Abstract
Over the past two decades, superhydrophobic surfaces that are easily created have aroused considerable attention for their superior performances in various applications at room temperature. Nowadays, there is a growing demand in special fields for the development of surfaces that can resist wetting by high-temperature molten droplets (>1200 °C) using facile design and fabrication strategies. Herein, bioinspired directional structures (BDSs) were prepared on Y2O3-stabilized ZrO2 (YSZ) surfaces using femtosecond laser ablation. Benefiting from the anisotropic energy barriers, the BDSs featured with no additional modifiers showed a remarkable increase from 9.2° to 60° in the contact angle of CaO–MgO–Al2O3–SiO2 (CMAS) melt and a 70.1% reduction in the spreading area of CMAS at 1250 °C, compared with polished super-CMAS-melt-philic YSZ surfaces. Moreover, the BDSs demonstrated exceptional wetting inhibition even at 1 400 °C, with an increase from 3.3° to 31.3° in contact angle and a 67.9% decrease in spreading area. This work provides valuable insight and a facile preparation strategy for effectively inhibiting the wetting of molten droplets on super-melt-philic surfaces at extremely high temperatures.
Highlights
Wetting of molten droplets on super-melt-philic surfaces is significantly inhibited.
Bioinspired directional structures without modifiers inhibit wetting above 1200 °C.
The structures remarkably inhibit the wetting even at 1400 °C.
The ability is attributed to anisotropic energy barriers provided by the structures.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Superhydrophobic phenomena are ubiquitous in nature [1–3]. The mechanism underlying the isotropic superhydrophobicity of lotus leaves, characterized by the synergetic effect of micro/nanopapillae and low-surface-energy waxy crystal, has been clarified [4]. Inspired by this natural phenomenon, artificial superhydrophobic surfaces have garnered significant attention due to their widespread practical applications in self-cleaning [5, 6], droplet manipulation [7–10], oil-water separation [11, 12], drag reduction [13, 14], anti-icing [15, 16], etc. Nowadays, it is easy to achieve room-temperature superhydrophobicity by constructing micro/nanostructures and implementing chemical modifications [17–23]. However, the attainment of similar superrepellent properties for molten droplets in high-temperature air environments (e.g. >1200 °C) remains tremendous challenges.
The most commonly used thermal barrier coatings (TBCs), composed of Y2O3-stabilized ZrO2 (YSZ), are essential for the dependable operation of gas-turbine engines used for aviation, power generation, and marine under high-temperature conditions [24–26]. When air containing CaO–MgO–Al2O3–SiO2 (referred as to CMAS) silicate is ingested into the engines, the TBCs are easily corroded by molten CMAS (commonly melting temperature >1200 °C), leading to the failure and peeling of the TBCs [27–29]. As an alternative, inhibiting the wetting and spreading of the CMAS melt on the super-CMAS-melt-philic TBCs is promising to alleviate corrosion from the source [30]. However, there are two major challenges in applying the superhydrophobic principle to decrease the wettability of the molten CMAS to the TBCs. A significant difference in physicochemical properties exists between molten CMAS and water, and commonly employed organic chemical modifiers for superhydrophobicity inevitably suffer oxidation and decomposition at high temperatures, resulting in limited options of modifiers. Although a few high-temperature resistant materials (e.g. precious metal Pt and rare earth-containing ceramic matrix composites) are available, they have also brought some confusions, such as reduced thermal cycling and thermal shock life, and high costs [31–33]. On the other hand, creating conventional micro/nanostructures on super-CMAS-melt-philic TBCs generally increases the wettability of CMAS according to the Wenzel model [34]. Although it is promising to decrease the wettability of molten CMAS through designing bioinspired reentrant structures that can transform superhydrophilic surfaces into superhydrophobic without using chemical modifiers, the complexity and material dependence of the current fabrication greatly hinder their applications on TBCs [35, 36]. Therefore, it is highly desirable to manufacture a surface that resists the wetting of molten CMAS with simplified structural morphology, facile fabrication, free of modifiers, and low costs.
Encouragingly, nature always brings us diverse inspirations to develop advanced artificial systems. Unlike lotus leaves, rice leaves show anisotropic wettability because the energy barrier provided by the directional macrogroove arrays hinders the movement of water in the direction perpendicular to the grooves [37]. However, the inhibition of high-temperature wetting solely by bioinspired structures and from the perspective of energy barriers has rarely been reported, despite its appealing potential for various applications. Here we proposed to construct bioinspired directional structures (BDSs) with anisotropic energy barriers on YSZ through facile femtosecond laser ablation. When compared to polished super-CMAS-melt-philic YSZ surfaces, the BDSs considerably boosted the contact angle (CA) of CMAS melt by 552.2% and decreased the spreading area (SA) by 70.1% at 1 250 °C. Surprisingly, the BDSs increased CA by 848.5% and decreased SA by 67.9% even at 1400 °C.
2. Results and discussion
2.1. Design of BDSs
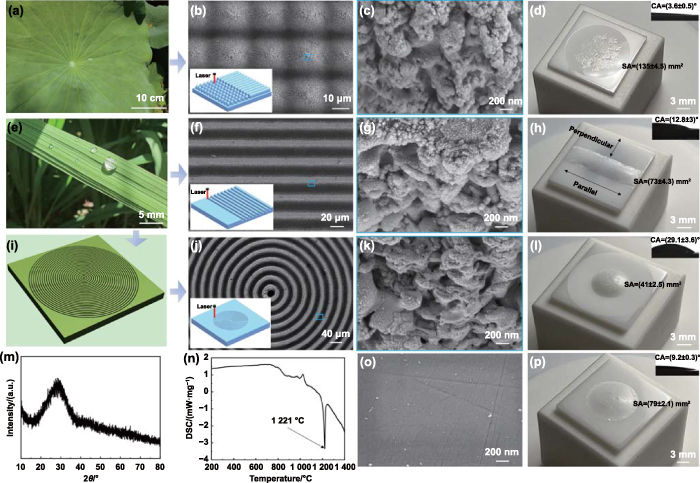
According to previous reports, lotus leaf-inspired micro/nanostructures can endow material surfaces with superhydrophobicity [38–40]. In this paper, femtosecond laser direct writing technology was employed in the following preparation of bioinspired structures due to its numerous merits for processing ceramic materials with high hardness, such as non-contact processing, high precision, low thermal effect, and environmental friendliness [41–43]. As shown in figures 1(a)–(d), we fabricated uniform conical microstructures with a height and spacing of about 20 μm and 22 μm, respectively (figure S1(a)) by mimicking lotus leaves. The applied scanning speed (SS) and number of scans (NS) in the fabrication process were 150 mm·s−1 and 5, respectively. The conical microstructures were covered by micro/nanoscale protrusions. However, molten CMAS tended to wet the as-prepared structural surfaces with a CA of 3.6°, which led to obvious spreading (SA = 134.9 mm2). This suggested that it was difficult for the designed structures to inhibit the wetting and spreading of molten CMAS on YSZ surfaces. Notably, it has been recently reported that the synergetic effect of lotus-leaf inspired structures and a carbon layer endowed YSZ with super-silicate-phobicity at 1200 °C, but the carbon layer would be rapidly oxidized once exposed to air at such a high temperature, which limited the practical application [44].
Figure 1. Design, fabrication, and properties of various bioinspired structures. (a) Photograph of lotus leaves. (b) and (c) SEM images showing the surface morphologies of the lotus leaf-inspired conical microstructures at different magnifications. (d) Actual photograph and CA (inset) of CMAS on the YSZ surface with conical microstructures. (e) Digital picture of rice leaves. (f) and (g) Low- and high-magnification SEM images of the rice leaf-inspired parallel grooves. (h) Actual photograph and CA (inset) of CMAS on the parallel grooves. (i) Schematic and (j) and (k) SEM images of the BDSs. (l) Actual photograph and CA (inset) of CMAS on the BDSs. (m) and (n) XRD spectrum and DSC curve of CMAS. (o) SEM image of PS. (p) Actual photograph and CA (inset) of CMAS on the PS. The insets in (b), (f) and (j) show the laser fabrication of various bioinspired structures. The characterizations of wetting and spreading behaviors were performed after heating the assemblies at 1250 °C for 5 min.
Download figure:
Standard image High-resolution imageInspired by the directional structure-induced anisotropic wettability of rice leaves, we prepared (SS = 110 mm·s−1, NS = 2) parallel microgrooves and investigated their anti-wetting property (figures 1(e)–(h) and S1(b)). Due to laser ablation, micro/nanoscale protrusions were distributed on the side walls of the grooves (width ≈ 23 μm, depth ≈ 19 μm, spacing ≈ 9 μm). It could be seen that molten CMAS wetted the grooves in the parallel direction. In contrast, the wetting (CA = 12.8°) and spreading (SA = 72.8 mm2) behaviors of molten CMAS were inhibited in the perpendicular direction. Considering the significant wetting inhibition property of the grooves along the perpendicular direction for molten CMAS, circular grooves (i.e. BDSs) were designed and fabricated (figures 1(i)–(k)). The width, depth, and spacing of the BDSs were about 20 μm, 20 μm, and 10 μm, respectively (SS = 110 mm·s−1, NS = 2, figure S1(c)). The similar micro/nanoscale protrusions with that on the parallel grooves were observed on the BDSs. Figure 1(l) shows the further inhibition of the BDSs to the wetting and spreading of molten CMAS (CA = 29.1°, SA = 40.7 mm2).
Figure 1(m) indicates the amorphous state of the prepared CMAS. A strong endothermic peak appeared at 1221 °C in the differential scanning calorimetry (DSC) curve (figure 1(n)) [45], which was attributed to the melting of CMAS. Obviously, the CA measurements after heating the samples at 1250 °C could acquire the wettability of molten CMAS on YSZ. To better illustrate the effects of the mentioned bioinspired structures on the wetting and spreading behaviors of molten CMAS, polished YSZ samples (figures 1(o) and S1(d)) were used to perform the relevant tests. It was observed from figure 1(p) that molten CMAS wetted the polished surfaces (PSs, CA = 9.2°, SA = 78.5 mm2). Obviously, the lotus leaf-inspired micro/nanostructures promoted the wetting of molten CMAS, while the BDSs significantly inhibited its wetting at a high temperature. The behavior of the lotus leaf-inspired structures promoting wetting was consistent with Wenzel model [46]:

where θA was the apparent CA, r was the ratio of the actual wetted area to the projected area of CMAS, θY (9.2°) was the intrinsic CA of molten CMAS on the PS. In brief, because the intrinsic wettability of YSZ surfaces was super-CMAS-melt-philic, rough structures generally enhanced the wetting of molten CMAS. In fact, both the micro/nanopapillae and low-surface-energy waxy crystal on the surfaces of lotus leaves played an important role in their superhydrophobicity.
2.2. Performance investigation of BDSs
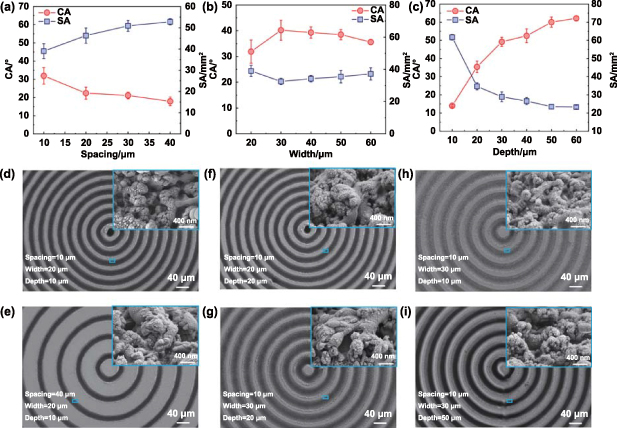
We systematically investigated the effects of different structure parameters on the wetting and spreading behaviors. The spacing between the BDSs ranged from about 10 µm to 40 µm (SS = 110 mm·s−1, NS = 2, figure S2). Figure 2(a) shows the CA and SA on BDSs (width ≈ 20 μm, depth ≈ 20 μm) with different spacing. It was observed that as the spacing increased, the CA decreased from 32.2° to 17.4°, while the SA increased from 39.0 mm2 to 52.8 mm2. Therefore, the spacing was set as about 10 µm to study the effect of width. As shown in figure S3, the width varied from about 20 μm to 60 μm and the depth was about 20 μm (SS = 110 mm·s−1, NS = 2). With the width increasing, the CA first increased and then decreased, and the SA first decreased and then increased (figure 2(b)). When the width was about 30 μm, the optimal CA and SA were 40.2° and 32.5 mm2, respectively. Figure S4 shows the change in the depth from about 10 μm to 60 μm (width ≈ 30 μm, spacing ≈ 10 μm). To obtain the different structural depth, the SS was selected as 200 mm·s−1, 110 mm·s−1, 150 mm·s−1, 170 mm·s−1, 210 mm·s−1, and 165 mm·s−1, respectively, and the corresponding SN was set as 1, 1, 2, 3, 5, and 4, respectively. The CA increased from 14.2° to 60.0° and the SA decreased from 61.8 mm2 to 23.5 mm2 with the depth increasing from about 10 μm to 50 μm (figure 2(c)). When the depth increased from about 50 μm to 60 μm, BDSs exhibited almost unchanged CA (62.1°) and SA (23.3 mm2). We compared the wetting and spreading behaviors between the PSs and the optimal BDSs (width ≈ 30 μm, depth ≈ 50 μm, spacing ≈ 10 μm). Notably, the BDSs significantly increased CA by 552.2% and decreased SA by 70.1%.
Figure 2. Effects of structural parameters on wetting and spreading behaviors of CMAS. (a)–(c) CA and SA as functions of structural spacing, width, and depth, respectively. SEM images of the BDS with spacing of (d) 10 μm and (e) 40 μm, width of (f) 20 μm and (g) 30 μm, and depth of (h) 10 μm and (i) 50 μm, respectively.
Download figure:
Standard image High-resolution imageTo clarify the effect of hierarchical structures, the formed structures were carefully analyzed. For the BDSs with typical spacing of about 10 μm and 40 μm, the enlarged scanning electron microscopy (SEM) images showed similar micro/nanoscale protrusions (figures 2(d) and (e)). When the width was about 20 μm and 30 μm, the micro/nanoscale protrusions showed no obvious change (figures 2(f) and (g)). Similarly, there was no obvious difference in micro/nanoscale protrusions for the BDSs with the depth of about 10 μm and 50 μm (figures 2(h) and (i)). The results showed that the changes in the anti-wetting property of the BDSs were attributed to the structural parameters of the microscale grooves.
2.3. Robust wetting inhibition property of BDSs
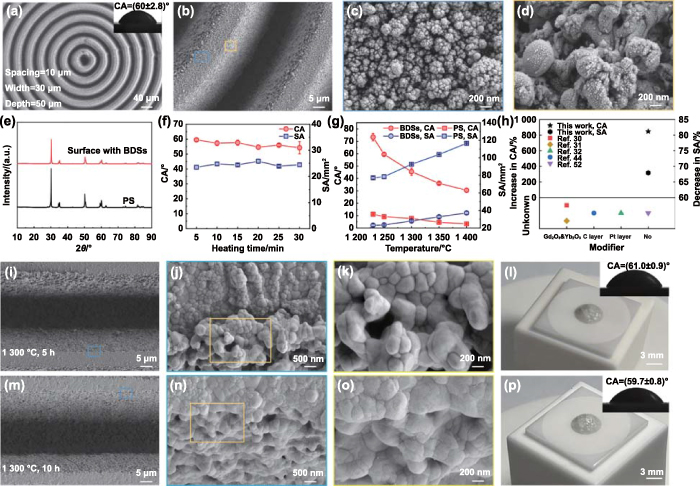
We used the optimal structural parameters to investigate the robustness of BDSs in wetting inhibition. Figures 3(a)–(d) show the surface morphologies and wettability of the optimal BDSs. The structures on the ridges were different from those on the side walls. When femtosecond laser with high energy density (>ablation threshold) irradiated the YSZ surfaces, the melting, vaporization, and removal of YSZ occurred. Due to the melting and solidification, some micro/nanoscale structures were formed in the grooves. Moreover, some of the products of vaporization fell back into the grooves and onto the ridges to form nanoparticles. The different formation mechanisms led to distinctive surface morphologies between the micro/nanostructure observed on the side walls of the grooves and on the ridges. It has been reported that laser ablation can induce the phase transformation of zirconia [47]. The transformation of t-ZrO2 to m-ZrO2 is accompanied by a volume change of 3% ∼ 5%, which easily leads to the cracking of YSZ coatings [48]. To investigate whether the femtosecond laser processing affected the phase composition, x-ray diffractometer (XRD) analysis was performed before and after laser texturing (figure 3(e)). Both the PS and BDSs consisted of t-ZrO2, indicating that the laser ablation did not cause a detrimental phase transformation of zirconia [49]. This was attributed to the rapid heating and the low thermal effect of femtosecond laser [50].
Figure 3. Robustness of BDSs in wetting inhibition. (a)–(d) SEM images showing the surface morphologies of BDSs at different magnifications. The inset shows the CA of molten CMAS on the optimal BDSs. (e) XRD spectra of PS and the surface with BDSs. (f) and (g) CA and SA as functions of heating time and temperature, respectively. (h) Comparison of the performance of BDSs with existing structured surfaces in inhibiting the wetting of molten CMAS at 1400 °C. 'Unknown' represents that the studies did not provide the experimental data under ∼1400 °C. (i)–(k) SEM images showing the surface morphologies of the BDSs after heating at 1300 °C for 5 h. (l) Actual photograph and CA (inset) of CMAS on BDSs after heating at 1300 °C for 5 h. (m)–(o) SEM images showing the surface morphologies of the BDSs after heating at 1300 °C for 10 h. (p) Actual photograph and CA (inset) of CMAS on BDSs after heating at 1300 °C for 10 h. The characterizations of wetting behaviors in (l) and (p) were performed after heating the assemblies at 1250 °C for 5 min.
Download figure:
Standard image High-resolution imageIn practical applications, TBCs may encounter more severe operating conditions, such as long-time operation in sandy environments and higher temperatures due to faster speed requirements. Therefore, it was necessary that the wetting and spreading behaviors of molten CMAS on the BDSs after prolonging heating duration and increasing heating temperature were investigated to assess the stability of the wetting inhibition capability of the BDSs. Figure 3(f) shows the changes in CA and SA with heating time. After heating at 1250 °C for 30 min, the CA of molten CMAS slightly decreased to 54.9° and the SA (24.5 mm2) remained almost unchanged. Figure 3(g) shows the CA and SA as a function of temperature. When the temperature was 1230 °C (slightly higher than the melting point of CMAS), the CA and SA reached at 73.4° and 22.9 mm2 after heating for 5 min, respectively. With the increase in temperature, the viscosity of CMAS decreased [51], resulting in better liquidity on YSZ surfaces. Consequently, the CA decreased to 31.3° and the SA increased to 37.5 mm2 as the temperature increased from 1230 °C to 1400 °C. Meanwhile, for the PSs, the CA decreased from 11.1° to 3.3°, and the SA increased from 77.0 mm2 to 117.0 mm2. Interestingly, the BDSs increased CA by 848.5% and decreased SA by 67.9% at 1400 °C, and this encouraging wetting inhibition capability has not been found on existing structured surfaces, even with the application of additional modifiers (figure 3(h)) [30–32, 44, 52].
It was widely accepted that micro/nanostructures play a vital role in surface wettability. Nanoparticles may undergo sintering in high-temperature environments, which leads to the change in micro/nanostructures and the deterioration of the wetting inhibition property. To investigate the effect of high-temperature treatments, the YSZ samples with the BDSs were exposed at 1300 °C for 5 h and 10 h. Figure 3(i) shows no obvious change in the microscale grooves after the high-temperature treatment for 5 h. However, the magnified SEM images displayed smoother nanoscale protrusions with increased size due to high-temperature sintering (figures 3(j) and (k)). After the high-temperature treatments, the wettability tests were carried out using the same experimental methodology as described in experimental section. It was found that the CA of molten CMAS on the BDSs still maintained at 61.0° (figure 3(l)). When the heating duration was increased to 10 h, the nanostructures exhibited more severe sintering phenomenon, while the surface wettability remained almost unchanged (figures 3(m)–(p)). The results further demonstrated that the ability of the BDSs to inhibit wetting was mainly attributed to the microscale grooves rather than the covered micro/nano protrusions.
2.4. Mechanism of BDSs inhibiting wetting
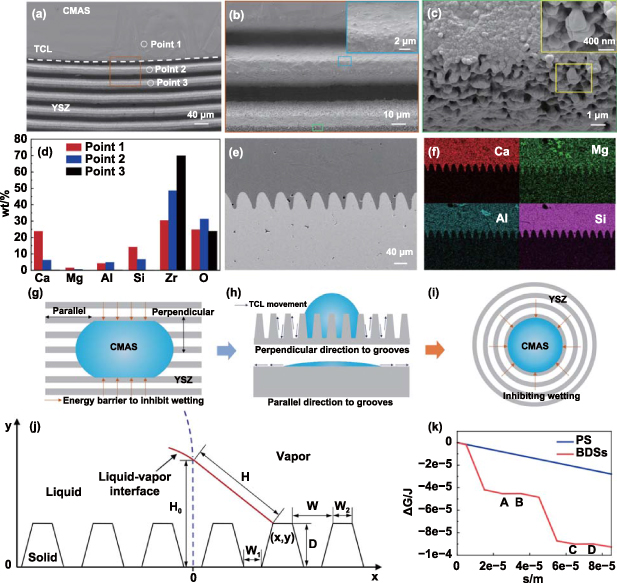
Figure 4(a) shows an apparent three-phase contact line (TCL). From the microscopic analysis for the wetting behavior, it was found that the first groove in front of the TCL was wetted by a thin layer of CMAS (figure 4(b)). Nevertheless, the wetting was inhibited in the second groove and the laser-ablated micro/nanostructures were clearly visible in the third groove (figure 4(c)). The results also implied that the molten CMAS wetted the BDSs in the Wenzel state. To further investigate the wetting behavior, elemental compositions of the point 1, point 2, and point 3 were characterized (figure 4(d)). At point 1, the typical element (Ca, Mg, Al, and Si) of CMAS showed relatively high proportions. The content of Ca, Mg, and Si obviously decreased at point 2. As expected, CMAS was not detected at point 3. To further assess the wetting state of molten CMAS on the BDSs, we characterized the longitudinal section of BDSs. It was observed that the BDSs were fully wetted by CMAS, confirming the Wenzel state of molten CMAS on the BDSs (figures 4(e) and (f)).
Figure 4. Microscopic wetting behavior of molten CMAS on BDSs and mechanism of BDSs inhibiting wetting. (a)–(c) SEM images showing the microscopic wetting behavior. (d) Element content at different positions of the surface with BDSs. (e) SEM image of the longitudinal section of as-prepared BDSs wetted by CMAS. (f) Elemental mapping corresponding to (e). (g) and (h) Illustration of anisotropic wetting behavior caused by energy barriers on parallel grooves. (i) Illustration of BDSs inhibiting wetting through energy barriers. (j) A longitudinal section view of the model BDSs. (k) ΔG as a function of s.
Download figure:
Standard image High-resolution imageAlthough molten CMAS wetting the BDSs in the Wenzel state, the wetting behavior was inconsistent with Wenzel model formula (1). Due to the presence of BDSs, r was larger than 1, so the calculated CA was theoretically less than 9.2°. However, the BDSs inhibited the wetting of CMAS in a CA of 60.0°. Therefore, we explained the underlying mechanism of BDSs to inhibit wetting from the perspective of energy barriers, as shown in figures 4(g)–(i). When a molten CMAS droplet wetted the parallel grooves, the movement of the TCL in the direction perpendicular to the grooves needed to overcome an energy barrier [53, 54]. In contrast, there was no energy barrier when the TCL moved in the parallel direction, and the grooves even promoted the wetting of molten CMAS because of capillarity, which was validated from figure 1(h). For BDSs, the wetting and spreading of molten CMAS were markedly inhibited due to the presence of energy barriers along the radial direction. To better understand the contribution of energy barriers to the wetting inhibiting, a thermodynamic model based on the change in total free energy of the system was developed. As shown in figure 4(j), the profiles in the perpendicular direction to the grooves were simplified into isosceles trapezoidal geometry. When the liquid front (initial length = H0) moved from the reference state to an adjacent configuration, the change in total free energy was given by [55]:

where ΔG1 represented the change in free energy resulting from a change in solid-vapor interfacial area and the corresponding change in solid-liquid interfacial area, ΔG2 was the interfacial energy change due to a change in liquid-vapor interfacial area, and gravity was neglected.
The formula of ΔG1, ΔG2 and ΔG were described as follows:
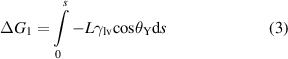


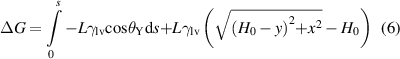
where L was the width of liquid front, γlv was liquid-vapor interfacial tension, s represented the change in surface topographic length when the liquid front moved from the reference position to an adjacent position, ΔH was the change in liquid front length, and H was length of the liquid front at any location.
Taking the optimal BDSs as an example, the total free energy change with s was calculated. The parameters were L = 1 m, γlv = 0.4 J·m−2 [56], W = 30 μm, W1 = 10 μm, W2 = 10 μm, D = 50 μm, H0 = 250 μm. As shown in figure 4(k), from point A to point B and point C to point D, the energy barrier was 2.2 × 10−7 J and 1.5 × 10−7 J, respectively. In contrast, no energy barrier was observed on the PS.
Notably, when considering practical applications, some limitations existed in the tests about the wetting inhibition ability of BDSs. Molten CMAS may come into contact with BDSs at any position, not just the center of the concentric rings. As shown in figure S5, when the CMAS cylinder was placed at the position ∼3.8 mm away from the center, the BDSs did not show wetting inhibition property at 1250 °C. This was because that the BDSs promoted the wetting along the circumferential direction through capillarity, which was confirmed from figure 1(h). To meet practical applications, we designed BDSs consisting of different numbers of concentric rings (width ≈ 30 μm, depth ≈ 50 μm, spacing ≈ 10 μm), and arranged them on YSZ surfaces (figure S6). The BDSs with 15 concentric rings showed 55.3% reduction in SA when compared with polished YSZ surfaces (figure S7), demonstrating the possibility of functioning in actual working conditions. In addition, molten CMAS would impact turbine blades at extremely high speeds, but the dynamic wetting and spreading processes were difficult to be simulated and observed through experiments. We will try to investigate the dynamic behaviors using numerical simulations in future.
In the present study, it was found that the BDSs provided energy barriers to inhibit the wetting of molten CMAS on super-CMAS-melt-philic YSZ surfaces at high-temperature above 1200 °C. Our design strategy might provide a new solution for inhibiting the wetting of high-temperature melt on super-melt-philic surfaces.
3. Conclusion
In summary, BDSs prepared with femtosecond laser direct writing technology were demonstrated to inhibit the wetting of molten CMAS on super-CMAS-melt-philic YSZ surfaces at high-temperature environments. Appropriate structural parameters were selected to achieve the obvious inhibition of wetting at 1250 °C. When compared with PSs, the BDSs featured with no additional modifiers considerably increased the CA by 552.2% and decreased the SA by 70.1%. Even if the BDSs were subjected to longer heating duration and higher temperature, they still exhibited good wetting inhibition property. Specially, the BDSs increased CA by 848.5% and decreased SA by 67.9% even at 1400 °C. We attributed the encouraging wetting inhibition property to the microscale BDSs that provided anisotropic energy barriers. The design of BDSs has broad prospects in the application of inhibiting the wetting of molten droplets on super-melt-philic surfaces at extremely high temperatures.
4. Experimental section
4.1. Materials and sample preparation
Bioinspired structures. YSZ plates with a dimension of 15 mm (length) × 15 mm (width) × 2 mm (thickness) were polished using 220#, 400#, 800#, 2000#, and 3000# grade sandpapers and diamond polishing pastes in sequence. Afterwards, femtosecond laser (PH1-20 W, light conversion) with a wavelength of 1028 nm, a pulse width of 290 fs, a repetition rate of 100 kHz, and an output power of 4 W was focused on the PSs to fabricate various bioinspired structures. The SS and NS were adjusted according to designed structure parameters.
CMAS cylinder. CaO, MgO, Al2O3, and SiO2 powders (66:18:13:90 in molar mass) were suspended in anhydrous ethanol, followed by sufficient mixing with the help of planetary ball mill (DECO-PBM-V-4 l, Changsha Deco Equipment Co., Ltd) at 300 r·min−1 for 8 h [57, 58]. The mixture was dried in a drying oven for 24 h at 60 °C. The obtained powders were heated in a high-temperature furnace (SX2-4-17TP, Nanyang Xinyu Furnace Co., Ltd) for 3 h at 1400 °C and subsequently cooled to room temperature. The formed block was crushed and ground into fine CMAS powders. The mixture of CMAS powders (50 mg) and 3% polyvinyl alcohol solution was pressed into a cylindrical mold to form a CMAS cylinder with a dimension of 3 mm (diameter) × 5 mm (height).
4.2. Characterizations
Morphological analysis was performed by SEM (Sigma 300, Zeiss) and LSCM (VK-X1000, Keyence). Elemental analysis was conducted using energy dispersive spectrometer (Xplore 30, Oxford). XRD (Ultima IV, Rigaku) was used to perform diffraction analysis. The melting behavior of CMAS powders was analyzed by DSC (STA449 F3, Netzsch) from room temperature to 1400 °C.
CA measurement was performed on optical contact angle meter (SDC-200 S, Sindin). A CMAS cylinder was placed onto each sample surface (figure S8). The whole assembly was placed in the high-temperature furnace and then heated to 1250 °C at a rate of 5 °C·min−1 with a dwelling time of 5 min. After being cooled to room temperature, the sample was taken out for CA measurement. The ambient temperature was controlled at (25 ± 1) °C. The average CA and SA values were calculated after at least five tests.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 52105212), Sichuan Science and Technology Program (No. 2023NSFSC0863), and China Postdoctoral Science Foundation (No. 2021M702712).
Conflict of interest
The authors declare no competing financial interest.
Supplementary data (3.2 MB PDF)