (Project Nos. PolyU 153030/15P and PolyU 153271/16P).