Abstract
This paper describes the development of a novel method of producing nanocomposites consisting of gold nanoclusters anchored on graphene oxide nanosheets in a cost-effective and reproducible manner. The novelty of the technique hinges on the covalent functionalization of atomically precise subnanometer gold clusters protected by glutathione (Au15SG13 and Au25SG18) on to graphene oxide (GO) nanosheets according to the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride crosslinking method, using the existing carboxylic groups present both at the surfaces of the nanoclusters and the GO nanosheets. The atomic precision of glutathione-protected gold nanoclusters was evidenced by electrospray ionization mass spectrometry. The formed hybrid nanocomposites were characterized by TEM measurements and exhibit nonlinear optical properties characteristic of GO, in particular a strong second harmonic scattering response as well as a multi-photon excited fluorescence spectrum characterized by a broad band in the visible range between 350 and 700 nm. Atomically precise nanoclusters covalently linked to GO nanosheets are therefore promising for new applications in the areas of optoelectronics and photovoltaics.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The large surface areas of graphenic materials provide ideal platforms to capture active molecules and clusters [1]. In particular, graphene−nanoparticle composites can be synthesized in situ or ex situ by combining graphene or its derivatives, with various types of nanoparticles including, but not limited to, quantum dots (QDs) [2, 3], noble metals [4–6], metal oxides [7, 8] or silica nanoparticles (SiNPs) [9, 10] depending on the expected final functional properties. The oxidation of graphene into graphene oxide (GO) leads to the formation of hydroxyl, epoxide and carboxylic groups [11] that serve as anchors for nanoparticles.
Ex situ methods (in which nanoparticles are synthesized separately and then deposited onto a graphene surface), offer a number of advantages when compared to the in situ ones (in which nanoparticles are grown directly on a graphene surface) such as significantly narrower size distribution as well as better control over the average size, shape or density of the nanoparticles that decorate the graphene sheets. In ex situ methods, the nanoparticles are synthesized in advance and subsequently attached to the surface of the graphene sheets via linking agents through either covalent or noncovalent interactions [12, 13].
While a plethora of ex situ methods have been reported for graphene-nanoparticles composites [13], only a few have been devoted to the anchoring of nanoclusters onto GO. Nanoclusters are an emerging class of noble metal based materials with atomically precise metallic cores protected by thiolated ligands showing in particular well-defined features in their optical absorption and emission spectra [14–17]. Notably, due to subtle interaction between the ligands and the metallic core upon photoexcitation, enhanced nonlinear optical properties have been observed [18–20]. As a result, synthesis of new hybrid materials made of nanoclusters and GO nanosheets may lead to the properties of both nanoscale materials.
Pradeep and co-workers have pioneered the field of nanoclusters anchored onto GO [21]. They have reported that atomically precise silver nanoclusters protected by glutathione can covalently functionalize solution phase thiolated graphene using a ligand exchange mechanism. In addition, bovine serum albumin protected Au25 and histidine protected Au10 have been self-assembled to anchor onto GO and form stable nanocomposites stabilized through electrostatic interactions [22]. In parallel, the co-reduction strategy to fabricate glutathione protected gold or silver nanoclusters on reduced graphene oxide in an aqueous medium has been reported by Song et al [23, 24]. More recently, L-cysteine capped copper nanoclusters have been attached to imidazole-functionalized partially reduced graphene oxide via electrostatic forces [25].
In this brief report, we propose a novel method to anchor atomically precise glutathione-protected Au25 and Au15 nanoclusters onto graphene oxide via the use of EDC to activate the existing –COOH groups at the surface of the nanoclusters and the GO nanosheets. This allows covalent bonding reactions between GO carboxyl groups and the reactive groups in glutathione-protected gold nanoclusters. The nonlinear optical response of these hybrid nanoclusters anchored to GO nanosheets, namely the second harmonic scattering response as well as the multi-photon excited fluorescence, is reported.
2. Experimental
2.1. Preparation of atomically precise glutathione-protected Au25 and Au15 nanoclusters –GO nanocomposites
Graphene oxide was synthesized using a modified Hummer's method described in details in our previous work, see [26]. GO (25 mg) was dispersed in 50 ml of water under sonication. Then, the solution was left to settle for one week before carefully taking the supernatant.
Ethylenediamine was coupled to the carboxylic group by a classical coupling method with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) to form GO-CO-NH-(CH2)2-NH2. More precisely, an excess of EDC (50 mg) and ethylenediamine (100 μl in 5 ml of water, pH adjusted to 6 with HCl) were added to the GO suspension and sonicated during 3 h. The solution was left standing overnight before centrifugation (4000 rpm, 3 washing cycles with water) to remove excess EDC and ethylenediamine.
GO-CO-NH-(CH2)2-NH2 was then dispersed in water, and excess of gold nanoclusters (AuNCs) (30 mg of Au15SG13 or Au25SG18) was added followed by EDC addition (10 mg). Au15SG13 and Au25SG18 were synthesized as reported in [19, 27]. The solution was left to react at ambient temperature for 24 h to form covalently bonded GO-AuNCs with the covalent linker -CO-NH-(CH2)2-NH-CO- (see figure 1 for reaction scheme). Then, the GO-AuNCs containing solution was centrifuged (4000 rpm) to remove excess reactant before being dispersed in water.
Figure 1. (a) Reaction scheme involving the covalent AuNCs anchoring to graphene oxide. Ethylenediamine is coupled to the carboxylic group of GO by a classical coupling method with EDC to form GO-CO-NH-(CH2)2-NH2. Then, excess of AuNCs is added followed by EDC to form GO-AuNCs covalent bond. For sake of simplicity, only one carboxylic group is shown on the surface of GO. (b) Schematic view of the GO-AuNC interface. The Au15 NC was built according to the structure featuring a cyclic [Au(I)-SR] pentamer interlocked with one staple trimer motif protecting the tetrahedral Au4 nucleus, together with another trimer motif.
Download figure:
Standard image High-resolution image2.2. Instrumentations
2.2.1. TEM
Transmission electron microscopy (TEM) measurements of AuNCs- GO drop-casted onto a silicon wafer were performed using a JEOL 2010 FTEM instrument.
2.2.2. UV–visible measurements
UV–vis spectra in solution were recorded using an AvaSpec-2048 fiber optic spectrometer with an AvaLight-DH-S deuterium-halogen light source.
2.2.3. Nonlinear optical measurements
The set-up for hyper Rayleigh scattering (HRS) and multi-photon excited fluorescence (MPEF) has been described in details in previous works [26, 28]. Briefly, the light source was a mode-locked femtosecond Ti:Sapphire laser delivering 140 fs pulses at a repetition rate of 80 MHz. After passing through a low-pass filter to remove any unwanted harmonic light generated prior to the cell, the fundamental beam at a wavelength of 800 nm and an average power of 400 mW was focused by a low numerical aperture microscope objective into a spectrophotometric cell containing the sample solution. The HRS and MPEF light were collected at an angle of 90° from the incident direction with a short focal length lens. The HRS light was separated from the excitation light by a high-pass filter and a monochromator set at the harmonic wavelength. The HRS light was then detected with a photomultiplier tube (model H11890-210, Hamamatsu) working in the single photon counting regime. For the MPEF signal, the wavelength of the spectrometer (Jobin-Yvon, model iHR320) was scanned between 350 nm and 750 nm with the same detection unit used.
3. Results and discussion
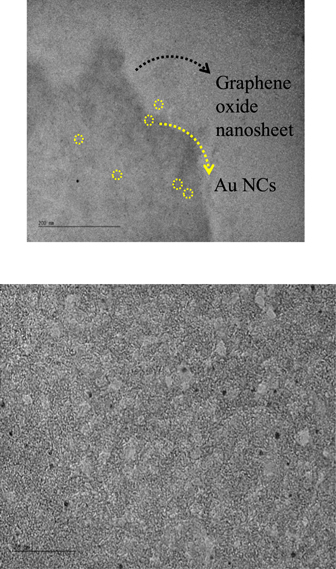
As mentioned in the introduction, a novel method is used to anchor atomically precise glutathione-protected Au25 and Au15 onto graphene. The nanoclusters are immobilized onto GO nanosheet's using EDC to activate and promote covalent bonding reactions between ethylenediamine, GO's carboxyl groups and reactive groups of glutathione-protected gold nanoclusters. Indeed, Au25SG18 nanoclusters possess 36 carboxylic groups that can serve as linkers. The produced Au NCs–GO nanocomposites were characterized by electron microscopy. TEM was used to investigate shape and size distributions of subnanometer Au25SG18 nanoclusters covalently anchored onto GO. It is clear from the TEM images of the GO-AuNCs hybrid nanosystems that the nanoclusters are of nearly spherical shape, highly mono-disperse in nature and no aggregation is present, see figure 2. The average diameter is calculated from the particle size distribution plot and is found to be 1.49 ± 0.36 nm, in good agreement with the size measurements performed on glutathione Au NCs [29]. Of note, the atomic precision (e.g. mono-disperse nature) of Au25SG18 (and of Au15SG13) was controlled by ESI-TOF MS analysis, see figure 3. The surface coverage of the AuNCs on the GO surface is rather low, e.g. ∼1 AuNC per 15 nm × 15 nm GO surface, and is limited to the presence of carboxylic functional groups covering the surface of GO. In our previous work [26], quantitative x-ray photoelectron analysis was conducted on GO nanosheets, leading to ∼6% of carboxylic groups with a total oxygen content of 25%. Thus the expected density of the COOH groups is ∼2 COOH per 2 nm × 2 nm. Even if these numbers are crude estimations, clearly the yield of covalent coupling is rather low, about one AuNC every 25 carboxylic groups. Nevertheless, a possible way out to increase the number of carboxylic acid groups on the surface of graphene, may emerge by photo oxidation of the same in presence of UV light. Also, oxidation of hydroxyl and epoxide groups on the surface of graphene, under basic conditions, may be performed to increase the yield of carboxylic acid groups on the surface of graphene. Also, comparative study highlighting the novelty of the current study vis-a-vis former studies has been given in the supplementary material (table S1 is available online at stacks.iop.org/NANOX/1/030005/mmedia).
Figure 2. TEM images of Au25 nanoclusters on the surface of a GO nanosheet. scale bar is 200 nm (top panel) and 20 nm (bottom panel).
Download figure:
Standard image High-resolution imageFigure 3. ESI-TOF MS analysis of Au15SG13 and Au25SG18 in negative mode. ESI-MS was performed on a commercial quadrupole time-of-flight (micro-qTOF, Bruker-Daltonics, Bremen, Germany, mass resolution 10,000). The samples were prepared to a final concentration of approximately 50 μM in methanol.
Download figure:
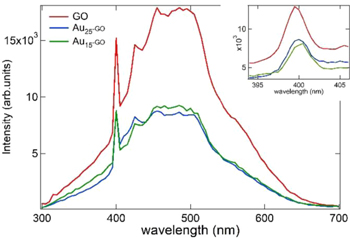
Standard image High-resolution imageThe extinction spectrum of GO is presented in figure 4 and is characterized by a large scattering due to the suspended nanosheets. This is manifested by the monotonous signal increase as the wavelength decreases. In addition, figure 4 shows the absorption spectrum of Au25SG18 synthesized by the proposed method. The spectrum shows well-defined and well-known features, in particular the broad band at 670 nm, due to the molecular transitions of the nanocluster [30]. The optical spectrum of the Au25-GO composites is similar to that of the neat GO nanosheets, without exhibiting the pronounced features of the nanoclusters. However, the extinction spectrum of the Au25-GO composites presents a weaker increase as the wavelength decreases as compared to the neat GO nanosheets corresponding to the possible signature of the nanoclusters. Clearly, the low Au25 nanoclusters surface coverage of the GO nanosheets can explain the absence of the broad band at 670 nm for the Au25-GO composites spectra.
Figure 4. Absorbance spectrum of freshly prepared Au25SG18 in aqueous phase, extinction spectra of neat GO nanosheets and of Au25SG18 covalently functionalized to GO nanosheets.
Download figure:
Standard image High-resolution imageFigure 5 presents the multi-photon excited fluorescence (MPEF) spectrum of GO and Au NCs-GO samples recorded for a laser excitation at 800 nm. A broadband in the visible range between 350 and 700 nm centred at about 500 nm is observed for both samples. Of note, the appearance of the fluorescence at wavelengths shorter than 400 nm indicates that at least three photons are required in the excitation step. In addition, the characteristic monochromatic HRS line is clearly observed at 400 nm. The MPEF spectra of GO and Au NCs-GO samples are very similar, although a clear global decrease is observed for the MPEF spectrum of the AuNCs-GO samples. MPEF spectra were recorded in water using the same initial concentration of GO nanosheets for all samples. Even if the final concentration of Au NCs-GO samples may be slightly overestimated (following the centrifugation steps), a global quenching on nonlinear optical MPEF and HRS signals upon covalent binding of the AuNCs on the GO nanosheets is clearly observed. We do not observe any significant size effect between the Au25 versus Au15 AuNCs on the global quenching. This steady state quenching may stem from an intra-composite energy or electron transfer process. This transfer has already been observed and investigated by time-resolved spectroscopic study of one-photon excited fluorescence on copper nanoclusters attached with imidazole-functionalized partially reduced graphene oxide via electrostatic attraction [25]. A similar quenching effect on the photoluminescence spectra was also reported for the Ag25SG18 nanoclusters bound to GO nanosheets [21].
Figure 5. MPEF spectrum of GO and Au NCs-GO samples in aqueous solution with excitation at 800 nm. The peak at 400 nm corresponds to the HRS line and is highlighted in inset.
Download figure:
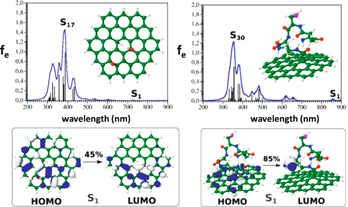
Standard image High-resolution imageIn order to better address the structure-property relationship in such nanocomposites, further theoretical investigations were explored. Investigating the entire GO nanosheet with covalently linked nanoclusters is out of the scope of this brief report. However, as a first step, we investigated (i) a graphene sheet oxidized with one epoxy and two hydroxyl groups and (ii) Glutathione (SG) covalently bound to pure graphene sheet fragment via the ethylenediamine linker. Semi-empirical-quantum mechanical (SEQM) PM7 method [31], as implemented in Gaussian [32], was employed to predict geometries and optical properties of these model systems. PM7 was chosen since recent studies [33–36] have demonstrated a fair accuracy compared to the hybrid B3LYP/6-31 G(d) DFT method for simple organic compounds, allowing for a good compromise between accuracy and calculation power. Geometries and optical spectra, e.g. one-photon absorption spectra (OPA), are shown in figure 6, along with molecular orbitals involved in the S1 excitation for models (i) and (ii). Clearly, while excitations are located within the graphene motifs in model (i), charge transfer (CT)-like excitations between the graphene motif and the SG ligand are making 85% of the S1 excitation. One can expect that in glutathione protected gold nanoclusters, the central metal core may act as reservoir of delocalized electrons leading to additional CT-like excitations.
Figure 6. SEQM PM7 OPA spectra together with structures obtained (upper part) and molecular orbitals involved in the S1 excitation of model graphene oxide (C54H24O3) and glutathione (SG) covalently bound to the pure graphene sheet (lower part). Broadening of the spectral lines is simulated with a Lorentzian profile with a half-width of 15 nm (blue lines). (S-magenta, C-green, O-red, N-blue, and H-white.)
Download figure:
Standard image High-resolution image4. Conclusions
In summary, through the presence of –COOH groups on the surface of both glutathione protected gold nanoclusters and GO nanosheets, we have explored a novel synthesis method to anchor atomically precise Au25 and Au15 NCs onto graphene via the use of EDC chemistry to activate and promote covalent bonding reaction with ethylenediamine. Interestingly, carboxylic groups are also present in many thiolated templates protecting gold nanoclusters, e.g. proteins [37, 38], glutathione [39], cysteine [19], captopril [40], mercaptobenzoic acid [41], mercaptoprionic acid [42] or thioglycolic acid [43]. Therefore, this method can be generalized for the covalent anchoring of atomically precise protected gold nanoclusters. The drawback however is currently the low surface coverage of AuNCs on GO nanosheets, as reported here. This low surface coverage is mainly due to the moderate number of –COOH groups resulting from oxidation in GO nanosheets (only 6%). But GO powders with increased oxidation levels can be prepared through variations of the Hummers method [44, 45]. Our results provide a general route for further experimental and theoretical exploration on the electronic and optical properties of these new nanocomposites. The nonlinear optical response (both SHG and MPEF) reported here may be of potential interest for biological bio-imaging and other bio-applications. Also, the quenching effect that reduces the nonlinear optical signals of the Au NCs–GO nanocomposites as compared to neat GO nanosheets may indeed find interest in the areas of optoelectronics and photovoltaics.
Acknowledgments
The authors would like to thank Nicholas Blanchard (iLM) for his help on TEM measurements. Martina Perić Bakulić thanks the French Embassy in Croatia for a scholar stipend of one month at Institut Lumière Matière. This research was partially supported by the project STIM—REI, Contract Number: KK.01.1.1.01.0003, funded by the European Union through the European Regional Development Fund—the Operational Programme Competitiveness and Cohesion 2014–2020 (KK.01.1.1.01). MPB acknowledges computational facilities of the HPC computer within the STIM-REI project. NK gratefully acknowledges the CNRS Visiting Professorship with Institut Lumière Matière. ST and NK also acknowledge the DST-Nano Mission and PURSE Schemes, Govt. Of India for project funding.