Abstract
CoFeCrNiSiB high-entropy alloy coatings with different content of REO (rare earth oxide) CeO2 were fabricated on the surface of corrosion-resistance steel plates by using laser cladding process. The surface properties of the cladded coatings including wear and corrosion resistance were evaluated by friction wear equipment and electrochemical workstation, respectively, while the microstructure was observed by scanning electron microscope, energy dispersive spectrometer, x-ray diffractor and transmission electron microscope. When the CeO2 content was not higher than 2%, the wear resistance and corrosion resistance of cladded coating was improved with the increased of CeO2 content. However, when the CeO2 content was between 2% and 4%, the wear resistance and corrosion resistance of cladded coatings decreased with the increased of CeO2 content. The corrosion resistance evaluation environment included 2 N(1 mol l−1) H2SO4 and 5 wt% NaCl solutions. Equi-axed grains of the cladded coatings got refined by adding CeO2, the grains were most refined at 2% in the range of 0%–4% CeO2 content. However, when too much CeO2 (>2%) was added, CeO2 would exist as an oxide in the intergranular area. Therefore, grain refinement could improve the surface properties of coatings, while the presence of intergranular oxides would make the surface properties of coatings worse.
Export citation and abstract BibTeX RIS
Introduction
In the special working environment, the surface properties of the workpiece such as surface strength, wear resistance and corrosion resistance were highly demanded [1–4]. Surface engineering technology could improve the surface properties of workpieces to meet the requirements of working in special environments [5, 6]. Laser cladding was a surface modification method that could significantly improve the wear resistance, corrosion resistance, heat resistance and oxidation resistance of the surface of the substrate. It not only meets the requirements for the specific properties of the material surface, but also saves a lot of valuable elements [7–9]. Compared to other surface treatment methods (vapor deposition, thermal spraying, etc [10–12], laser cladding had the characteristics of metallurgical bonding between coating and substrate, high precision, low dilution rate, rapid melting and cooling process.
Rare earth [13–15] had been widely used in the field of material processing, and surface modification was an important field. Zhang [16] had studied the influence of rare earth oxide Y2O3 on the microstructure and high temperature oxidation properties of cladding coating. The results showed that ceramic particles and microstructure of coatings can be fined by rare earth oxide Y2O3. Volume fraction of ceramics increased with the increase of Y2O3. The high temperature oxidation resistance of the coatings was improved by Y2O3. As well as, the cumulative oxidation mass of cladding coating with 2 wt% Y2O3 decreased by one-third of the coating without addition of Y2O3. However, there were still few research had been done on the effects of rare earth addition on the microstructure and surface properties of Co-based alloy laser cladded coatings.
In this paper, a co-based alloy cladded coating with different rare earth CeO2 content was prepared by laser cladding on H13 steel substrate and the microstructure and surface properties of the cladded coating was studied.
Experiments
H13 steel plate with dimension of 200 mm × 100 mm was chosen as the substrate material. the composition of as-received H13 steel was 0.32–0.45 C, 0.20–0.50Mn, 0.25–0.5 Si, 4.75–5.50 P, 1.10–1.75Mo,0.80–1.20 V, 0.03S, Fe rest (wt%). The cladding material was consisted of Co34Cr29B14Fe8Ni8Si7 (at%) with sizes ranging from 30 μm to 48 μm and the rare-earth alloy powders (CeO2). The powder was prepared by ball milling of CeO2 and pure powders of various elements of which was pre-placed on H13 steel. Contents of each rare-earth powder (mass fraction, %) were 0%, 1%, 2%, 3%, and 4%. The gas flow rate of pure argon as protection gas was 20 l min−1 during cladding with SL-80 Nd:YAG laser machine with the parameters as follow: scanning speed of 10 mm min−1, defocusing distance of 20 mm, light spot diameter of 2.2–2.5 mm, overlapping ratio of 50% between adjacent tracks and preset powder thickness of 200 μm. Cross sectional microstructure of the cladded coatings was characterized by TESCOULD VEGA II scanning electron microscopy (SEM) and EDAX energy dispersive spectroscopy (EDS). The phase composition of the cladded coatings was analyzed by D/max 2500 x-ray diffractometer (XRD, Cu Kα radiation, scanning within 2θ = 10°−90°). Micro-hardness distribution in the cross section of the coatings was obtained using TH701 micro-hardness tester with impression load of 0.2 k. Corrosion resistance of the upper layer to 3.5 wt% NaCl solution and 1 mol l−1 H2SO4 solution was analyzed at electrochemical workstation (IM6E, Germany) which included working electrode, a Saturated Calomel Electrode (SCE) as the reference electrode and a platinum electrode as the auxiliary electrode to form a three-electrode system. All electrochemical experiments were performed at under room temperature according to the Chinese national technique standard GB/T 24196–2009. Wear resistance of the coatings was measured using ball-on-disc type friction and wear testing machine (HT-1000). Zirconia ceramic balls (diameter of 5 mm) were selected as the counterpart material. The experimental parameters were: loaded mass of 1150 g, frictional radium of 3 mm, rotational speed of 560 r min−1, temperature of room temperature and wearing time of 30 min, 60 min and 90 min. The samples were pre-ground for 5 min under the same load before the test, thus eliminating the effects of differences in surface conditions.
Result and discussion
Microstructure analysis
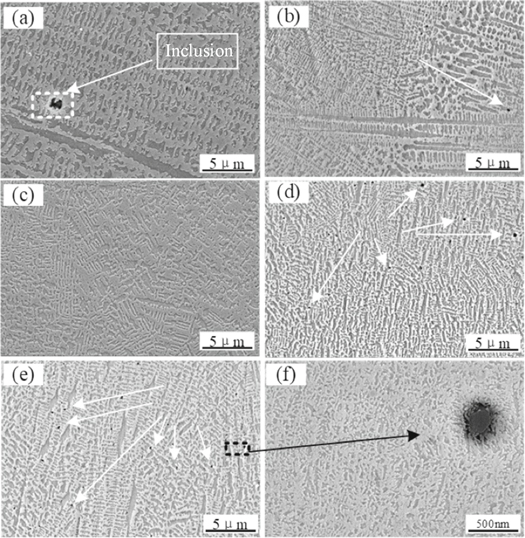
The morphology of the upper and middle parts of the laser cladded coating with different content of CeO2 was showed in figure 1. The microstructure of the cladded coating was significantly changed after the addition of CeO2. The microstructure of the coating was refined, the secondary dendrite spacing was reduced, the number of eutectic structures between the dendrites was increased, the directionality of the dendrites was gradually weakened, and the dendrite structure was gradually small and disorderly and the microstructure had no apparent directional dendrites when the content of CeO2 was 2%. However, when the content of CeO2 was more than 2%, the effect of refining structure did not increase with the increase of the content of CeO2. On the contrary, the primary dendrite was more developed and the dendrite orientation was more obvious. The effect of rare earth oxide addition on refining the microstructure was 2% > 1% > 3% > 0% > 4%. the grain was coarser than that without adding CeO2 when the content of rare earth oxide was 4%, The refinement of grain by rare earth was the key to improve the properties of cobalt-based alloy cladded coating. The black dots in figure 1(a) were inclusions, and it could be found that CeO2 also had significant metamorphism on inclusions. The shape of inclusions was substantially spherical after adding CeO2. Moreover, when the content of CeO2 was less than 2%, the amount of inclusions formed by oxygen and sulfur decreased as the CeO2 content increased. When the CeO2 content was 2%, the inclusions completely disappear. The effect of deoxidation and desulfurization by CeO2 will decrease, and the amount of slag will increase as the CeO2 content increased. In the range of CeO2 content in this experiment, the optimum addition amount of rare earth oxide CeO2 was 2%.
Figure 1. Microstructure of cladded coating (upper and middle). (a) Without CeO2; (b) 1% CeO2; (c) 2% CeO2; (d) 3% CeO2; (e) 4% CeO2; (f) inclusion amplification region in figure (e).
Download figure:
Standard image High-resolution imageTable 1. EDS results of A and B in figure 2(c).
| Co | Si | Fe | Ni | Cr | |
|---|---|---|---|---|---|
| A | 16.10 | 5.62 | 63.78 | 5.20 | 9.03 |
| B | 19.18 | 3.57 | 49.69 | 4.88 | 17.55 |
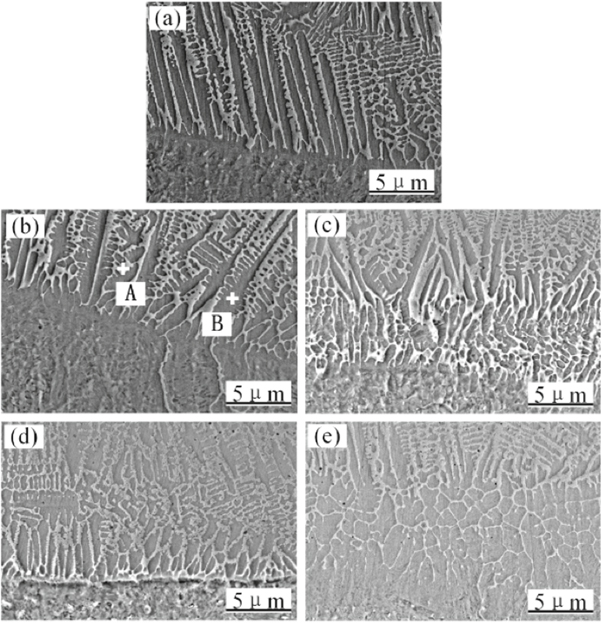
The microstructure and morphology of the fusion zone of the cladded coating after adding different content of CeO2 was showed in figure 2. The height of columnar crystals varied with the content of CeO2: with the increase of rare earth element content, the height of columnar crystals tended to decrease and the orientation of columnar crystals decreased. Point A was the point in bright dendrite region, and point B was located on gray dendrite. The contents of alloying elements were different between dendrites. Fe, Ni and Si were more in the interdendrite region where point A was located, while Co and Cr were more in the dendrite where point B was located. The reason for this difference in element distribution was that Co and Cr had higher melting points and therefore exist in the dendritic region of Pre-eutectic structure, while Fe, Ni and Si were elements with low melting points, so they tend to concentrate in the interdendritic region of post-eutectic structure. The Fe element in dendrite was mainly composed of (Fe, Ni) and other Fe solid solutions, while the Cr element was mainly concentrated in the dendrite region, which made the dendrite region a higher corrosion resistance.
Figure 2. Microstructure of cladded coating (bottom). (a) Without CeO2; (b) 1% CeO2; (c) 2% CeO2; (d) 3% CeO2; (e) 4% CeO2.
Download figure:
Standard image High-resolution imageThe main phases on the surface of the cladded coating were FeNi3, Co and Cr2Ni3. When the CeO2 content was 4%, two phases Cr1.12Ni2.88 and Ni2.9Cr0.7Fe0.36 appeared, which may be caused by the uneven composition. The phase composition of the cladded coating did not change after adding CeO2, indicating that the rare-earth oxide did not change the phase composition while improving the structure, figure 3.
Figure 3. XRD patterns f cladded coating. (a) without CeO2; (b) 1% CeO2; (c) 2% CeO2; (d) 3% CeO2; (e) 4% CeO2.
Download figure:
Standard image High-resolution imageThe micro-hardness distribution from surface to substrate of cladded coating with different content of rare earth oxide was showed in figure 4. The micro-hardness of each coating showed the same rule: the micro-hardness decreased gradually from the surface to the substrate, and dropped sharply in the fusion line and then became stable. Proper addition of rare earth elements could effectively improve the micro-hardness of the cladded coating. When 2% CeO2 was added, the micro-hardness of the cladded coating increased the most than that without CeO2. Then the micro-hardness of the cladded coating decreased gradually with the increase of CeO2. When the content of CeO2 was 4%, the micro-hardness of the cladded coating was even lower than that without adding CeO2.
Figure 4. Micro-hardness profile of the cladded coatings.
Download figure:
Standard image High-resolution imageTo compare the corrosion resistance of the specimens in terms of corrosion kinetics, the polarization curves of the cladded coatings were measured. The polarization curves could represent the change of electrode potential with the change of current density on the electrode. Figure 5 was polarization curves measured in H2SO4 and NaCl solutions for samples with different CeO2 contents.
Figure 5. Electrochemistry test result of the substrate and coatings, (a) polarization curves in 1 mol l−1 H2SO4 solution, (b) polarization curves in 3.5 wt% NaCl solution.
Download figure:
Standard image High-resolution imageFrom the polarization curve of H2SO4 solution shown in figure 5(a), the curve was mainly composed of the cathodic polarization curve of the downward trend and the anodic polarization curve of the upward trend. In this experiment, the shape of the cathodic polarization curve was basically the same, and the slope of the curve was smooth and stable. This showed that all the cathodic polarization processes were basically the same and there were no other changes during the whole cathodic polarization process. The cathodic reaction was mainly hydrogen evolution reaction. In this experiment, with the increase of voltage, the samples were anodized. All the anodic polarization curves were basically the same. They could be divided into activation zone, passivation zone and dimension passivation zone. The reason why the samples were passivated in 2N H2SO4 solution was that the increase of loading voltage led to the formation of passivation film on the surface, on the other hand, the content of Cr and Ni in the cladded coating was relatively high. The existence of two elements that could easily passivate the surface.
The polarization curves of the cladded coating in NaCl solution in figure 5(b) were basically the same as those in H2SO4, but in 5% NaCl solution, the anode curve did not show passivation with the increase of voltage.
The corrosion current density (μA/cm2) could be obtained by fitting the polarization curves of figure 5 with Tafel fitting method. The corrosion current density could be used to characterize the corrosion resistance of the cladded coating in the current solution from the corrosion kinetics. Therefore, the coating with the content of 2% CeO2 showed the best corrosion resistance while that with 4% was the worst.
Figure 6 was the friction coefficient and wear loss of laser cladded coating with different content of CeO2 in cobalt-based alloy powder under the above experimental conditions. It could be seen from the figure that the magnitude of friction coefficient was 2% < 1% < 3% < 0% < 4% with the content of CeO2, which was consistent with the effect of rare earth elements on micro-hardness and corrosion resistance. This showed that a proper amount of CeO2 could effectively reduce the friction coefficient of the cladded coating, and the best addition amount was 2%. The friction coefficient increased again with the increase of CeO2 until the content of that was 4%, it was even worse than that without CeO2.
Figure 6. Results of friction and wear test, (a) variation process of friction coefficient along time, (b) wear weight loss of different tested surfaces.
Download figure:
Standard image High-resolution imageThe wear loss of laser cladded coating with the same CeO2 content at different wear time was shown in figure 6(a). It could be seen that the wear rate and wear time of each specimen were linearly correlated at different time. When the CeO2 content was not higher than 2%, the wear loss of cladded coating was decreased with the increased of CeO2 content. However, when the CeO2 content was between 2% and 4%, the wear loss of cladded coatings increased with the increased of CeO2 content. Therefore, the coating with the content of 2% CeO2 showed the best wear resistance while that with 4% was the worst.
Discussion
The refinement of the grain could contribute to the effect of rare earth on nucleus growth:
Rare earth elements were adsorbed on the solid-liquid interface to reduce the surface free enthalpy, which reduced the driving force of grain growth and restricts the growth of grain.
Rare earth elements adsorbed on the solid-liquid interface, which decreases the surface atomic activity and thus reduced the diffusion ability. At the same time, the diffusion of other elements atoms in the coating was weakened by the segregation of rare earth elements, which hinders the growth of grains.
The segregation of rare earth elements at the solid-liquid interface increased the composition undercooling of the frontier liquids, decreased the stability of the solid surface and promoted the outward dendrite growth of grains, so the secondary dendrite spacing decreased.
The influence of rare earth elements on the hardness of cladded coating was discussed from the following points:
The refinement of grains could lead to the increase of hardness. The relationship between yield strength and grain size of metal materials was as follows: Formula (1):

σs -yield strength of materials
σi -Friction Resistance of Material Dislocation
k -Correlation constant
d -grain diameter
It could be seen from the above formula that the smaller the grain size, the higher the yield strength. Generally, the change of strength and hardness was the same. In this experiment, the addition of rare earth elements refined the grain size of the coating, and the effect of fine grain strengthening increased the hardness value of the coating.
The role of solid solution strengthening. On the one hand, rare earth elements could dissolve into solid solution and play a role of solid solution strengthening. Laser cladding was a non-equilibrium process, and it was possible to obtain supersaturated solid solution of rare earth elements. Because of the large atomic radius of rare earth elements, strong lattice distortion and remarkable solid solution strengthening would occur when solid solution was formed. On the other hand, in addition to the solid solution strengthening of rare earth elements themselves, rare earth elements could further strengthen the solid solution strengthening by influencing the content of other alloying elements. The addition of rare earth elements in laser cladded coating changed the content of Cr and Ni, and the addition of appropriate CeO2 increased the content of Cr and Ni, and the effect of solid solution was enhanced. When the content of these two elements decreased, the effect of solid solution was weakened. The experimental results showed that the variation of Cr and Ni content caused by rare earth was consistent with that of hardness.
According to empirical electron and solid molecule theory [17], the structure radius of rare earth atoms was not fixed. During the cladding process, the rare earth atoms were adsorbed on the atoms of Fe, Ni, Cr, etc. Some of the electrons of rare earth atoms would break away from the binding of the nucleus or migrate, and the radius of rare earth atoms would be reduced, and entered the solid solution through vacancies and double vacancies to form a displacement solid solution. When more light elements such as B and C were introduced into the lattice gap, the lattice size changed, resulting in a solution strengthening mechanism that enhanced the microhardness.
The effect of CeO2 on corrosion could be discussed from its effect on the structure, chemical composition, phase structure and slag inclusion of the cladded coating.
As we had discussed, we could see that CeO2 had a significant impact on the microstructure. The dendrites in the cladding could be refined obviously when the amount of CeO2 was moderate. With the increased of the number of crystals, the composition segregation was improved, and the grain boundary was prolonged, so that the impurity density at the grain boundary decreased. This reduced the tendency of grain boundary corrosion.
The content of Cr, Ni, Si and other alloying elements in the cladded coating would increased by adding appropriate CeO2, which could effectively improve the corrosion resistance.
The effect of rare earth elements on inclusions in the coating also affected the corrosion resistance.
In this experiment, when the content of rare earth oxide was 2%, the structure was the most refined, the content of alloying elements was increased mostly, and the inclusions were also greatly reduced. Therefore, the comprehensive effect of 2% CeO2 was that the corrosion resistance of cladded coating alloy in the corrosion medium solution used in this experiment was the strongest.
To investigate the effect of Ce content on the friction and wear morphology of laser cladded coatings at different time, the wear scar morphologies of each cladded coating at 60 min and 90 min were observed by electron microscopy. Figure 7 was the morphology of each cladded coating enlarged 500 times after 60 min wear, and figure 8 was the morphology enlarged 500 times and 2000 times after 90 min wear.
Figure 7. The SEM morphology of worn surfaces in the cladded coatings in 60 min with different content of CeO2, (a) without CeO2; (b) 1% CeO2; (c) 2% CeO2; (d) 3% CeO2; (e) 4% CeO2; (f)substrate.
Download figure:
Standard image High-resolution imageFigure 8. The SEM morphology of worn surfaces in the cladded coatings in 90 min with different content of CeO2, (a) without CeO2; (b) selected zone in (a); (c) 1% CeO2; (d) selected zone in (c); (e) 2% CeO2; (f) selected zone in (e); (g) 3% CeO2; (h) selected zone in (g); (i) 4% CeO2; (j) selected zone in (i) .
Download figure:
Standard image High-resolution imageFigure 7 showed the morphology of furrows at 60 min of friction and showed that the furrows were not obvious at figures 7(a)–(d), but became clear at figure 7(e), the furrows of the substrate were also clearly visible.
Figure 8 showed the furrows morphology after 90 min and the enlarged morphology of some areas. When the time increased from 60 min to 90 min, the furrows of each cladded coating were clear. Table 2 showed the EDS results of points on two typical wear morphology regions, A and B, selected in figure 8.
Table 2. EDS results of A and B in figure 7.
| Co | Cr | Fe | Ni | Si | O | |
|---|---|---|---|---|---|---|
| A | 29.04 | 24.76 | 30.10 | 6.59 | 5.86 | 3.65 |
| B | 8.00 | 7.59 | 25.57 | 2.00 | 2.36 | 54.48 |
The proper addition of CeO2 could refine the structure of the laser cladding layer, reduce the spacing of secondary dendrites, and round the shape of slag inclusions. It could improve the strength of the cladding layer as well as the plasticity and toughness of the cladding layer. The improvement of toughness was manifested in the wear morphology that plastic deformation is more sufficient.
However, due to the low solid solubility of rare earth elements, most of them existed in the form of compounds at grain boundaries. When CeO2 was excessive, a large number of rare earth compounds were segregated at grain boundaries, which had a negative impact on the deformation ability of grain boundaries and makes wear worse.
In addition, excessive CeO2 increased the amount of slag inclusions, decreased the technological properties of the alloy, and decreased the strength, plasticity and toughness, which resulted in uneven wear furrows.
Conclusion
- 1.The Cobalt-based alloy with different content of CeO2 cladded coating was fabricated by using laser cladding process and exhibited different microstructure. The effect of rare earth oxide addition on the structure refinement was 2% > 1% > 3% > 0% > 4%.
- 2.When the CeO2 content was not higher than 2%, the micro-hardness, wear resistance and corrosion resistance of cladded coating was improved with the increased of CeO2 content. However, when the CeO2 content was between 2% and 4%, the micro-hardness, wear resistance and corrosion resistance of cladded coatings decreased with the increased of CeO2 content.
- 3.The wear morphology showed that the wear forms of cladded coating with 0% and 4% content of CeO2 were abrasive wear and oxidative wear, and that of cladded coating with 1%, 2% and 3% content of CeO2 were abrasive wear.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2016EEQ03), China Postdoctoral Science Foundation-General Program (No. 2018M641822) and the Natural Scientific Research Innovation Foundation in HIT (No. HIT.NSRIF.201703). Special appreciation shall be sent to Dr Jia Liu who had been giving wholehearted supports to the first author.