Abstract
Efficient water splitting for H2 evolution over semiconductor photocatalysts is highly attractive in the field of clean energy. It is of great significance to construct heterojunctions, among which the direct Z-scheme nanocomposite photocatalyst provides effective separation of photo-generated carriers to boost the photocatalytic performance. Herein, Z-scheme hydrated tungsten trioxide/ZnIn2S4 is fabricated via an in-situ hydrothermal method where ZnIn2S4 nanosheets are grown on WO3⋅xH2O. The close contact between WO3⋅0.5H2O and WO3⋅0.33H2O as well as ZnIn2S4 improve the charge carrier separation and migration in the photocatalyst, where the strong reducing electrons in the conduction band of ZnIn2S4 and the strong oxidizing holes in the valence band of WO3⋅0.33H2O are retained, leading to enhanced photocatalytic hydrogen production. The obtained WO3⋅xH2O/ZnIn2S4 shows an excellent H2 production rate of 7200 μmol g−1 h−1, which is 11 times higher than pure ZnIn2S4. To the best of our knowledge, this value is higher than most of the WO3-based noble metal-free semiconductor photocatalysts. The improved stability and activity are attributed to the formation of the Z-scheme heterojunction, which can markedly accelerate the interfacial charge separation for surface reaction. This work offers a promising strategy towards the design of an efficient Z-scheme photocatalyst to suppress electron–hole recombination and optimize redox potential.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Future perspectives
Photocatalytic hydrogen production from water provides a green and sustainable way to convert solar energy to hydrogen. The separation and transfer of charge carriers are highly important for the reaction. Constructing Z-scheme heterojunction nanocomposites can not only promote the charge separation, but also optimize the redox capacity, and thus is one of the most attractive strategies towards efficient hydrogen evolution. All-solid-state Z-scheme photocatalytic systems are especially more attractive for efficient charge separation since they can shorten the electron transfer path and avoid the undesired backward reactions caused by redox couples as compared with the Z-scheme systems with a shuttle redox mediator. Strategies to construct all-solid-state Z-scheme systems with efficient charge separation and transfer efficiency are of great significance to the field of light driven hydrogen evolution.
1. Introduction
Hydrogen is a kind of clean, eco-friendly, sustainable, and green energy source, which has a broad development prospect [1–4]. Photocatalytic hydrogen production from water provides a green and sustainable way to convert solar energy to hydrogen [5–9]. Although numerous semiconductor photocatalysts for hydrogen production have been developed, hydrogen evolution is still limited by the low catalytic efficiency [10–13]. Insufficient separation and utilization of charge carriers is one of the critical issues that inhibit the further improvement of photocatalytic performance [14–17]. Therefore, it is important to construct photocatalysts with unique architectures to suppress the rapid recombination of photo-generated electron and hole pairs and extract them to the maximum extent for participation in water reduction.
Heterojunction hybrid structures have received intensive attention, which can steer the charge kinetics by proper selection of the semiconductor materials [18–21]. Among them, Z-scheme heterojunction nanocomposites are of particular importance due to their ability to promote not only the charge separation, but also the redox capacity. In a typical Z-scheme heterojunction nanocomposite, due to the well matched band structure of the components, photo-generated electrons and holes with weak redox capacity recombine with each other, leaving the electrons at the more negative conduction band (CB) and the holes at the more positive valence band (VB) separated, which results in long-lived charge separation and improved redox capacity [22–27]. Compared with the Z-scheme systems with a shuttle redox mediator, the all-solid-state Z-scheme photocatalytic systems with two semiconductors in direct contact are much more attractive for efficient promotion of the charge separation since they can shorten the electron transfer path and avoid the undesired backward reactions caused by the redox couples [28–33]. Although various kinds of all-solid-state Z-scheme photocatalytic systems have been developed, an efficient Z-scheme system for hydrogen production under visible light, a rational selection of semiconductors as well as a delicate synthesis to optimize their interfaces are not easy to achieve. How to make full use of the advantages of a solid-state Z-scheme system while suppressing undesirable potential barriers for charge transfer is still challenging.
WO3 is one of the potential visible light photocatalysts due to its tunable band gap (2.4–2.8 eV) in the visible region along with its high stability and nontoxicity [34, 35]. Various nanostructures of WO3 have been prepared for different applications. Although rarely studied, hydrated tungsten trioxide is reported to possess better photocatalytic properties than tungsten trioxide itself [36–38]. Inspired by the verified band structures of different hydrated tungsten trioxides, we hypothesize that in-situ synthesis of different hydrated tungsten trioxides in one-pot could possibly construct WO3⋅xH2O-based heterojunctions with tight interfaces to avoid charge recombination and realize boosted charge separation, which is thus promising as the oxidation counterpart to develop an efficient Z-scheme photocatalyst. Metal sulfides are regarded as good candidates for photocatalytic hydrogen production due to their strong absorption in the visible range. ZnIn2S4 (ZIS), a typical layered structure, is one of the important members in the community of ternary chalcogenides. Its suitable band gap (2.34–2.48 eV), remarkable chemical stability and nontoxicity rank ZIS as an attractive photocatalyst in different applications, such as, organic transformation, CO2 reduction and hydrogen evolution [39–43]. Especially, the use of ZIS for photocatalytic hydrogen evolution from water has received increasing attention. The well matched band structures of both ZIS and WO3⋅xH2O offer a great opportunity to construct WO3⋅xH2O/ZIS as a new kind of efficient Z-scheme photocatalyst.
In this study, we report a new design of a solid-state Z-scheme WO3⋅xH2O/ZIS photocatalytic system with dramatically enhanced photocatalytic H2 evolution. WO3⋅0.33H2O and WO3⋅0.5H2O with strong interfacial interaction were obtained by a one-pot hydrothermal reaction, which ensured the fast electron transfer from the CB of WO3⋅0.33H2O to the VB of ZIS, mediated by WO3⋅0.5H2O. Thus, the optimized WO3⋅xH2O/ZIS showed the best photocatalytic performance with a hydrogen evolution rate of 7200 μmol g−1 h−1, which is about 11 times higher than that of pure ZIS. This study provides fresh impetus for designing and developing functional photocatalytic systems for energy conversion.
2. Experimental
2.1. Preparations
All the reagents were of analytical grade and used without further purifications. WO3⋅0.33H2O–WO3⋅0.5H2O (denoted as WO3⋅xH2O) nanocomposites were synthesized according to previous literatures [44] with a slight modification, where 660 mg (1 mmol) sodium tungstate dihydrate and 100 mg polyvinylpyrrolidone (PVP) (Fw = 55 000) were dissolved in 12 ml deionized water and 2 ml acetic acid. The above solution was stirred for 60 min and then transferred into a Teflon-lined stainless steel autoclave (25 ml), followed by heating at 200 °C for 8 h. After cooled down to room temperature, the sample was separated by centrifugation and washed with distilled water and absolute ethanol several times and dried at 60 °C in an oven. To prepare WO3⋅0.5H2O, 0.5 g Na2WO4 · 2H2O and 1.35 g NH2CONH2 were dissolved in 2.5 ml water, and 43.0 ml ethanol was slowly added to form a white suspension. After ultrasonication for 10 min, 4.0 ml 3.0 mol l−1 HCl was added into the suspension, which was then maintained at 200 °C for 24 h. For WO3 ⋅0.33H2O, 2.2 mmol sodium oleate were dissolved in 20 ml of distilled water at 50 °C and 0.5 g of Na2WO4·2H2O were added. After vigorous stirring for 5 h, the pH value was adjusted to 1 by adding dilute HNO3 and the volume of the suspension was adjusted to 40 ml by adding deionized water. After being stirred for 4 h, the precursor was sealed in a 50 ml Teflon-lined autoclave and heated at 180 °C for 20 h.
To prepare WO3⋅xH2O/ZIS nanocomposites with different mass ratios, 50 mg WO3⋅xH2O was dispersed in 30 ml distilled water and sonicated for 60 min. Subsequently, different amounts of Zn(NO3)2, In(NO3)3 and L-cysteine were added and the suspension was sonicated for several minutes. After that, the resultant suspension was sealed in a Teflon-lined stainless steel autoclave and heated at 180 °C for 18 h. The product was collected by centrifugation, washed with deionized water and ethanol consecutively, and finally dried at 60 °C. The WO3⋅xH2O/ZIS nanocomposites with different mass ratios are denoted as WO3⋅xH2O/ZIS-Y, where Y represents different mass rations of WO3⋅xH2O and ZIS. For comparison, the WO3⋅0.33H2O/ZIS and WO3⋅0.5H2O/ZIS nanocomposites were synthesized following the similar procedures of WO3⋅xH2O/ZIS. Pure ZIS was synthesized following the similar procedures with the absence of WO3⋅xH2O.
2.2. Characterizations
The crystal structures of as-prepared samples were determined by Bruker D8 ADVANCE x-ray diffraction with Cu Kαradiation (l = 0.15418 nm), which was operated at 40 kV and 40 mA. The morphologies of the samples were obtained by a field emission scanning electron microscopy (JSM-6700F). Transmission electron microscopy (TEM) and high resolution transmission electron microscopy images were obtained in a JEOL model JEM 2010 EX instrument with an accelerating voltage of 200 kV. To prepare the TEM sample, the sample powder suspension was sonicated in ethanol and a drop of it was dripped on a 3 mm-diameter fine mesh copper grid mounted with a carbon film. X-ray photoelectron spectra (XPS) was obtained by a Thermo Scientific ESCA Lab 250 system, with a monochromatic Al Kα as the x-ray source and a hemispherical analyzer. Ultraviolet-visible diffuse reflectance spectroscopy (UV–vis DRS) of as-synthesized materials were recorded at the wavelength range of 250–800 nm. BaSO4 was used as a reflectance standard in the UV–vis diffuse reflectance experiment. Electrochemical impedance spectroscopy (EIS) and transient photocurrent responses were measured on an electrochemical analyzer (CHI760) in a standard three-electrode system using the prepared samples as the working electrode with an active area of ca. 1.0 cm2, a Pt wire as the counter electrode, and Ag/AgCl (saturated KCl) as a reference electrode. Na2SO4 (0.2 M) aqueous solution was used as the electrolyte. Impedance data were fitted with ZSimpWin software (Princeton Applied Research). Photoluminescence (PL) spectra were measured by a fluorescence spectrometer (Hitachi F-4500) with an excitation source at the wavelength of 355 nm. The PL decay dynamics were studied by a time-correlated single photon counting module. A 300 W Xe arc lamp served as a light source for photocatalytic hydrogen evolution and photocurrent.
2.3. Photocatalytic hydrogen evolution
Photocatalytic experiments for hydrogen evolution were carried out in a closed gas circulation and evacuation system fitted with a top Pyrex window. The photocatalytic activity was evaluated with 50 mg photocatalyst suspended in 100 ml aqueous solution containing 15 vol.% of lactic acid as the sacrificial agent. The suspension was irradiated with a 300 W Xe lamp equipped with a 420 nm cut-off filter to provide the visible light irradiation. The temperature of the reactant solution was maintained at room temperature by flowing cooling water during the photocatalytic reaction. The amount of generated H2 was analyzed by a gas chromatograph (Bruker 450-GC) with a thermal conductivity detector (molecular sieve 5 A, argon carrier gas 99.999%). The apparent quantum yield (AQY) at 420 nm monochromatic light was estimated by the following equation
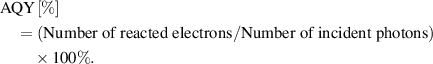
3. Results and discussion
The XRD pattern of the obtained WO3⋅xH2O nanocomposite shows a mixture of WO3·0.5H2O (JCPDS 44-0363) and WO3⋅0.33H2O (JCPDS 35-1001) (figure 1(a)). The well preserved XRD patterns of WO3⋅xH2O in WO3⋅xH2O/ZIS nanocomposites revealed the unaffected crystalline phase during the hybridization process. The two diffraction peaks at 20.7° and 47.7° correspond to the (102) and (108) crystallographic planes of hexagonal ZIS phase, respectively (JCPDS 49-1562), indicating the successful hybridization of ZIS with WO3⋅xH2O. The peak intensities of ZIS gradually become stronger as the concentration of the ZIS precursor is increased, indicating the formation of WO3⋅xH2O/ZIS-Y (figure 1(b)).
Figure 1. XRD patterns of (a) WO3⋅xH2O, ZnIn2S4 and WO3⋅xH2O/ZIS-0.7 nanocomposites; (b) WO3⋅xH2O/ZIS-Y.
Download figure:
Standard image High-resolution imageThe morphology of the WO3⋅xH2O/ZIS was examined by FESEM. As shown in figure 2(a), pure WO3⋅xH2O shows the stacking of nanosheets with smooth surfaces. Flower-like ZIS nanosheets are tightly integrated on the surface of WO3⋅xH2O as observed in WO3⋅xH2O/ZIS-0.7, confirming the successful hybridization instead of simple physical mixing (figure 2(b)). The strong interconnection between ZIS nanosheets and WO3⋅xH2O is further confirmed by TEM (figure 2(c)). Interplanar spacings of 0.312, 0.295, and 0.324 nm are observed, which correspond to the d-spacings of (200) plane of WO3⋅0.33H2O, (222) plane of WO3⋅0.5H2O and (012) plane of ZIS, respectively (figure 2(d)). In addition, clear boundaries between the above three components can be observed, indicating intimate contacts formed in the ternary heterojunctions.
Figure 2. Typical FESEM images of (a) WO3⋅xH2O and (b) WO3⋅xH2O/ZIS-0.7 nanocomposites; (c) TEM, (d) HRTEM for WO3⋅xH2O/ZIS-0.7 nanocomposites.
Download figure:
Standard image High-resolution imageThe surface chemical composition and electronic state of WO3⋅xH2O/ZIS nanocomposites are studied by XPS analyses, in comparison with ZIS and WO3⋅xH2O (figure 3). The XPS spectrum of WO3⋅xH2O/ZIS-0.7 in the W 4 f region shows peaks at 35.0 eV (4 f7/2) and 37.2 eV (4f5/2), which correspond to the typical binding energies of W6+ states (figure 3(a)). Compared with WO3⋅xH2O, the binding energies of W6+ in WO3⋅xH2O/ZIS-0.7 shift slightly to higher values, indicating decreased electron density around W [45]. Meanwhile, the characteristic binding energies of In3+ 3d (444.5 and 452.0 eV) in WO3⋅xH2O/ZIS-0.7 slightly shift to lower binding energies as compared with pure ZIS, while there are no changes for Zn2+ 2p (1021.4 and 1044.7 eV) and S2− 2p (161.3 eV and 162.6 eV) (figures 3(b)–(d)). The shift of the binding energies of W6+ and In3+ in different directions suggests the strong electronic interaction between WO3⋅xH2O and ZIS in the WO3⋅xH2O/ZIS nanocomposites [30, 46]. Given the well matched band structures of WO3⋅xH2O and ZIS and their strong electronic interaction, we expect that the ternary heterojunction structured WO3⋅xH2O/ZIS could show excellent photocatalytic performance.
Figure 3. XPS spectra of WO3⋅xH2O, ZnIn2S4 and WO3⋅xH2O/ZIS-0.7: (a) W 4f, (b) Zn 2p, (c) In 3d, and (d) S 2p.
Download figure:
Standard image High-resolution imageThe photoelectric properties of the as-synthesized ZIS, WO3⋅xH2O and WO3⋅xH2O/ZIS nanocomposite are examined by UV–vis DRS as shown in figure S1. Pure WO3⋅xH2O shows an absorption edge at about 400 nm. Incorporation of the visible light responsive ZIS onto WO3⋅xH2O obviously results in enhanced absorption in the visible range, which increases with increasing amount of ZIS incorporated, in agreement with the observed color change of the samples.
The photocatalytic hydrogen production activity is evaluated in a closed gas circulation system with lactic acid as the sacrificial agent under visible light irradiation. Figure 4(a) compares the photocatalytic H2 production rates of the whole set of samples. In consistent with previous reports, ZIS is active for the visible light induced hydrogen evolution but with low activity of 660 μmol g−1 h−1, owing to its poor charge separation capability. Hybridization of WO3⋅xH2O and ZIS results in significantly improved hydrogen evolution activity. Increasing the loading amount of ZIS onto WO3⋅xH2O first leads to an increase and then a decrease in the hydrogen evolution rate, with a surprisingly high activity of 7200 μmol g−1 h−1 achieved over WO3⋅xH2O/ZIS-0.7, which is about 11 times that of pure ZIS (figures 4(a) and (b)). It is noteworthy that this hydrogen evolution rate value is higher than most of the WO3-based noble metal-free photocatalysts (table S1 and figure 5(a)). Moreover, the AQY for the WO3⋅xH2O/ZIS-0.7 reaches 9.3% at 420 nm. The decreased hydrogen evolution rate of WO3⋅xH2O/ZIS at higher loading amount of ZIS is probably due to the shielding of excess amount of ZIS over the nanocomposite, which acts as charge recombination center. The photocatalytic stability of WO3⋅xH2O/ZIS-0.7 was investigated by intermittent evacuation and exposure to atmospheric conditions every 4 h for four times. As shown in figure 5(b), WO3⋅xH2O/ZIS-0.7 can be reused for at least four times without obvious loss of the hydrogen evolution activity. In addition, the recycled WO3⋅xH2O/ZIS-0.7 shows similar XRD patterns and morphology with the fresh one (figure S2), further confirming the high stability of WO3⋅xH2O/ZIS-0.7 for the photocatalytic hydrogen evolution.
Figure 4. (a) Time-dependent photocatalytic hydrogen production and (b) photocatalytic hydrogen evolution rates of WO3⋅xH2O, ZnIn2S4 and WO3⋅xH2O/ZIS-Y samples.
Download figure:
Standard image High-resolution imageFigure 5. (a) Comparison of the photocatalytic H2 evolution rate of WO3-based semiconductors photocatalyst; (b) Recycling test of H2 evolution over the WO3⋅xH2O/ZIS-0.7 sample.
Download figure:
Standard image High-resolution imageTo explore the underlying mechanism of the enhanced hydrogen evolution activity of WO3⋅xH2O/ZIS hybrids, the hydrogen evolution performance of WO3⋅xH2O alone is investigated. As expected, pure WO3⋅xH2O has hardly any photocatalytic H2 evolution activity due to its weak visible light absorption and its CB being more positive than the hydrogen evolution potential. Therefore, the synergistic effect between WO3⋅xH2O and ZIS is regarded to be a crucial factor for the excellent photocatalytic activity observed in WO3⋅xH2O/ZIS. In addition, since WO3⋅xH2O exists in different crystalline phases and may have great influence on the photocatalytic performance, we study the photocatalytic activities of heterojunctions formed between single phase WO3 hydrate and ZIS (that are, WO3·0.5H2O/ZIS-0.7 and WO3·0.33H2O/ZIS-0.7) to understand the role played by the WO3·xH2O hybrid. As shown in figure 4(b), both samples are active for hydrogen evolution, but are much inferior to WO3·xH2O/ZIS-0.7. These results reveal that the Z-scheme nanostructured ternary heterojuctions formed among WO3·0.5H2O, WO3·0.33H2O and ZIS in the WO3·xH2O/ZIS-0.7 nanocomposites are indispensable for the excellent photocatalytic activity, which help promote efficient interfacial charge separation and transportation.
Steady and transient PL analyses are performed to reveal photophysical characteristics of the photogenerated electron–hole pairs. As shown in figure 6(a), pure WO3⋅xH2O, ZIS and WO3⋅xH2O/ZIS-0.7 show PL emission at around 355 nm, with WO3⋅xH2O/ZIS-0.7 demonstrating the lowest PL intensity, indicating suppressed recombination of the charge carriers and hence suppressed radiative emission [47]. In addition, the time-resolved transient PL decay spectra show that WO3⋅xH2O/ZIS-0.7 exhibits shorter emission lifetimes (t1 = 0.9 ns, t2 = 1.1 ns) as compared with ZIS (t1 = 1.0 ns, t2 = 9 ns) and WO3⋅xH2O (t1 = 1.0 ns, t2 = 10 ns). The average emission lifetime, which reflects the overall emission decay behavior[48], of WO3⋅xH2O/ZIS-0.7 (1.0 ns) is much shorter than that of ZIS (7.2 ns) and WO3⋅xH2O (8.5 ns) (figure 6(b)). This is probably because that the strong interfacial contact in the local heterostructure accelerates the charge transfer across the interfaces. The promoted charge transfer process is also revealed by the transient photocurrent responses and the EIS, in which the WO3⋅xH2O/ZIS-0.7 electrode shows the highest photocurrent intensity and the smallest semicircle radius (figures 6(c) and (d)).
Figure 6. (a) Photoluminescence (PL) spectra, (b) time-resolved PL decay spectra (excitation at 350 nm), (c) transient photocurrent responses with light illumination, (d) EIS of WO3⋅xH2O, ZnIn2S4 and WO3⋅xH2O/ZIS-Y samples.
Download figure:
Standard image High-resolution imageTo get more insight into the enhanced charge flow process, band structure study is performed. The band gap energies (Eg) of WO3.0.33H2O and WO3⋅0.5H2O are reported to be approximately 2.63 and 2.95 eV, respectively [49, 50]. We use Mott-Schottky method to further determine their VB and CB edges. As shown in figures S3(a) and (b), the flat band energy (Efb) values are determined from the linear intercept of the axis, which are −0.35 and −0.25 eV vs. NHE at pH = 7 for WO3⋅0.33H2O and WO3⋅0.5H2O, respectively. Therefore, based on the experimental results of photocatalytic H2 production, the possible charge transfer process across the ternary WO3⋅xH2O/ZIS heterojunction under visible light is proposed and illustrated in figure 7. Under the light irradiation, electrons are excited to the CB and holes are left in the VB of WO3⋅0.33H2O in the WO3⋅xH2O/ZIS ternary structure. The tight contact between WO3⋅0.33H2O and WO3⋅0.5H2O provides a short migration path of the photo-generated carriers, which increases the opportunity of photo-induced electrons in the CB of WO3⋅0.33H2O to migrate through WO3⋅0.5H2O and then recombine with the holes in the VB of ZIS. The migration of charge carriers can promote the electron accumulation in the CB of ZIS, and the holes are retained in the VBs of WO3⋅0.33H2O, which are consumed by the sacrificial electron donor. Therefore, the photocatalytic reaction of the as-prepared WO3⋅xH2O/ZIS nanocomposite follows a direct solid-state Z-scheme mechanism, which not only accelerates the separation and transfer of photo-generated charges but also retains the electrons with strong redox ability for efficient photocatalytic H2 production. It can be concluded that the enhanced photocatalytic activity of the as-prepared WO3⋅xH2O/ZIS nanocomposites can be ascribed to the enhanced visible-light harvesting and highly effective charge-separation resulting from the solid-state Z-scheme junction in the ternary structure.
Figure 7. Schematic illustration of the hydrogen evolution process for the WO3⋅xH2O/ZIS under visible light irradiation.
Download figure:
Standard image High-resolution image4. Conclusion
In summary, a novel Z-scheme WO3⋅xH2O/ZIS ternary heterojunction has been constructed for photocatalytic H2 production. The close-contact between WO3⋅0.33H2O and WO3⋅0.5H2O provides a short migration path in the material interfaces from the CB of WO3⋅0.33H2O to the VB of ZIS, mediated by WO3⋅0.5H2O, which effectively accelerates the separation of the charge carriers. Benefiting from the unique spatial structure and charge migration path (enhanced separation efficiency of photo-generated electron–hole pairs), the obtained WO3⋅xH2O/ZIS nanocomposites exhibit more efficient photocatalytic performance for photocatalytic H2 production than pure WO3⋅xH2O and ZIS under visible light irradiation. This Z-scheme nanostructured heterojunction shows great potential to realize more efficient H2 production.
Acknowledgments
The work was supported by Research Grants Council of the Hong Kong Special Administrative Region, China (PolyU152140/19E).
Supplementary data (0.2 MB DOCX)








