Abstract
This paper reviews Kretschmann-based SPR sensor utilizing ZnO thin films and nanostructures for performance enhancement. The advancement in surface plasmon resonance technology relies on low-cost, high sensitivity and high selectivity sensor. Metal oxide has been incorporated in SPR sensor to be used for detection of biological and chemical compounds. ZnO as one of the metal oxides is an attractive material due to its unique physical and optical properties. Numerous techniques for fabrication and characterization of ZnO on SPR gold substrate have been studied. The mechanism for gas and biomolecules detection depends on their interaction with ZnO surface, which is mainly attributed to the high isoelectric point of ZnO. There are several types of ZnO nanostructures which have been employed for SPR application based on the Kretschmann configuration. In future, the thin film and nanostructures of ZnO have potential applications for miniature design, robust, high sensitivity, and low-cost portable type of SPR biosensor to be used for on-site testing in real-time and label-free manner.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Optical biosensors based on surface plasmon resonance (SPR) are becoming popular in recent years. The real strength of SPR lies in its highly efficient, label-free and real-time measurement. The development of SPR is important in many fields such as agricultural and food safety [1], medical diagnosis [2], environmental safety control [3] and industrial monitoring [4]. The major application of SPR biosensor is for detection of a variety of biomolecules such as lipid [5], peptides [6], receptor [7], nucleic acid [8], protein [9] and antibodies [10]. The SPR technique has not only been used for biosensing purposes but also in characterizing a broad range of material thin films [11]. The most commonly used configuration to excite SPR is by prism coupling through Kretschmann configuration in attenuated total reflection method [12]. As p-polarized laser light passes through a prism and a thin metal (Au) substrate, reflection of the incident light is detected by a photodetector. When the incident angle of light reaches the resonance angle, coupling of the light energy with the metallic layer generates a surface plasmon wave at the interface between the metallic surface and the sensing medium. A small change in refractive index at the surface leads to a shift in resonance angle, which is the incident angle that supports the formation of surface plasmon wave [13].
The first commercially available SPR instrument, Biacore, was launched to the market by Biacore AB (Sweden) in 1990 [14, 15]. Currently, there are a considerable number of SPR instruments produced by different manufacturers [16–19]. The SPR instruments comprised of three integrated components: i) the SPR optics consist of a high refractive index prism, ii) the SPR sensor (or sensor chip) coated with metallic film at appropriate thickness, and iii) a liquid flow channel containing the analytes or samples to be tested. The instrument can be employed in either angular interrogation mode or wavelength interrogation mode for detection of biomolecules at high sensitivity. The main advantage of angular interrogation versus wavelengths interrogation is the high optical power which is coupled into the sensing region, hence increasing the sensitivity. More recently, BioNavis (Finland) has developed a research line of multi-parametric SPR using a unique arrangement of goniometric optics configuration consists of multiple lasers in a spot to allow cross-correlational measurement of sensing parameters [20–40]. Even so, there are several drawbacks of the commercial setup as the instrument is bulky in size with a restricted range of incident angle making it unconducive for remote sensing [41]. Moreover, the sensor chips are costly and usually not reusable [42]. However, many other useful parameters such as the thickness and refractive index of a new sensing layer can be determined using the commercial setup. Else than that, the commercial setup provides key insights into the kinetics of molecular binding between the analytes and ligand.
Some of the limitations of the prism-based SPR sensor can be overcome by using optical fiber SPR sensor based on Kretschmann configuration. Total internal reflection takes place at the interface between core and cladding in the optical fiber. It allows light to be guided through the optical fiber and an evanescent wave is propagated along the interface. An optical fiber SPR sensor can be fabricated by replacing the cladding from a small part of the optical fiber with a metal film. Meaning that the unclad core is coated with a metallic layer which creates a surface for the excitation of SPR. However, unlike the SPR sensor using prism coupling, a shift in resonance wavelength is observed instead of the resonance angle. For this reason, a polychromatic light source is applied to the fiber optic. Occurrence of biomolecule interaction changes the refractive index of the sensor metal surface at the cladding, and hence the detection of biomolecules can be made. Compared to the prism coupling based SPR sensor, optical fiber has the advantages of easy fabrication and possibility of miniaturization that is very useful for remote sensing. However, the molecular kinetics of the analyte-ligand interaction cannot be determined using fiber-optic sensing and can only be executed using prism-based SPR sensing [43]. Moreover, optical fiber sensing has its own challenges such as easy breakage of single mode fiber (SMF) when bent in order to increase detection sensitivity as well as difficulty in coating multiple layers on the thin fiber. Therefore, it is best to have a fundamental sensing approach beginning with prism-based SPR sensing and subsequent deployment using optical fiber sensing once all relevant sensing layers have been optimized.
A SPR sensor is made up of a glass substrate coated with a nanoscale-thick metallic layer. The metallic layer in the SPR sensor is the backbone for SPR excitation because it contains a large number of free electrons that contribute to the negative permittivity of the materials. It is the interaction of free electrons in the metallic layer with incident light that excite SPR phenomenon. Thin film of gold (Au) and silver (Ag) are commonly used as the plasmonic material since they have a lower d-electron energy bands as compared to the conduction band of other metals and their bulk plasma frequency are 9.5 eV and 6.5 eV, respectively, sufficient for designing an SPR sensor for detection in the visible wavelength [44–46]. However, there are several limitations of using Au or Ag alone for SPR biosensor such as poor adhesion metal layer on substrate surface and required surface modification as the sensing probe.
The emergence of nanotechnology leads to the advancement in application of nanomaterials in SPR sensor due to its high tunability of material properties at the nanoscale. In recent years, metal oxides are among the various engineered nanomaterials being used for the improvement of SPR sensors. Metal oxides are also frequently used as semiconductor materials as they exhibit a change in electrical conductivity in proportion to the composition of the analyte surrounding them. In particular, research utilizing zinc oxide (ZnO) as a metal oxide semiconductor, have gained popularity due to its attractive intrinsic properties in microelectronics and biosensing applications. ZnO as an n-type semiconducting metal oxide is well known for its direct and wide band gap (∼3.4 eV) and high exciton energy (60 meV) at room temperature. It has been vastly studied for photonic applications in the near UV–vis wavelengths such as light emission diodes (LEDs) [47], luminescence [48] and ultraviolet lasers [49]. Its high resistance to heat protects it from electronic degradation when functioning. Moreover, among many II-IV semiconductor, ZnO is the hardest material with Mohs scale of hardness of ∼5 [50]. ZnO, as a low-cost material with its biocompatibility and biodegradability, are safe biomaterials to be used for in vivo biosensing and biodetection. Zhou and coworkers studied the stability of ZnO nanowire in various solutions, namely deionized water, ammonia, NaOH solution, and horse blood serum [51]. Results showed that ZnO could be dissolved in water, ammonia and NaOH solution. When ZnO came into contact with horse blood serum, it sustained in the liquid for a few hours before it degraded into mineral ions. If the ZnO was applied for in situ biosensing and detection in biological system, the ions particularly the Zn ions, can then be absorbed by the body and provide nutritional value to the body. In fact, ZnO is also commonly found in daily personal care and cosmetic products.
While Au (or Ag) is the material required for SPR excitation, there is no SPR effect in ZnO. The large band gap of ∼3.4 eV makes ZnO transparent in the visible range and so it does not absorb light at visible wavelengths. In other words, ZnO does not have conduction band electrons capable of resonating with the electric field of light at visible wavelengths. Nevertheless, ZnO is widely used in combination with Au or Ag to enhance the performance of prism and fiber optic based-SPR sensors as shown in figure 1. The current paper reviews Kretschmann-based SPR sensor utilizing ZnO thin films as well as nanostructures for performance enhancement in Au or Ag based SPR biosensor. Firstly, the fundamentals of SPR phenomenon is briefly described. In the following section, the approaches for enhancing sensing performance of Au or Ag based-SPR sensor using ZnO are presented.
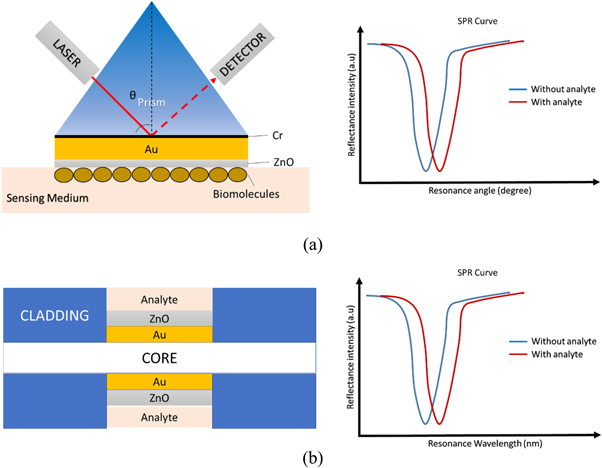
Figure 1. The schematic diagram of SPR sensor using Kretschmann configuration. (a) Prism-based SPR sensor consists of a prism, Au and ZnO layers and its SPR curve when refractive index changes in the presence of analyte; (b) Fiber optic based-SPR sensor consists of core and cladding with unclad region coated with Au and ZnO layers and its SPR curve when refractive index changes in the presence of analyte.
Download figure:
Standard image High-resolution image2. Performance parameter of SPR biosensor
Upon interaction between the evanescent wave from the light and surface plasmon of the plasmonic layer, surface plasmon wave is generated and propagated at the metal/dielectric interface if the resonance condition is met. For prism-based SPR sensor working in angular interrogation mode, the resonance condition is described in equation (1).
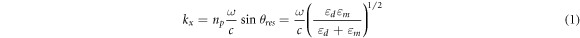
where  is refractive index of the prism, θres is the resonance angle,
is refractive index of the prism, θres is the resonance angle,  is the angular frequency, c is the speed of laser light in the vacuum, and
is the angular frequency, c is the speed of laser light in the vacuum, and  and
and  are the dielectric constants of the metal and the dielectric, respectively. For fiber optic-based SPR biosensor working in the wavelength interrogation mode the resonance condition is described in equation (2) [52].
are the dielectric constants of the metal and the dielectric, respectively. For fiber optic-based SPR biosensor working in the wavelength interrogation mode the resonance condition is described in equation (2) [52].
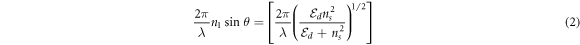
where  is the refractive index of the core of the fiber, λ is the SPR wavelength of the incident beam,
is the refractive index of the core of the fiber, λ is the SPR wavelength of the incident beam,  is the angle of incidence of the beam,
is the angle of incidence of the beam,  is the real part of the dielectric constant of the metal coated on the unclad core of the fiber and
is the real part of the dielectric constant of the metal coated on the unclad core of the fiber and  is the refractive index of the dielectric or the sensing medium around the metal layer. In equations (1) and ), the left side of the equation represents the wavevector of evanescent wave from the incident light while the right side of the equation represents the wavevector of surface plasmon on the metallic surface [53].
is the refractive index of the dielectric or the sensing medium around the metal layer. In equations (1) and ), the left side of the equation represents the wavevector of evanescent wave from the incident light while the right side of the equation represents the wavevector of surface plasmon on the metallic surface [53].
Several important performance parameters have to be considered when evaluating the sensing performance of the SPR sensor. A good SPR signal can be measured from the minimum reflectivity and full width at half maximum (FWHM). A deep and sharp reflectance curve improves the SPR sensing performance. The minimum reflectivity is attributed by the merging of photon energy from the incident light with the surface plasmon. A smaller value of minimum reflectance can be obtained by optimizing the thickness of the plasmonic layer, e.g., ∼50 nm Au thin film for prism based SPR sensor [54]. The sharpness of an SPR reflectance curve is indicated by a small value of FWHM. It depends on the complex dielectric constant of the metallic layer. In general, a larger value of real part (negative) along with a small value of the imaginary part of the dielectric constant results in a sharper reflectance curve. Sensor sensitivity is described by the shift in the resonance angle or wavelength as a function of changes in the refractive index depending on the analyte attached to sensing surface. Figure of merit is a typical quality parameter for assessing the performance of the SPR sensor. It can be calculated by dividing the sensor sensitivity value with FWHM. Meanwhile, the limit of detection provides information about the lowest concentration of targeted analyte that can be measured by the biosensor. The smaller the limit of detection value, the better the sensing performance of the biosensor.
Prior to the deposition and characterization of an SPR biosensor, ZnO is optimized using numerical methods to achieve a high-performance sensor. This can be achieved by simulation of the SPR curve with different thicknesses of the ZnO layer using Finite Difference Time-Domain (FDTD) method from Lumerical [55–57]. As the thickness of the ZnO increases, the FWHM and resonance angle of the SPR signal becomes larger which would affect sensor reliability and sensitivity. Therefore, optimization of the ZnO thickness by taking into account the sensor performance and its practicality into consideration is crucial prior to ZnO thin film deposition and characterization.
3. ZnO Thin film deposition and characterization
There are many methods that have been applied for the fabrication of ZnO thin films such as pulsed laser deposition (PLD), sputtering, thermal evaporation and condensation, solid state reaction and chemical method. Several chemical deposition techniques which are available for the fabrication of ZnO thin films are such as chemical vapor deposition (CVD), electrodeposition [58], spray pyrolysis and sol-gel deposition techniques. Spray pyrolysis is the preferable technique for deposition of metal oxide films due to its simple, low cost, and production process can be easily scaled up [59]. In this technique, the solution is sprayed on to the substrate that is heated at high temperature. As a smaller grain size is desirable to improve sensor performance, the temperature of the substrate is the parameter to control the grain size in the pyrolytic reaction.
The physical route for fabrication of ZnO thin film include thermal evaporation technique and Rf (radio frequency) magnetron sputtering. The thin layer grown by Rf magnetron sputtering technique has high purity, good reproducibility, and accurately controlled thickness. Thickness of the thin film can be measured by quartz crystal thickness monitoring [60]. Characterization of the ZnO layer on the SPR sensor using FTIR gives a peak at around 418 and 550 cm−1 due to the vibrations of the Zn–O bonding [61]. XRD pattern of ZnO thin film on Au SPR sensor produces a peak at 2θ = 34.1° in agreement with 002 plane of the hexagonal wurtzite structure of ZnO. This value indicates that the ZnO thin film grown is in the c-axis orientation [62].
4. ZnO for sensitivity enhancement
Due to the demand of ultra-high sensitivity sensor for detection of biological and chemical compounds at low concentration and small molecular weight, several sensitivity enhancement approaches have been explored [63]. There are many reasons for a poor sensing performance of SPR sensor. One of the reasons could be the poor adhesion strength between metal and substrate causes instability of the sensor which degrades its performance. Chromium (Cr) and Titanium (Ti) are widely used as the adhesion material. Nonetheless, there are several drawbacks including metal interdiffusion and low portion of light is transmitted to the surface of gold layer. Moreover, these materials produce large FWHM value and low sensitivity. A few metal oxides are used as adhesive layer between substrate and the metallic layer such as Titanium dioxide (TiO2) and silicon dioxide (SiO2). Mauya et al combined SiO2 with low refractive index over TiO2 with high refractive index to form a TiO2/SiO2 composite layer. Compared to the individual TiO2 and SiO2, the composite layer works better as the adhesive layer. The plasmonic effect near the TiO2/SiO2 interface provided light trapping effect to allow generation of more surface plasmon. Consequently, it enhanced the resonance angle shift and thus the SPR sensing.
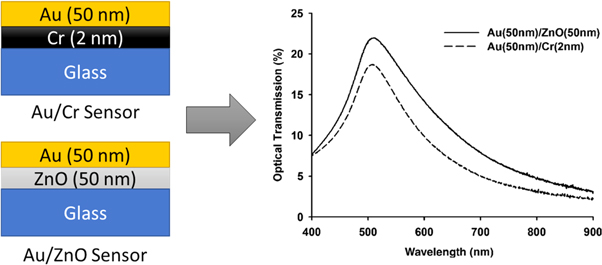
Besides, ZnO as a semiconducting metal oxide has also been used as the adhesion material owing to the non-centrosymmetric property of ZnO which enhances the performance of the sensor. The ZnO/metal interface help to promote phonon interaction. Figure 2 shows the transmission spectra of Cr and ZnO as the adhesion layer for 50 nm gold film obtained from UV–vis spectrophotometer measured in 400–900 nm wavelength [64]. Both Au/Cr and Au/ZnO sensors exhibit transmission peak around 500 nm which is corresponding to the optical transmission of gold thin film at the green region of the electromagnetic spectrum. Despite that, the gold sensor with 2-nm Cr shows lower transparency than the sensor with ZnO because a portion of light was absorbed by the Cr layer. Hence, the light collecting ability makes ZnO a better adhesive material than the conventional Cr. In fact, ZnO creates a larger shift in resonance angle and smaller FWHM in SPR curve which leads to enhancing the sensitivity, detection accuracy and the sensing performance of the sensor [65].
Figure 2. The comparison of transmission spectrum of Au/Cr and Au/ZnO sensors with the respective transmission spectra. Figure was adapted from Chang et al [64].
Download figure:
Standard image High-resolution imageFurther, the SPR excitation using Ag as the plasmonic metal layer face the challenge of oxidation under air exposure which reduced the sensor sensitivity and limit their applications [66, 67]. Compared to conventional Au-based SPR sensors, Ag-based SPR sensors produce higher sensitivity with a sharp dip in the SPR curve with smaller FWHM value. However, it is seldom used due to its poor chemical stability and biological affinity. The problem of low chemical stability in Ag can be eliminated by coating an ultrathin metal oxide layer such as TiO2 [68] and SnO2 [69, 70] to act as a protective layer. Else than that, coating a ZnO layer enhances the sensitivity of the Ag-SPR sensor as the ZnO is a high- indexed material [71]. Further examples on the usage of ZnO for sensitivity enhancement are listed in table 1.
Table 1. The summary of SPR uses of metal oxide in Kretschmann configuration.
| Nanostructure materials | Size | Operating wavelength | Role | Target Analytes | Performance (Sensitivity/ FWHM/LOD) |
|---|---|---|---|---|---|
| ZnO thin film [72] | 16 ± 1 nm | 632.8 nm | Gas sensing layer | methanol, ethanol, isopropanol and hexane vapors | Large response toward ethanol and isopropanol |
| ZnO nanorod array [73] | 546.6 × 53.6 nm | 633 nm | Increased surface sensing area | DNA hybridization | 3 times increased sensitivity |
| ZnO-Au nanocomposite solution [74] | 0.0625–1 ml | — | ZnO/Au/Protein probe | Rabbit IgG | 16-fold lower LOD |
| ZnO film [75] | 400–800 nm | mid-IR | Plasmonic layer | — | — |
| ZnO thin film [76] | 50 nm | 830 nm | Intermediary layer | Ethanol | 0.56° FWHM |
| Ga-doped ZnO thin layer [77] | 80 nm | 1550 nm | Plasmonic materials | — | — |
| ZnO thin film [78] | 200 nm | 407 nm, 470 nm, 533 nm, and 633 nm | Immobilization layer for dielectric constant determination | Glucose oxidase, cholesterol oxidase, urease, and uricase | — |
| ZnO island [79] | 0.50–3 μm | — | Gas sensing layer | CO2 gas | Shift of SPR angle to higher angle upon absorption of CO2 gas |
| ZnO nanorod and flower-like structure [80] | Nanorod: 200–300 nm diameter and 3–5 μm length | 650 nm | Gas sensing layer | CO2 gas | Shift of SPR angle to lower angle upon absorption of CO2 gas |
| ZnO thin film [81] | 200 nm | 633 nm | Gas sensing layer | CO gas | 0.091 °/ppm sensitivity |
| ZnO thin film [61] | 200 nm | 633 nm | Immobilization layer | Cholesterol | 0.36 ° mM−1 sensitivity |
| ZnO thin film [82] | 200 nm | 633 nm | Immobilization layer | N. meningitidis DNA | 0.03°/(ng/μl) sensitivity |
| ZnO thin layer [81] | 200 nm | 633 nm | Gas sensitive layer | CO gas | 0.091 °/ppm sensitivity |
| ZnO thin layer [64] | 50 nm | 400–900 nm | Intermediate layer | CA15–3 | 0.025 U/mL limit of detection |
| ZnO thin film [83] | 32 nm | 633 nm | Adhesive layer | ss-DNA | 66°/RIU sensitivity |
| ZnO thin film [84] | 5 nm | 632.8 nm | Adhesive layer | Pseudomonas and pseudomonas like bacterial | Sensitivity: 187.43 °/RIU, DA: 2.05 deg−1, FOM: 29.22 RIU−1 |
| ZnO thin layer [42] | 200 nm | 632.8 nm | Immobilization layer | Glucose | Limit of detection 20 mM |
| ZnO thin layer [85] | 20 nm | 833 nm | Gas sensing layer | Nitric oxide gas | Low sensitivity |
5. ZnO for gas sensing
The first application of ZnO thin film for SPR sensor was as a sensing layer for gas detection. Generally, selection of material for sensing a particular gas is determined by the interaction of its surface active side formed by ions O−, O2−, H+, and OH− with gas molecules. SPR based gas sensor using ZnO is widely used for sensing for both reductive and oxidative gases [72]. The key property of ZnO for gas sensing is the reversible interaction of its surface with the gas molecule due to covalent bonding or dipole-dipole interaction [86]. As a n-type semiconductor, ZnO has high level of defects to provide a good surface for gas molecule absorption. As the molecules of gas is absorbed on the ZnO surface, changes in the dielectric constant of the ZnO takes place and results in refractive index changes. However, the absorption of gas on defect surface is only applicable for gas with higher molecular weight such as H2S. Gases with lower molecular weight such as H2 is not possible to be absorbed on the ZnO defect surface as the gas molecule migrates easily.
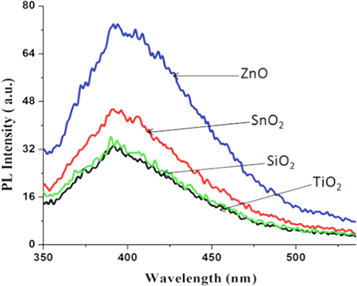
Excessive inhalation of H2S gas is poisonous and harmful to human health. It can cause respiratory problem, loss of consciousness, and fatality. Therefore, environmental monitoring of H2S gas is very important. The H2S gas sensing principle of ZnO can be explained by redox reaction that occurs between the gas molecule and ZnO. The surface of ZnO provides the oxygen supply for reduction and oxidation reaction. The reaction between H2S and ZnO gives off ZnS, SO2 and H2O. The H2O formed has no effect on the sensing surface, but the formation of SO2 changes the dielectric constant/refractive index at the ZnO sensing layer [86]. Hence, shifts in the resonance angle or resonance wavelength can be observed. Tabassum et al demonstrated that the defect level on ZnO surface is sufficiently high for gas detection [87]. According to the photoluminescence spectra (figure 3) obtained from various metal oxides including ZnO, TiO2, SiO2 and SnO2, ZnO showed the highest value of peak photoluminescence intensity (PPI). The highest the PPI the higher the defect level. Hence, compared to other metal oxides, ZnO provides the highest number of active sites for H2S absorption.
Figure 3. The photoluminescene spectra of different metal oxides showing PPI of ZnO is the highest among the four oxides. Adapted from Tabasum et al [87].
Download figure:
Standard image High-resolution imageThe ZnO coated Au sensor has also been used for the detection of carbon monoxide (CO) gas [81]. Similarly, the detection principle for CO gas is due to the variation in the refractive index at the ZnO-CO interface. There are two reasons for changes in the refractive index; firstly, the changes of bulk media from air to CO gas increased the refractive index, secondly, the absorption of CO gas at ZnO surface changes the refractive index at the sensing layer. The interaction of CO gas with ZnO surface lead to reduction reaction. When the oxygen on the film surface absorb the trapped electron, electron density is lowered. The total reduction of electron density at the interface reduced the refractive index. As a result, the resonance angle shift to lower angle as the CO gas concentration increased. Detection of CO gas showed that the ZnO sensor has good stability and high sensitivity (0.092 °/ppm) at 0.5 to 100 ppm concentration range. The fabricated sensor also showed negligible response towards other gases such as NH3, CO2, NOx, H2 and liquefied petroleum gas, indicating high selectivity of the sensor toward CO gas.
Meanwhile, nitric oxide (NO) gas is an extremely harmful gas as it is a highly-reactive free radical that is damaging to human body. Usually NO gas is formed as a by-product from the combustion process in fossil fuel plant and thus environmental monitoring for this gas is crucial. Feng et al prepared a SPR sensor with ZnO nanofilm for detection of NO gas. The electrical and optical properties of ZnO changed when exposed to NO gas. When NO reacted with ZnO (20 nm) on Au layer, shift in resonance angle took place. Detection mechanism of NO gas by ZnO thin film involves an increased conductance and permittivity of the ZnO layer due to reduction in the height of the Schottky barrier [85]. In a system under oxygen condition, the oxygen species was ionosorbed on the surface of ZnO layer, and it acted as an electron acceptor due to their relative energetic position with the respect to the Fermi level. The electron supply for this process originating from intrinsic oxygen vacancies of ZnO were released from the conduction band and were trapped at the surface. Then, the reducing NO gas reacted with the ionosorbed oxygen species which eventually desorbed as NO2. As NO gas concentration increased, the absorbed oxygen decreased which caused the surface trapped electrons to be released back to the bulk. The reflectance intensity increased and resonance angle was shifted to larger angle when the NO reacted with the ZnO. Hence, the conductivity of the ZnO film increased.
Chlorine (Cl2) gas sensor using fiber optic based SPR sensor has been studied [88]. The unclad region of the fiber optic was coated with Ag (40 nm) and ZnO (15–30 nm) layers. When the Cl2 gas was passed through the sensor chamber and come into contact with the probe, it reacted with the ZnO thin film. The reaction resulted in formation of zinc chloride and oxygen. The formation of zinc chloride changed the dielectric constant of the sensing layer, and hence shift in SPR curve occur. A selectivity study was conducted to compare the performance of the Cl2 sensor for other gases such as hydrogen sulphide, ammonia, methane, hydrogen and nitrogen. Based on the reaction type of these gases with ZnO, hydrogen sulphide, hydrogen, ammonia and methane act as reducing agent, while Cl2 acts as oxidizing agent. Nitrogen are unreactive to the probe as it is an inert gas. The fiber optic gas sensor with Ag (40 nm) and ZnO (18 nm) gave the highest sensitivity because there was a very large shift in resonance wavelength for Cl2 gas compared to others gases. The advantages of this sensor include high sensitivity, good selectivity, fast response, reusable, low cost, as well as can be used for online monitoring and remote sensing.
As mentioned above, ZnO is used for detection of both reductive and oxidative gas based on reversible redox reaction with the ZnO. When exposed to oxidative gas such as Cl2 the ZnO surface acted as electron acceptor. While exposure to reductive gasses such as NO, the oxygen species will interplay with these gas molecules and release trapped electron back to the conduction band. The interaction of both oxidative and reductive gases with the surface of ZnO results in changes in the refractive index of the ZnO surface, which can be observed by a shift in the resonance spectra.
6. Immobilization of biomolecules on ZnO layer
SPR biosensor is used for detection of biomolecules. For such detection, the ligand is immobilized on the sensor surface while the analyte flows over the sensor surface though a flow cell. Binding between the ligand and analyte changes the refractive index at the sensor surface. Hence, detection of the biomolecule interaction (association and disassociation) can be observed from the shift in the resonance angle.
The major consideration for enhancing the sensitivity and efficiency of SPR biosensor is the selection of a suitable matrix for immobilization of biomolecules such that the immobilized biomolecules are stable and retain its biological functionality. Study of different biomolecular interaction can be made simply by modifying the surface of the biosensor that allow different kinds of surface functionalization thereby reducing changes of the experimental setup [89]. By choosing the appropriate immobilization matrix, the adhesion, protein-protein interaction and cell-cell interactions can be improved. Several types of immobilization matrix are suitable for designing a SPR biosensor chip. Using a good immobilization strategy with the right selection of immobilization surfaces, biomolecules can be immobilized on the sensor surface without disturbing its conformation and biological activity [90]. For example, carboxymethyl dextran (CMD)-coated gold SPR sensor increases the number of immobilized biomolecules. Biomolecules immobilized on the CMD surface should carry amine, carboxyl, aldehyde or thiol groups [91–97]. The most commonly used surface chemistry to immobilize biomolecule on CMD sensor is amine coupling process using mixture of EDC and NHS. In this method, the carboxyl group on the sensor surface will be activated to allow linkage between the ligand and carboxyl group taking place. The remaining non-activated carboxyl group are blocked by ethanolamine to avoid non-specific binding. The CMD SPR sensor can be regenerated using glycine-HCl solution. Example of biomolecules that can be immobilized by this sensor is monoclonal MMP-9 antibodies [98]. The antibody prepared in acetate buffer was injected over the activated surface. However, one drawback of using CMD surface is the formation of heterogenous surface when protein or peptide possess different functional group on its surface resulting in random-orienting immobilization and sometimes, to the extent that it blocks the active site of the protein [99]. Streptavidin SPR sensors are used to immobilize biotinylated ligand [100, 101]. As tetrameric molecule, streptavidin has four biotin binding sited present in pairs at each site of the molecule. This makes streptavidin having strong affinity toward biotin. The streptavidin can withstand harsh chemical regeneration and maintain its reproducibility from cycle to cycle. Streptavidin sensors have been used for detecting small molecules. The streptavidin binds to biotinylated protein once it attaches to the biotin layer on the sensor surface [102]. Chelated nitrilotriacetic acid (NTA) sensor chip can be used for the detection of histidine-tagged protein. The NTA is pre-immobilized on the dextran coated sensor. Also, functionalization of the SPR sensor is also achieved using thiol group of self-assembled monolayers by thermal evaporation deposition method. Other than that, semiconductor ZnO has been used for immobilization of ligand at the sensing region in SPR sensor. Currently, several works have been done using ZnO/Au SPR biosensor for detection of biomolecules using prism coupling method. Various biomolecules have been immobilized on ZnO surface for SPR detection involving glucose [42], DNA [73], antibody [74], and cholesterol [61].
Enzyme as a biological catalyst provides an outstanding selectivity toward targeted analytes. In SPR biosensor, enzyme is incorporated as the biorecognition element on the immobilization matrix for detection of a specific analyte [103]. The immobilization matrix used for enzyme immobilization can be achieved using ZnO layer. The characteristic property of ZnO makes it suitable for immobilization of biomolecules as its isoelectric point (IEP) value of 9.5 is high enough to allow protein attachment through electrostatic interaction. Biomolecules to be attached on the ZnO have low IEP value. The ZnO bears a positive charge at neutral pH and thus a strong electrostatic interaction occurs for attachment of the biomolecules on the surface of ZnO. Various biomolecules have been immobilized on the surface of ZnO for the designation of a SPR biosensor. Moreover, the dielectric property of the biomolecules immobilized on the surface of ZnO as a dielectric layer can be determined using the SPR technique.
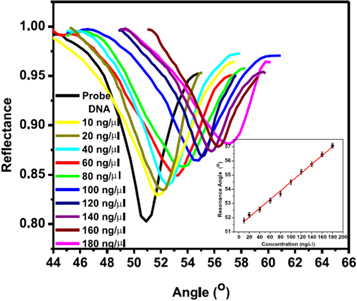
Kaur et al detected Neisseria meningitidis and cholesterol using ZnO/Au SPR sensor [61, 82]. The sensor was coated with ZnO nanofilm on the layer of gold. For N. meningitidis detection, immobilization of single-stranded probe DNA (ssDNA) having IEP value of ∼4.5 was performed on the surface of the ZnO thin film by electrostatic interaction. Physical adsorption method was applied for the immobilization of the DNA probe by incubating the sensor in DNA probe solution at room temperature for three hours. Excessive and unbound DNA probe was removed from the ZnO surface by washing with buffer and dried. Confirmation test using FTIR spectroscopy showed corresponding adsorption peak for cytosine, adenine, and guanine indicating that the DNA probe are present on the surface of the sensor. For detection of the complementary target DNA, the fabricated sensor was incubated in room temperature for 35 s to allow DNA hybridization to take place. Immobilization of DNA probe on the ZnO surface increases the resonance angle from 46.59° to 51.29° due to changes in the refractive index of the surface as shown in figure 4. The minimum reflectance increased significantly as the DNA probe was immobilized on the surface and complementary target DNA was hybridized with the DNA probe. The increase in minimum reflectance is the result of damping of surface plasmon wave due to a reduction in back scattered field [104]. The sensor sensitivity increased with deposition of a thicker ZnO layer due to increase in resonance angle. Incubation of the fabricated sensor in non-complementary DNA target gave no shift in resonance angle indicating selectivity of the biosensor toward N. meningitidis DNA strand.
Figure 4. Damping of SPR spectrum due to back scattering of light and results in the reduction of the SPR signal. Adapted from Kaur et al [82].
Download figure:
Standard image High-resolution imageIn case of cholesterol detection, the enzyme cholesterol oxidase was electrostatically immobilized on the ZnO surface [61]. The immobilized enzyme on the ZnO layer retained its structural conformation and biological functionality. A successful immobilization of cholesterol oxidase enzyme on the ZnO surface was indicated by 1071 and 1654 cm−1 peaks in the FTIR spectra corresponding to the vibration and stretching of carbonyl of the protein amide I band. Upon binding of cholesterol to the active site of cholesterol oxidase, the enzyme catalyzed cholesterol into cholestenone. This result in refractive index change at the sensing surface. The nanostructured and porous topography of the ZnO layer promote binding interaction between cholesterol to the enzyme active site. As the concentration of cholesterol increased, the resonance angle increased linearly from 46.65° to 50.45°. Limit of detection was 0.12 mM. The surface of the biosensor can be quickly regenerated by injection of PBS to wash the cholesterol from the surface. The sensor can be reused up to 15 times with a good consistency in signal measurement.
Measurement of glucose concentration is very important for disease control in diabetic and chronic kidney disease (CKD) patients. There are various glucose sensors available in the market for glucose measurement based on electrochemical or optical techniques such as electrochemical signal transduction (EST) [105] and fluorescence signal transmission (FST) [106, 107]. SPR detection of glucose can be made using glucose oxidase enzyme based on the catalytic reaction of glucose oxidase on glucose, and hence it is very specific and sensitive to the glucose [42]. Glucose oxidase enzyme having IEP ∼4.22 are immobilized on the ZnO layer having IEP 9.5 through physical absorption method. The enzyme glucose oxidase is prepared in phosphate buffer saline (PBS) solution and is used for immobilization of the ZnO surface. The electrostatic interaction between the enzyme and ZnO surface helps to retain the biological activity of the enzyme. During the detection process, the glucose binds to the active site of glucose oxidase and the enzymatic reaction forms the complex molecules gluconic acid and H2O2. The reaction causes reduction in refractive index at the sensing region and results in smaller resonance angle shift. The ZnO layer has high refractive index which is 1.952 + 0.02i while the refractive indices of gluconic acid and H2O2 are 1.461 and 1.414, respectively. The complex formation at the sensing layer reduces the effective refractive index at the sensing region. In the presence of glucose solution at concentration range of 0–300 mg dl−1, it left shifted the resonance angle from 53.1° to 50.1°. To prevent unwanted interference from glucose, the sensor surface was flushed with PBS to wash out the remaining glucose on the surface of the sensing layer from previous concentration for each measurement. As the concentration increased from 0 to 300 mg dl−1, the resonance angle and minimum reflectance decreased linearly. By fixing the resonance angle, the decrease in minimum reflectance implies an increase in the SPR signal as glucose concentration increased. The SPR response is very sensitive to any changes at the sensing region. Therefore, the increase in the SPR signal was due to the change in refractive index of the bulk media that lead to the shift in the plasmon frequency. Meaning that the binding of glucose to the active site of the glucose oxidase enzyme and the redox reaction that took place increased the electron density at the sensing region, and hence changed the plasmon frequency. Further, the immobilization of glucose oxidase enzyme on the surface of Au sensor has also been attempted. However, no significant change in the resonance angle value was observed in the case of Au sensor as the sensor was tested with glucose solution at a concentration of 0 to 300 mg dl−1 as compared to the sensor with the addition of ZnO layer. Hence, the addition of ZnO is play an important role for performance enhancement of the conventional Au SPR sensor and results in realization of an efficient glucose biosensor design with limit of detection of 20 mM.
7. ZnO nanomaterial-based SPR sensing
Other than thin film, ZnO nanostructures can exist in numerous morphologies, inclucing nanospheres, nanowires, nanorods, nanoneedles, nanocones, nanobelts, nanocomnes, nanorings nanosprings and nanocages [108]. The ZnO nanostructures available at varies morphologies can combined with noble metal to enhance the performance of SPR sensor. The physical and chemical properties of ZnO nanostructures changed as their size, shape and surface chemistry changed. An increased surface area of the ZnO at sensing region can enhance the sensitivity of the SPR sensor and provides a good platform for biomolecules immobilization due to its biocompatibility and biodegradability [51, 109, 110]. ZnO at nanoscale confers specific property that allows for signal enhancement [111]. This is because the increase in the amount of interactions between immobilized ligands and the biomolecules to be detected enhanced the SPR signal. Wang and coworkers studied the effects of surface topography of ZnO nanostructures for protein adsorption [112]. Four different nanostructures were used, namely nanoparticles, nanorods, nanosheet and nanobeams. ZnO nanorod gave the highest amount of protein absorbed as it probably has the highest number of adsorption site compared to the other three nanostructures. Byun et al demonstrated fabrication of SPR sensor with ZnO nanorod arrays on Au layer for detection of DNA hybridization [73]. Detection of DNA hybridization gave a sensitivity of three times higher than that of SPR sensor without the ZnO NRAs.
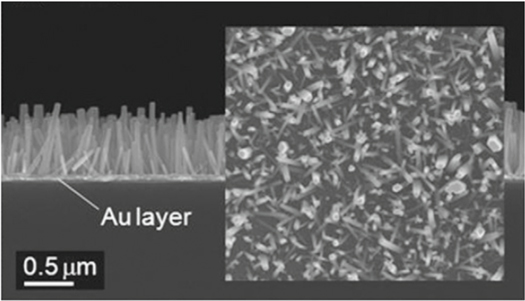
Wet chemical growth technique is commonly employed for fabrication of the ZnO nanorod arrays (NRAs) [73]. In this technique, ZnO seed layer was first grown on Au coated glass substrate by RF magnetron sputtering which provide better control of thickness with good reproducibility and high stability. Immersion of the sample into zinc nitrate hydrate and hexamethylenetetramine aqueous solution vertically allows ZnO NRAs to develop as shown in the SEM image in figure 5. ZnO nanoparticles can be coated on the sensor surface by dipping it in ZnO nanoparticle solution at a temperature of 60 °C. The structure morphology and optical property of the ZnO can be analyzed by SEM. By employing XRD the presence of the ZnO nanoparticle on the substrate can be confirmed. EDX can provide information regarding the chemical composition and sample purity. Besides that, XPS is another powerful technique that can be used to check the chemical composition of the fabricated sensor [113].
Figure 5. SEM image of ZnO NRAs grown from the sputtered ZnO seed layer Au coated glass substrate. Adapted from Byun et al [73].
Download figure:
Standard image High-resolution imageUsha et al fabricated and characterized a fiber optic SPR sensor using Ag/ZnO nanorod/GOx for blood dextrose sensing to be used in insulinoma test and hypoglycemic condition [114]. The sensor was fabricated by coating the unclad region of fiber with a layer of Ag and hydrothermally grown ZnO nanorod. The ZnO nanorods acted as a high index medium for sensitivity enhancement as well as a matrix for the enzyme GOx immobilization. Due to the high isoelectric point of ZnO nanorod, it can physically adsorb the GOx which sequentially depend on the enzyme mass and the surface area of the ZnO nanorod layer coated. The enzymatic reaction between GOx and dextrose changes the dielectric constant of the ZnO nanorod which lead to a shift in resonance wavelength. A set of control experiment was conducted to clarify the performance of the sensor based on different shapes of ZnO, namely ZnO thin film (12 nm), ZnO nanoparticles, and ZnO nanorod for immobilization of GOx. The shifts of the resonance wavelength of the dextrose SPR sensor with ZnO thin film were 7.5 nm, while the sensor with ZnO nanoparticles produced shift of around 6 nm only. In case of dextrose SPR sensor with ZnO nanorod, there was a significant difference of around 64 nm shift in resonance wavelength. It was also reported that the fiber optic SPR sensor for dextrose detection has better limit of detection of 0.012 mM compared to existing dextrose SPR sensors.
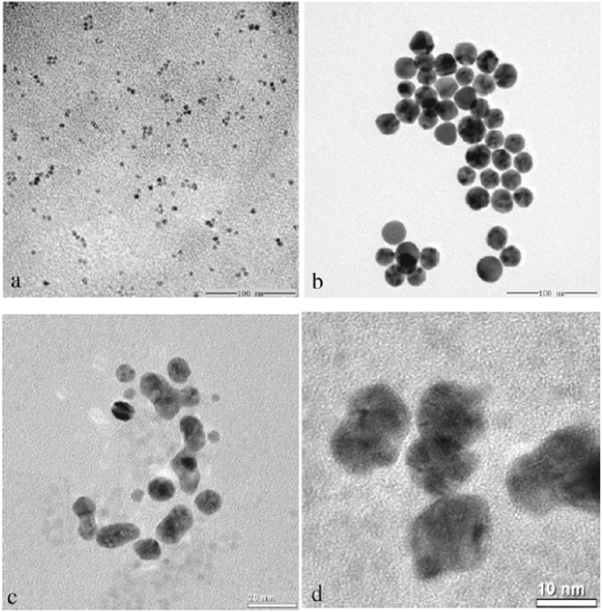
In addition, enhancement of the optical properties of nanostructure semiconductor can be made by placing metal nanoparticles on the semiconductor nanoparticles surface. In fact, coating ZnO with metal nanoparticles was found to enhance the ability of ZnO in ultraviolet emission [115]. Figure 6 shows ZnO nanocrystals modified with Au nanoparticles. Wang et al created a protein probe by binding protein with the ZnO/Au nanocomposites in covalent bond [74]. In the study, 2-mercaptopropionic acid (MPA) was utilized to bring together the Au nanoparticles of the ZnO/Au nanocomposites due to a definite and strong interaction between gold and thiol group. The antigen was able to bind to the ZnO/Au nanocomposite once the activation of carboxyl group in MPA was achieved by NHS and EDC. The interaction between the ZnO/Au antigen with the immobilized antibody on the SPR biosensor then results in a change in refractive index at the sensing region. Eventually, a shift in the resonant wavelength was observed. The detection limit for rabbit IgG measured by the developed biosensor was 16-fold lower compared to a SPR biosensor with individual Au layer.
Figure 6. Transmission electron microscope (TEM) images of ZnO nanocrystals (a), Au nanoparticles, ZnO/Au nanocomposites (c), and high-resolution images of ZnO/Au nanocomposites (d). Images adapted from Wang et al [74].
Download figure:
Standard image High-resolution image8. ZnO in LSPR
The utilization of ZnO thin film and nanostructures in combination of Au or Ag film for SPR sensing in visible region have been discussed. More recently, localized surface plasmon resonance (LSPR) utilizing semiconductor metal oxide appeared to have potential to be developed as optical sensing in the near infrared (NIR) range. LSPR occurs when collective oscillation of conduction-band electrons in confined metallic nanostructures interact with the electric field of light. LSPR sensing in NIR range offer several benefits [116]. Firstly, the penetration of evanescent wave into the analyte is deeper which could enhance the sensitivity. The penetration depth of ∼2 μm could be achieved which is much larger than the penetration depth in visible region (∼200–300 nm). Secondly, sensing in NIR range can help to avoid photodamage and phototoxicity in living cells. However, a vast majority of LSPR sensing was carried out in visible region as nanostructure of noble metals such as Au and Ag have plasma frequency at visible range. LSPR in metal can be tuned only by changing the size, morphologies of the nanomaterials and refractive index of the media, due to the carrier density in the metals are constant. This limits the tunability of the plasma frequency at a broad spectral range. For this reason, chemical and biomedical application of LSPR in NIR range required new materials supporting plasma frequency at NIR region.
On the contrary to noble metals, nanostructured semiconductor metal oxide offer advantage for LSPR in NIR region due to the tunability of free carrier densities by controlling dopant concentration. This allow the plasmon resonance in the semiconductor metal oxide to expand over a broad spectral range from the NIR to mid-infrared by varying various hosts and dopant atoms, and manipulation of doping level [117–119]. Carrier densities around 1021 cm−3 excited LSPR in the NIR. ZnO, cadmium oxide and indium oxide are amongst the semiconductor metal oxides which can be doped using n-type dopants such as indium doped tin oxide (ITO) and aluminum-doped ZnO (AZO), germanium-doped ZnO (GZO) or indium doped ZnO (IZO). AZO is noted to be beneficial due to its comparable optical and electronic properties, low cost, biocompatibility, and Earth-abundant material. Attempt of LSPR gas sensing has been made using ZnO doped with gallium, aluminium and germanium nanocrystals for detection of hazardous gases including hydrogen (H2) and nitrogen dioxide (NO2) [120]. The changes in dopant-induced plasmon resonance in the NIR was monitored. Due to the highly sensitive LSPR to chemical and electrical changes took place at the nanocrystals surface, the presence of toxic gases was detected. Having shown for the first time the application of tunable surface plasmon absorption in NIR for AZO nanocrystal opens up the possibility of exploring promising practical application of AZO in NIR LSPR sensing for chemical and biological compounds. Moreover, AZO nanocrystal can be used as the alternative to the commonly used transparent conductor ITO in optoelectronic devices for lower cost and more environmental friend. However, although the fabrication of ITO nanocrystal had advance significantly, it is still a challenge to control doping along with size and shape in ZnO based transparent conductor nanocrystal, i.e. AZO, IZO, or GZO. Moreover, the equipment required for NIR sensing, such as the laser and the detector, is more costly than sensing in visible wavelength, which limits the practical utilization of LSPR for NIR sensing.
9. Future prospect and conclusion
Today, the application of commercially available Kretschmann-based SPR instruments are limited for biosensing and analytical research in laboratory. In order for it to be used for on-site testing with a portable design, there is a need to find a replacement for the bulkier SPR instrument. Upon determination of analyte-ligand correlation using Kretschmann-based SPR, deployment can be made to optical fiber optics-based sensor for portability purposes. Although current research trends aim at designing miniaturized portable sensor using low cost and robust nanostructured materials with optical fiber-based SPR sensor, initial fundamental design needs to be performed using prism-based SPR sensing. Using nanostructured materials for designing a biosensing system holds promise of maximum sensor efficiency. The system is benefited by the high surface-to-volume ratio of the nanostructured material and the sensing medium having dimensions close to the size of biomolecules. ZnO and its nanostructures in different sizes and morphologies has proved its multilevel application in gas and biological sensing due to its extraordinary properties. Table 1 summarizes the ZnO nanostructures and thin film used for difference purposes in enhancing the performance of SPR sensor. In many efforts of investigation, the use of ZnO nanomaterials not only improved SPR sensing performance but also lead to new application well beyond the scope of chemical and biological sensing. The bottleneck of the ZnO/Au SPR sensors is the sensing application is limited only to visible wavelength. While some of the detection required a deep penetration depth, the SPR sensor has to be developed such that it has plasma frequency at near-infrared region. The future direction of the ZnO sensor can be directed toward chemical and biological sensing in NIR region using LSPR. For example, it can be useful for real time monitoring of dynamic processes of living cells.
Acknowledgments
This research was funded by Malaysian Ministry of Education & Universiti Kebangsaan Malaysia with grant numbers of FRGS/1/2019/STG02/UKM/02/8, AKU254: HICoE Phase II MEMS for Biomedical Devices, DIP-2016-022 & GUP-2016-062 Kulim Hi-Tech Pte. Ltd is also acknowledged for the technical assistance.