Abstract
Accidental marine oil discharge causes severe environmental pollution and thus remediation is essential. However, the removal and recycling of the spilled oil using economical and highly efficient methods are still major challenges. In this work, an oil absorbent of corn straw fibers (CSF) were prepared via a one-step direct modification with hydrophobic octyltrimethoxylsilane (OTS). With the surface modification, the CSF exhibited both superhydrophobicity and superoleophilicity, and the contact angles of water and oil on the as-prepared samples were 156 ± 1° and 0°, respectively. Owing to the oleophilicity and hydrophobicity features of the CSF, it could be used to selectively absorb oil in water. The adsorption capacity of the as-prepared absorbents for diesel oil, the recyclability, and the reaction mechanism between OTS and the CSF were investigated in detail. The oil absorbent could still remain superhydrophobic even after eleven successive cycles of oil-water separation. With the characteristics of easy biodegradability, durability, and recyclability, as well as the facile procedures involved, oil absorbent of CSF prepared by direct OTS-modification is expected to be used for oil spill cleanup and recovery.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Environmental pollution caused by oil spills has attracted global attention, and the discharges of toxic chemicals severely impair human health and have disastrous impacts on the ecosystem. In industry, oil skimmers or booms have often been employed to purify oil-water mixtures, however, these methods require input energy and high pressure in operation and are time-consuming and inconvenient [1]. In dealing with emergency situations involved in oil-leakage accidents, high-porosity materials, such as sponges [2], foams [3], and textiles [4], have been commonly employed for oil spill cleanup. However, these materials could simultaneously absorb water and oil, leading to incomplete separation between the oil and water, low adsorption capacity, poor oil/water selectivity, and low efficiency. Furthermore, a large number of traditional methods, including filtration, flotation, and gravity separation, and electrochemical methods have also been employed for oil-water separation, but the operations process were time-consuming and tedious [5]. Moreover, in practical applications, the subsequent disposals of the oil absorbent wastes by direct burning or underground burying easily lead to the secondary pollution, as toxic gases are being released and the soil is contaminated. Therefore, how to develop a material which selectively absorbs oil or water from water-oil mixtures is highly demanded in water-oil separation. Owing to the intrinsic immiscibility between water and oil, developing a material with selective affinity toward water or oil may be a promising way to improve oil-water separation efficiency [6].
Wettability is defined as the ability of a liquid to maintain contact with a solid surface. It is closely related to surface chemistry and surface architecture [7], and is commonly characterized by contact angle (CA). A surface featuring a water/an oil CA larger than 150° and a sliding angle lower than 10° is called superhydrophobic/superoleophobic, and when the CA is lower than 10°, the surface is defined as superhydrophilic/superoleophilic [8, 9]. To date, materials with superhydrophobic/superoleophilic surfaces have been widely designed, prepared, and applied in oil-water separation due to their distinctive oil/water selectivity [10]. The investigated materials include cotton fabric [11], porous sponges [12, 13], commercial melamine foams [14], metal meshes [15], wood slice [16] and membrane [17, 18]. However, the facts are that most of the above-mentioned materials have poor biodegradability, and the involved complex construction processes as well as high cost also limit their practical application in the cleanup of oil spills. Regarding the traditional methods for fabricating superhydrophobic-superoleophilic materials, the indispensable process of constructing the hierarchical structures with micro/nanometer scales is highly complex, and which is unfavorable for the massive production. Therefore, it is necessary to provide a more facile and low-cost method to obtain superhydrophobic-superoleophilic materials without constructing hierarchical structures.
Corn straw is an abundant, inexpensive, and readily available natural source of lignocellulosic biomass [19]. In common farming activities, only a small amount of corn straw is consumed as animal feed, household fuel, or raw material for manufacturing paper, and a large amount of corn straw is simply disposed by direct burning in situ, which is a great waste of bio-resources and results in serious environmental pollution. Confronted with this kind of predicament, how to improve and develop a technique for preparing oil absorbents from CSF and broaden the applied fields of corn straw as a bio-resource is highly necessary. Zang et al, prepared an oil absorbent by depositing hollow, spherical ZnO particles on the surface of CSF and subsequently subjected the fibers to hydrophobic modification using hexadecyltrimethoxysilane, the as-prepared CSF could absorb crude oil with high uptake capacities [20]. Li and coworkers [21] treated CSF by acetylating cellulose fibers of corn straw, which was oleophilic and did not get wet with water, and provided a potential application of straw residues as natural absorbents in oil cleanup. Tian et al [22] prepared CSF with superior superhydrophobicity and superoleophilicity by the deposition of SiO2/ZnO composite particles and subsequent modification with ocytltrimethoxysilane. Herein, we present a more facile method to prepare an oil absorbent of CSF by a one-step direct surface modification. The proposed technique here is fluorine-free and the cost is relatively low. With the inherent properties of easy biodegradability and low density, the obtained CSF with simultaneous superhydrophobicity and superoleophilicity can serve as an oil absorbent with high efficiency, low operation cost, and good recyclability. The techniques are expected to provide a new avenue for the preparation of oil absorbents, as well as to enrich and expand the cyclic utilization of corn straws as bio-resources.
2. Experimental
2.1. Materials
Pristine corn straw was obtained from a local farm in Ganzhou District, Zhangye City, China. Ethanol with analytical purity was provided by Tianjin Hengxing Chemical Reagent Co. Ltd. Commercial Octyltrimethoxysilane (OTS) was purchased from Jinzhou Jianghan Fine Chemical Reagent Co. Ltd. and directly used without further treatment.
2.2. Surface modification
First, 3.0 g of CSF, which was ground and sieved with a 60 mesh stainless steel mesh, were directly immersed in a 5% (v/v) ethanol solution of OTS for 24 h at room temperature under vigorous stirring. Then, the CSF were separated and dried in an oven at 70 °C for 24 h.
2.3. Characterization
Surface morphologies were observed using scanning electron microscopy (SEM, Quanta 450 FEG). The surface chemical composition was analyzed via X-ray photoelectron spectroscopy (XPS, PHI-5702) with Al Ka radiation. Contact angles and sliding angles were measured by an SL200KS (Kino, USA) contact angle meter equipped with a video camera and a tilting stage. A water droplet of 8 μl volume was dropped onto the target surface, and the reported contact angle was the mean value obtained from parallel measurements at five different positions.
3. Results and discussion
3.1. Surface morphology
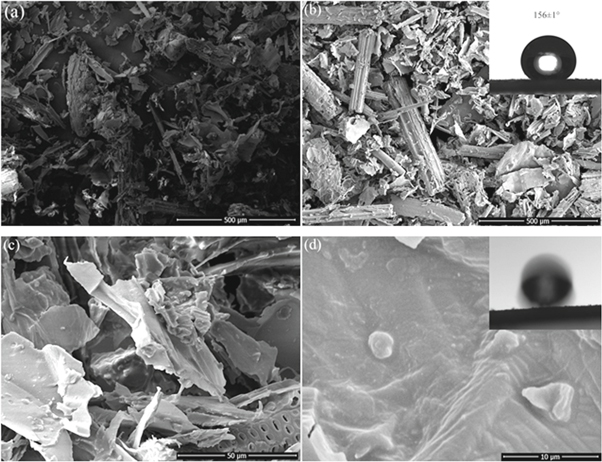
Surface wettability is dominated by surface chemistry and surface architecture [7]. The hierarchical microstructures play an important role in obtaining superhydrophobic surfaces [23]. Figure 1(a) shows the surface morphology of CSF fibers with irregular shapes, the length of fibers varied from 20 μm to 200 μm. Pristine CSF is mainly composed of cellulose, and there are massive hydrophilic hydroxyl groups (–OH) on the fiber surface [24]. When water droplet is dropped onto the particles of pristine CSF, it will spread because of the inherently hydrophilic surface. The morphologies of CSF modified by OTS were almost the same as those of the pristine corn straws, as shown in figure 1(b). High-resolution images reveal that the CSF was composed of numerous clusters, micropores, and microspheres, as depicted in figure 1(c) and figure 1(d). With the OTS modification, the morphologies of the CSF did not significantly change, but the wettability of the fibers sharply changed from superhydrophilicity to superhydrophobicity with a water CA of 156 ± 1°, as illustrated in the inset of figure 1(b). On the superhydrophobic surface of the CSF particles, water droplets were difficult to stay steadily, with a head-down tilt of 9°, the water droplet began to glide off, as shown in the inset of figure 1(d).
Figure 1. SEM images of pristine CSF (a); OTS-modified CSF (b)–(d); The insets of figures 1(c) and (d) correspond to the contact angle and sliding angle on superhydrophobic CSF, respectively.
Download figure:
Standard image High-resolution image3.2. Chemical composition
With respect to the microstructures of the pristine CSF, the morphologies of the CSF did not change with OTS modification, but its wettability sharply changed from superhydrophilicity to superhydrophobicity, which reveals that the surface chemical composition with low surface energy plays a key role in the performance of wettability.
CSF with hierarchical structures are mainly composed of cellulose, hemicelluloses, and lignin, and which contain massive hydrophilic hydroxyl groups [25]. According to Wenzel's theory [26], grinding makes the hydrophilic CSF more hydrophilic because of the increases of surface roughness. Therefore, when water droplets were dropped onto the particles of pristine CSF, the water droplets could penetrate into the microstructures, indicating the intrinsic superhydrophilic performance. However, when the pristine CSF were immersed in an ethanol solution of OTS for 24 h, the hydrolyzed OTS reacted with the –OH on the surface of the superhydrophilic CSF through a condensation reaction. With the condensation reaction, long alkyl chains were self-assembled on the surface of the hierarchical structures of CSF. The reaction mechanism between –OH and the hydrolyzed OTS is illustrated in figure 2(a). With the one-step direct modification, the surface energy of the CSF were sharply decreased due to the self-assembled alkyl chain, the surface energy of –CH2 and –CH3 in the long chain is 36 mN m−1 and 30 mN m−1, respectively [27]. Owing to the synergistic effect of hierarchical structures and the chemical composition with low surface energy, the wettability of the CSF switched from superhydrophilicity to superhydrophobicity.
Figure 2. Mechanism between OTS and CSF (a); XPS spectra of pristine CSF (b); XPS spectra of OTS-modified CSF (c).
Download figure:
Standard image High-resolution imageIn order to verify the reaction between hydrolyzed OTS and –OH on the surface of CSF, XPS was used to analyze the surface chemical composition. As for the pristine CSF, the characteristic peaks located at 284.6 eV and 530.3 eV are attributed to C1s and O1s in cellulose, respectively, as depicted in figure 2(b). With the OTS modification, additional peaks appeared at 102.2 eV and 151.5 eV, which correspond to Si 2p and Si 2 s, respectively, were observed, as shown in figure 2(c). The characteristic peaks of Si 2p and Si 2 s in the XPS spectra verify the condensation reaction between –OH and hydrolyzed OTS.
3.3. Wettability analysis
As described above, the superhydrophobicity of OTS modified CSF was originated from the synergistic effect of hierarchical structures and the surface chemical composition with low surface energy. The water droplet maintained a spherical shape when it was dropped onto the surface of the superhydrophobic CSF. According to the Cassie-Baxter model [28], with the effect of chemical heterogeneities on the surface, the fiber surface had a rough microstructure. That is to say, the contact between the water droplet and the superhydrophobic CSF was a composite contact of solid-air-liquid, and the water droplet was difficult to penetrate into the rough microstructure due to the trapped air in apertures. That is, the composite contact area of solid-air-liquid was mainly occupied by the air trapped in the microstructure of the superhydrophobic CSF, and the air underneath the water droplets could act as an 'air pillow' to support and prevent water droplets from penetrating into the microstructure. Consequently, the water droplet on the superhydrophobic surface was hard to remain steady in the composite state; with a slight tilt, and the water droplet began to move and rolled off the surface. The roll-off behavior of the water droplet on the superhydrophobic CSF is closely related to the metastable state energy and the barrier energy required for the water droplet to move from one metastable state to another [29]. The higher CA and the lower sliding angle indicate that the water droplets on the OTS-modified CSF possessed high metastable state energy and low barrier energy, and the hierarchical structures with low surface energy decreased both the triple contact line and the wetted surface fraction [30]. However, when the oil droplets of diesel and other organic solvents, including hexadecane, pentadecane, tetradecane, tridecane and dodecane, were dripped onto the superhydrophobic CSF, the droplets could penetrate into the microstructures quickly, indicating the superhyrophobic surface was superoleophilic. Furthermore, water possesses a higher surface energy than oil, for example, the surface energy of water and diesel is 72.8 mN m−1 and 26.8 mN m−1, respectively [9], and the lower surface tension means that the oil droplets are easier to spread over the solid surface. In fact, different from the technique for obtaining superhydrophobic surfaces by constructing hierarchical structures with micro/nanometer scales, a peculiarly shaped structure known as a re-entrant, overhanging, or mushroom-like structure is required in constructing superoleophobic surfaces [31, 32]. The as-prepared CSF, with the simultaneous properties of superhydrophobicity and superoleophilicity, could be used as an oil absorbent in oil spill cleanup.
3.4. Oil spill cleanup
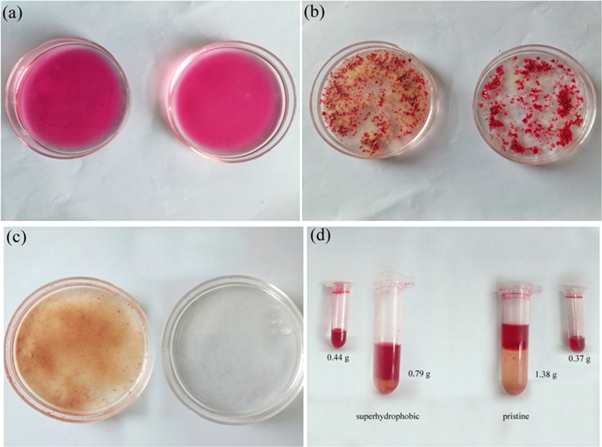
To test the adsorption capacity of CSF in the removal of spilled oil from water, diesel oil was used as a sample, in the experiment, the diesel was dyed with oil-red-O for clear observation. First, 1.00 ml diesel oil with a mass of 0.84 g was dripped into the water, and the oil kept floating because of the lower surface tension than that of water and the immiscibility between water and oil, as shown in figure 3(a). Afterward, both pristine CSF and superhydrophobic CSF were carefully strewn into the floating diesel. It was found that the pristine CSF with hydrophilicity kept floating and began to absorb the diesel, but some samples landed into the bottom of the Petri dishes due to the adsorption of water. However, the OTS-modified CSF kept floating and selectively absorbed the dropped oil due to the superhydrophobicity and superoleophilicity. Figure 3(b) shows the cases of pristine CSF and superhydrophobic CSF in water after the adsorption of dyed diesel. For the thorough removal of the diesel, 0.65 g pristine CSF and 0.39 g superhydrophobic CSF were eventually consumed, and the corresponding adsorption capacities were about 1.29 g g−1 and 2.15 g g−1, respectively. Finally, the diesel-containing CSF were carefully taken out by using a superhydrophobic copper mesh. Compared with the water treated by CSF, the water treated by superhydrophobic CSF was cleaner and there were no obvious residues left on the water surface, as shown in figure 3(c). By high-speed centrifugation, 0.79 g and 1.38 g oil-water mixtures were finally obtained from the superhydrophobic and the pristine CSF, and the diesel separated from the above oil-water mixtures was 0.44 g and 0.37 g, respectively, as shown in the inset of figure 3(d).
Figure 3. Oil spill cleanup by CSF. (a) Diesel oil floating on water; (b) CSF after absorbing diesel; (c) Water surface after removing CSF; (d) Oil-water mixtures and diesel obtained from CSF. The diesel was dyed by oil-red for clear observation.
Download figure:
Standard image High-resolution imageAs described above, both the superhydrophobic CSF and oil could be recovered by using high-speed centrifugation. Interestingly, the OTS-modified CSF kept superhydrophobic after diesel-corn straw fibers separation by high-speed centrifugation, and the superhydrophobic CSF could be recycled by annealing at 120 °C for 2 h. In the subsequent recycling operation, the diesel adsorption capacity was estimated to be about 2.1 ∼ 2.3 g g−1, and the recovery efficiency exceeded 60%, the adsorption capacity and recovery efficiency varied with cycles was shown in figures 4(a) and (b). The water droplet could always maintain a spherical shape on the CSF, and the CA was larger than 150°, which demonstrates the durability of superhydrophobicity in oil removal. The variation of water CA with the number of cycles is illustrated in figure 4(c). Figure 4(d) displays an image of spherical water droplet on recovered corn straw after diesel-corn straw fibers separation.
Figure 4. (a) Adsorption capacity; (b) recovery efficiency; (c) contact angle variation with cycles; (d) spherical water droplet on recovered superhydrophobic CSF.
Download figure:
Standard image High-resolution imageTo evaluate the maximum adsorption capacity of the CSF for diesel, 1.00 g of CSF was put in a 159 mesh nylon bag and dipped into 50 ml diesel for 30 min. Then, the nylon mesh was taken out and suspended in air for 30 min. Owing to the oil adsorption, the mass of the superhydrophobic CSF and pristine CSF increased from the original 1.00 g to the final 3.06 g and 3.10 g, respectively. The maximum diesel adsorption capacity of the CSF was estimated by using the following equation:
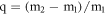
where m1, m2 denotes the mass of the original CSF and the mass of the diesel-containing CSF, respectively. With the measured values, the maximum diesel oil adsorption capacities of the superhydrophobic CSF and the pristine CSF were estimated to be about 2.06 g g−1 and 2.10 g g−1, respectively. As for the maximum oil adsorption, there was no large difference between the superhydrophobic CSF and the pristine CSF. However, as for the removal of the oil in water, the superhydrophobic CSF could be recycled, and the adsorption capacity and recovery efficiency were high due to water repellency and selective affinity to oil. In addition to diesel oil, the superhydrophobic CSF can also be employed to remove other organic solvent such as hexadecane, pentadecane, tetradecane, tridecane and dodecane, and the corresponding adsorption capacity is 1.86, 1.83, 1.79, 1.80 and 1.77 g g−1, respectively, as shown in figure 5(a). After being adsorbed by the as-prepared corn straw, the above organic solvent can also be recovered by centrifugal separation, and the corresponding recovery efficiency is 61.5%, 68.2%, 66.8%, 69.9% and 61.2%, respectively, as depicted in figure 5(b). As for the recovered CSF, it remained superhydrophobic and the water droplet dripped on the CSF was spherical, as shown in figure 5(c). Additionally, the as-prepared CSF remained superhydrophobic even when it was exposed to air at room temperature for 90 days, which reveals its stability. In order to further evaluate the stability of superhydrophobic CSF in corrosive solution, the as-prepared CSF was immersed in a solution of HCl or NaOH and kept stirring for 24 h at room temperature. It was found that the water contact angles on the as-prepared CSF did not significantly changed in pH range of 0 ∼ 12, the water contact angles exceed 150°. However, in the NaOH solution at pH ∼14, water contact angle decreased from ∼156° to 97°, as shown in figure5(d). The results indicates that the as-prepared corn straw kept stable both in acidic and weak basic environment, but unstable in strong basic environment due to the breakage of long alkyl chain self-assembled on CSF. With the advantages of water repellency, oil affinity, and relatively high stability, as well as biodegradability, the superhydrophobic CSF is expected to serve as natural oil absorbent in practice for the removal of spilled oil in water.
Figure 5. (a) Adsorption capacity; (b) recovery efficiency of as-prepared CSF to organic solvent; (c)Water drop on recycled CSF after separating tetradecane; (d) Water contact angles of as-prepared CSF being immersed in acidic or basic solution for 24 h.
Download figure:
Standard image High-resolution image4. Conclusion
An oil absorbent of superhydrophobic-superoleophilic CSF was prepared using a facile and environmentally friendly method of direct modification. The contact angles of water and oil on the as-prepared sample were 156 ± 1° and 0°, respectively. The superhydrophobicity was ascribed to the synergistic effect of hierarchical structures and the low surface energy of long-chain alkyl self-assembled on the CSF surface. Owing to the superhydrophobic and superoleophilic features of the as-prepared CSF, it could selectively absorb diesel in water. In the oil removal process, both the as-prepared CSF and absorbed oil could be recycled via high-speed centrifugation, the oil adsorption capacity was 2.1 ∼ 2.3 g g−1, and the oil recovery efficiency exceeded 60% in every cycle. The method presented here is facile, and the raw material of corn straw is biodegradable, thus, the modified CSF is expected to serve as a candidate material for the design and preparation of natural oil absorbents.
Funding information
This work was supported by the National Natural Science Foundation of China (No.41761061), CAS 'Light of West China' Program, the Scientific Research Foundation of the Higher Education Institutions of Gansu Province (No. 2016B-091), the General Program of the Key Laboratory of Hexi Corridor Resources Utilization of Gansu Universities (No. XZ1603), and National training Program of Innovation and Entrepreneurship for Undergraduates (No. 201910740009).