Abstract
During thyroid surgery, some parathyroid glands fail to maintain their function, therefore, they are unavoidably detached from the patient. For the purpose of re-preservation of the function, they are minced into small segments and transplanted into the fat or muscle layer. Yet, this method of auto-grafting the parathyroid glands is frequently unsuccessful due to its poor interaction and engraftment with the native tissue, eventually leading to the dysfunction of the parathyroid hormone (PTH) secretion. In this study, we suggest a methodology to restore parathyroid activity through the introduction of the 'tissue printing' concept. Parathyroid glands of patients with secondary hyperparathyroidism were minced into the fragments smaller than 0.5 × 0.5 mm, which is in common with the traditional surgical method. These parathyroid tissues (PTs) were uniformly mixed with the adipose-derived decellularized extracellular matrix (adECM) bioink that protects the PTs from hostile in vivo environments and promote initial engraftment. PTs-encapsulated adECM bioink (PTs-adECM) was then printed onto the pre-designed polycaprolactone (PCL) mesh to produce patch-type PTs construct, which functions as a mechanical support to further enhance long-term in vivo stability. The engineered patch was transplanted subcutaneously into rats and harvested after 4 weeks. In vivo results showed that the engineered patches were well engrafted and stabilized in their original position for 4 weeks as compared with PTs only. Immunohistochemistry results further revealed that the concentration of PTH was approximately 2.5-fold greater in rats engrafted in the patch. Taken together, we envision that the novel concept 'tissue printing' over cell printing could provide a closer step towards clinical applications of 3D bioprinting to solve the unmet need for parathyroid surgery method.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Parathyroid hormone (PTH), which is secreted by the four parathyroid glands attached to the thyroid gland, plays an important role in regulating blood calcium levels [1, 2]. About 0.5% to 4.4% of patients who have undergone thyroidectomy experience permanent hypoparathyroidism with little or no secretion of PTH [3]. Due to a lack of PTH, the patients must take calcium and Vitamin-D supplements for the rest of their lives [4]. However, these supplements have been associated with severe side effects, including constant numbness and muscle stiffness [5]. To avoid these side effects, surgeons have attempted to preserve the parathyroid glands and maintain blood flow to these glands during thyroidectomy [6]. However, unwanted parathyroid damage often occurs during surgery. Once damaged, the parathyroid glands should be detached from their original location and transplanted into muscle or fat bed, which is termed as parathyroid autotransplantation in clinics [7–11].
For the parathyroid autotransplantation, it is necessary to mince the detached parathyroid tissue (PT) into 0.5 × 0.5 mm fragments with fine scissors. The minced PTs are eventually transplanted on the muscle or fat layer. However, the transplanted PTs do not remain stable and most of PTs disappear somewhere else due to poor integration and engraftment into the subcutaneous or muscle tissue. For this reason, the remaining small quantities of PTs do not function immediately and lose their hormonal function [12]. These patients must therefore re-take calcium and vitamin D, suggesting the need for technical advances to ameliorate their initial engraftment and hormonal function.
Three-dimensional (3D) bioprinting technique enables us to precisely deposit multiple cells and biomaterials at pre-defined locations [13, 14]. This technique has been widely used to engineer various tissue constructs mimicking, for example, skin [15, 16], cartilage [17], blood vessels [18], and liver [19, 20], improving tissue growth, regeneration, and function. To date, however, this technique has not been utilized for the functional improvement of endocrine tissues, particularly for parathyroid engraftment, tissue integration, and hormone secretion. To resolve the unmet need for clinical parathyroid surgery, in this study, we suggest a new methodology that PT itself are printed rather than parathyroid cell printing. Specifically, human PT was isolated and carefully minced. Following this, the minced PTs were encapsulated in the adipose-derived decellularized extracellular matrix (adECM) bioink that protects the PTs from hostile in vivo environments and promotes initial parathyroid engraftment onto the native subcutaneous tissue. PTs-encapsulated adECM bioink (PTs-adECM) was then printed onto a pre-designed polycaprolactone (PCL) mesh, which functions as a mechanical support to further enhance long-term in vivo stability. In vitro and in vivo results showed that these engrafted patches secrete PTH, suggesting autotransplantation may reverse the effects of surgery-associated permanent hypoparathyroidism. We speculate that the novel concept of tissue printing over cell printing would provide a closer step towards clinical applications of 3D bioprinting technique to resolve the issue of clinical parathyroid surgery. By omitting cell isolation and culture in vitro, furthermore, this tissue printing-based approach might minimize several steps required for clinical approval.
2. Materials and methods
2.1. Study design
The study was a cooperative research work performed by staff at two institutions, Seoul National University Bundang Hospital (SNUBH) and the Pohang University of Science and Technology (POSTECH). Human parathyroid samples were provided by SNUBH and the 3D tissue printing processes for the fabrication of PTs-printed patches, composed of printable adECM bioink and porous PCL support, were performed at POSTECH. The study protocol was approved by the Institutional Review Board of SNUBH (B-1904/535-301).
2.2. Preparation of adECM bioink
Porcine adECM was prepared as described in another article with slight modifications [21, 22]. In brief, fresh adipose tissue collected from a slaughterhouse was centrifuged to remove oil and blood, washed with 1× phosphate-buffered saline (PBS) solution and decellularized with 0.5% sodium dodecyl sulfate (SDS) solution for 48 h. The decellularized tissue was treated with isopropanol for 48 h to remove lipids, washed several times with 1× PBS solution, sterilized by treatment with 0.1% peracetic acid solution in 4% ethanol for 4 h, and washed several times with 1× PBS and distilled water. Lyophilized adECM was frozen in liquid N2 and crushed into a powder using a mortar and pestle. To formulate 2% (w/v) adECM bioink, each 100 mg aliquot of adECM powder was added to a solution of 10 mg of pepsin (P7125, Sigma-Aldrich, St. Louis, MO, USA) in 0.5 M acetic acid and incubated for 3 d. After complete solubilization, the pH of the adECM solution was adjusted by dropwise addition of cold 10 M NaOH solution on ice to avoid thermal gelation. The pH-adjusted adECM bioink was stored at 4 °C until being used.
2.3. Rheological characterization of adECM bioink
Rheological examinations were performed using an Advanced Rheometric Expansion System (TA Instrument, USA) with a 25 mm diameter parallel plate geometry. To evaluate the viscosity, steady shear sweep analysis of adECM pre-gel was performed at a constant temperature of 15 °C. Dynamic frequency sweep analysis was conducted to measure the frequency-dependent storage and loss moduli of dECM gels in the range of 1–1000 rad s−1 at 2% strain after incubation for 30 min at 37 °C. A temperature sweep experiment was performed to study the gelation kinetics of dECM pre-gels. The storage and loss moduli were measured to evaluate the structural stability after the thermal gelation process. All measurements were conducted in triplicate.
2.4. Preparation of minced parathyroid glands (PTs)-encapsulated adECM bioink
The study goals and protocols were explained in advance to four patients with primary and secondary hyperparathyroidism between May and October 2019 at Seoul National University Bundang Hospital (SNUBH). The participating patients were provided written informed consent. Three and one-half parathyroid glands were removed from the patients with secondary hyperparathyroidism during surgery.
Patients' parathyroid glands from SNUBH were stored in complete Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin (P/S). The intact parathyroid glands were cut into fragments smaller than 0.5 mm in diameter and were passed through 500 µm nylon mesh filter bags to prevent nozzle clogging prior to adECM encapsulation. The filtered PTs (30 mg ml−1) were uniformly mixed into 2% (w/v) adECM bioink at a volume ratio of 1:10 while avoiding unwanted air bubbles. Specifically, 60 mg of PTs in 200 μl of culture medium was mixed into 2 ml of 2% adECM bioink.
2.5. Tissue printing process for engineering PTs-printed patches
In this study, the Food and Drug Administration (FDA)-approved PCL was used as a supporting framework to prepare transplantable PTs-adECM, owing to its acceptable bioprintability, biodegradation behavior, and biocompatibility [23]. A porous PCL mesh (10 mm diameter, 200 µm porosity, and 1 mm thickness) was printed using an in-house 3D bioprinting system [23]. This mesh acted as a mechanical support to enhance and stabilize the integration of printed PTs-encapsulated adECM into the host subcutaneous tissue. Following this process, the PTs encapsulated in adECM bioink were sequentially printed onto the supportive PCL mesh to form well-distributed minced PTs. These patches were validated by comparison with PTs alone. The workflow of this study is shown (figure 1).
Figure 1. Workflows of this study. (A) Mincing of PTs into pieces smaller than 0.5 × 0.5 mm. (B) Encapsulation of PTs in adECM bioink (PTs-adECM). (C) Biofabrication processes to engineer transplantable PTs-printed patch. (D) Analysis of parathyroid engraftment and hormone secretion of PTs-adECM and PTs alone under in vitro and in vivo conditions.
Download figure:
Standard image High-resolution image2.6. Cell viability assay
Groups of PTs-adECM and PTs alone were cultured for 7 d in complete 154CF medium (Life Sciences; Cascade, NY, USA), containing human keratinocyte growth supplements and 0.07 mM calcium chloride. Cell viability over 7 d was monitored by staining using Live/Dead Cell Assay Kits (Invitrogen; Carlsbad, CA, USA), where living and dead cells colored green and red, respectively. The stained images of the respective groups were quantified using Image J software (version 1.48).
2.7. Evaluations on tensile properties of PTs alone and PTs-printed PCL patch
Tensile testing was performed on PTs alone and PTs-printed PCL patch using a universal mechanical testing machine (3340 Series Single Column Systems, Instron, USA). The tensile strengths of two groups were measured at a steady strain rate of 2 mm min−1. Young's modulus was then calculated from the initial slope of the linear region (5%–15% strain) in the stress–strain curve (data not shown).
2.8. PTH secretion analysis in vitro
PTH secreted by cells cultured in vitro was qualitatively assessed through immunofluorescence staining examination. Briefly, the samples were incubated overnight at 4 °C anti-human PTH primary antibody (cat. no. ab154792, Abcam; Cambridge, MA, USA, 1:500 dilution) and washed three times in 1× PBS for 5 min each. The samples were then incubated for 1 h at room temperature with Alexa Fluor 488-conjugated anti-rabbit secondary antibody (cat. no. ab150077, Abcam; Cambridge, MA, USA, 1:200 dilution) and washed three times in 1× PBS for 5 min each. Nuclei were subsequently stained by incubation with 4', 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich), and the stained samples were imaged using a confocal microscope (Carl Zeiss LSM 880, Jena, Germany). To quantitatively compare the secretion of PTH, the soluble protein extract from the tissues (10 mg piece per 1 ml extraction buffer at the beginning) was analyzed on days 1, 4, and 7 using enzyme linked immunosorbent assays (ELISA) Kits (MyBiosource; San Diego, CA, USA) according to the manufacturer's instructions.
2.9. Animal experiments
All animal protocols were approved by the POSTECH ethics Committee (POSTECH-2018-0031). PT alone and PTs-printed patches were subcutaneously implanted into 24 healthy male Sprague-Dawley rats (n = 3 per group for each time point). The rats were sacrificed after week 0, 1, 2, and 4; as a control group, week 0 means the time point immediately after the implantation. Initial parathyroid engraftment, long-term stabilization, and hormone secretion were further evaluated.
2.10. Histological analysis
Samples were fixed with 4% solution of paraformaldehyde (PFA) for hematoxylin and eosin (H&E) and immunohistochemical (IHC) staining. Representative images of stained PTH in each group were captured using an automatic digital slide scanner (Panoramic Viewer; 3DHistech, Budapest, Hungary). IHC optical density scores were quantified as described in detail in literature [24].
2.11. Statistical analysis
All data were expressed as mean ± standard deviation (SD). Differences between groups were determined using a Student's t-test. Significance between groups was established for p < 0.01. All the measurements were repeated at least three times and representative results were shown.
3. Results
3.1. Investigations on adECM bioink rheology, cell viability, and PTH secretion
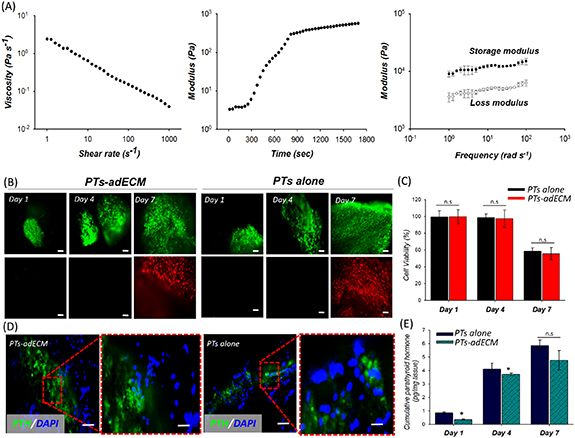
The prepared adECM pre-gel at 15 °C showed shear thinning behavior in the established shear rate range (figure 2(A)). Gelation kinetics of the dECM pre-gels was successfully demonstrated at varying temperatures from 4 °C to 37 °C. The result revealed that the dECM pre-gel started gelation immediately at 15 °C and formed a crosslinked gel at 37 °C within 30 min. In addition to the shear thinning behavior and gelation kinetics, the storage and loss moduli after the gelation were investigated. The storage modulus was larger than the loss modulus, meaning that the prepared adECM bioink could maintain its stable structure after the thermal gelation. With the prepared printable bioink, we finally embedded PTs in adECM bioink (PTs-adECM). The PTs alone were also prepared in vitro. To determine an appropriate time for subcutaneous implantation after extraction from patients, cell viabilities of the two groups were assessed on days 1, 4, and 7. After 4 d, few dead cells (red) were observed, with most of the cells in the two groups surviving (green) and at a comparable level (figure 2(B)). By day 7, however, the dead cells in the two groups had increased with no significant difference between the groups. The quantitative results of cell viability over 7 d supported this observation (figure 2(C)). These findings indicate that, to maintain parathyroid function, PTs cultured in vitro should be transplanted into subcutaneous tissue within 7 d.
Figure 2. In vitro evaluations of PTs alone and PTs-adECM cultured for 7 d. (A) Rheological evaluations (shear thinning (left), gelation kinetics (middle), and storage/loss modulus (right)) of 2% adECM. (B) Immunofluorescent images of living (green) and dead (red) cells after 1, 4, and 7 d. (C) The quantified cell viability of PTs-adECM and PTs alone over 7 d. (D) Immunofluorescence staining of PTH (green) in the two groups. (E) ELISA quantification analysis of PTH secreted by PTs alone and PTs-adECM after culture for 1, 4, and 7 d.
Download figure:
Standard image High-resolution imageQualitative comparison of the levels of PTH secreted by PTs-adECM and PTs alone showed that PTH was expressed by PTs in both groups (figure 2(D)). Quantitative analysis of cumulative PTH secreted into the culture medium over 7 d showed that both PTs-adECM and PTs alone released similar amounts of normal PTH into the medium, indicating that PTs remained functional after incorporation into adECM bioink (figure 2(E)). Because PTs-adECM is also bioprintable, it may allow the incorporation of a printable PCL support that can provide better mechanical stabilization in vivo.
3.2. In-depth evaluations on the prepared PTs-printed patch
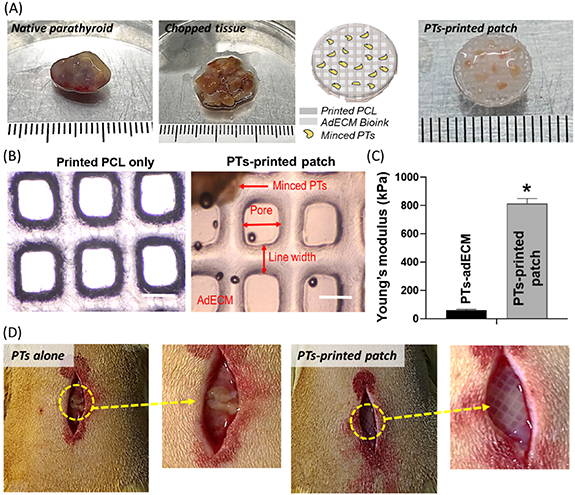
To prepare functional and implantable tissue patches containing PTs, the minced PTs-adECM was printed onto the pre-designed supportive PCL membrane in the functional and implantable form (figure 3(A)). As a control group, a group of PTs alone was prepared. The well-positioned PTs in 2% adECM, designed pores, and line width were observed in the prepared PTs-printed patch and compared with the printed PCL only (figure 3(B)). The observations demonstrated that the tissue printing process was successfully implemented. Due to the influence on the PCL support, Young's modulus of PTs-printed patch was approximately 16.3 folds larger than that of PTs-adECM only (without PCL support) (figure 3(C)). Obviously, this mechanical enhancement indicates that the engineered patch could provide better stabilization and integration to the native subcutaneous tissue. The PTs-printed patch and PTs alone were cultured and stabilized in vitro for 1 d. Finally, they were implanted subcutaneously in the rats for further comparison and observations (figure 3(D)).
Figure 3. Detailed processes from fabrication of an implantable PTs-printed patch to implantation into rats. (A) Intact human parathyroid glands were minced and encapsulated in the adECM bioink followed by printing on a supportive PCL mesh of diameter 10 mm. (B) Further comparisons and observation of printed PCL only and PTs-printed patch, scale bar: 200 μm. (C) Calculated Young's moduli of PT-adECM (without PCL support) and PTs-printed patch (with PCL support). (D) Subcutaneous positioning of engineered patches and PTs alone into rats. *p < 0.01 as compared with PTs-adECM.
Download figure:
Standard image High-resolution image3.3. Parathyroid engraftment, integration, and PTH secretion in vivo
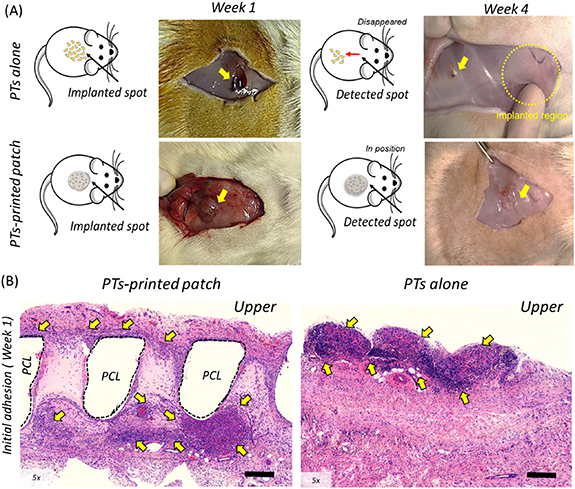
After one week, both the printed patches and PTs alone generally remained in their original position (figure 4(A)). At week 4, however, the group of PTs alone had migrated considerably from their original site of injection and the numbers of PTs had markedly decreased. By contrast, the engineered patches remained in their original position at week 4 and were well-integrated into the host tissue, indicating that these preparations, consisting of PTs-printed patches, adECM, and supportive PCL, were rapidly immobilized within the host tissue.
Figure 4. Gross images and histological evaluation before and at one week after implantation of PTs-printed patches and PTs alone. (A) Monitoring after one and four weeks to evaluate the engraftment and integration into subcutaneous tissue. (B) H&E staining to evaluate initial adhesion (week 1) of PTs-printed patch and PTs alone. Yellow arrows represent the identified PTs alone and printed patch on week 1 and 4 after the subcutaneous implantation.
Download figure:
Standard image High-resolution imageIn addition, the initial in vivo integration of PTs-printed patches and PTs alone into subcutaneous tissue was evaluated by H&E staining at week 1. The PTs-printed patches were located at the inner region of the host tissue with the PTs present throughout the implanted patches (figure 4(B)). Implantation of PTs alone, however, resulted in the presence of aggregates found on the surface of the host tissue, despite the minced tissues being initially well distributed.
We hypothesized that the well-integrated PTs-printed patches would eventually show normal parathyroid functions, such as the secretion of PTH. Histologic evaluation showed that PTH was not expressed prior to subcutaneous implantation of PTs-printed patch or PTs alone (figure 5(A)). On week 1, PTH was locally expressed in rats implanted with PTs-printed patch, whereas little PTH was expressed following implantation of PTs alone. These findings suggest that the engineered patch could better interact with the host tissue and provide relatively prompt stabilization under in vivo conditions.
Figure 5. IHC evaluation of PTH expression for four weeks after implantation of PTs-printed patches and PTs alone. (A) Immunohistochemistry (IHC) for PTH for four weeks. Brown color indicates secreted PTH. Scale bars represent 200 μm. (B) Quantification evaluation based on IHC optical density scores. *p < 0.01 compared with implantation of PTs-printed patches.
Download figure:
Standard image High-resolution imageEvaluation of the degree of PTH secretion on weeks 2 and 4 after implantation of PTs-adECM PCL revealed that PTH was expressed over a wider area after two weeks than one week, indicating that the stabilized PTs had greater functional activity after two weeks than one week. Similarly, PTH was still expressed throughout the implanted region at week 4. However, PTH expression in the control group implanted with PTs alone was extremely limited on week 2 with no significant increase at week 4. More importantly, it was difficult to locate the position of the implanted PTs.
To corroborate these results, the IHC optical density scores for PTH secretion were compared in the two groups (figure 5(B)). Although these scores did not differ significantly in the two groups before subcutaneous implantation, these scores differed significantly four weeks after implantation. These observations indicate that these engineered patches were well stabilized in vivo, resulting in the secretion of PTH.
4. Discussion
Patients with permanent hypothyroidism take considerable quantities of calcium and vitamin D throughout their lives, which can result in severe side effects and poor quality of life [25]. To date, the clinical method for the treatment of permanent hypoparathyroidism has merely consisted of re-implantation of the minced PTs into a muscle or subcutaneous layer [8]. Because this method is frequently unsuccessful, new methods are necessary for auto-transplantation. In the field of tissue engineering together with 3D bioprinting, most of the researchers have relied on cell isolation and culture before bioink encapsulation and biofabrication. Under in vitro condition, however, it might be difficult to grow and culture several cell types isolated from tissues/organs, particularly in the case of PT [26]. We herein suggested a novel approach of 'tissue printing' rather than cell printing to restore the parathyroid activity. This approach enabled direct printing of tissue itself without cell isolation and in vitro culture to produce a patch type PTs, showing the improved engraftment, stabilization, and enhanced PTH secretion when compared with the PTs alone. This bioprinting methodology, which is able to resolve the issue of traditional method, might mark a new era in the clinical-relevant technological advances. Furthermore, by omitting cell isolation and culture in vitro, this tissue printing-based approach might diminish several steps required for clinical approval.
Although direct 3D bioprinting of the parathyroid gland is preferable, there may be situations where this is impossible or may take several days. We observed that, similar to PTs alone, 3D printed PTs patches continued to secrete PTH in the laboratory for 7 d. However, the numbers of dead and dying cells started to increase on day 7, suggesting that 3D printing of the parathyroid gland and tissue transplantation should be performed within 7 d. Obviously, these in vitro results show the maintenance period of PTs under in vitro conditions before the transplantation. In the clinical view, however, immediate auto-transplantation of PTs will have to take place in the operation room. Because 3D bioprinting process only requires 3–5 min to print a PTs patch, it would be advantageous under the urgent and pragmatic situations.
We confirmed that tissue printed transplantable patches loaded with minced PTs engrafted and integrated into the subcutaneous tissue in vivo. These PTs-printed patches likely have a histologically fixed structure. In the field of tissue engineering, micro-porosity made by biocompatible and bioresorbable PCL scaffolds have endowed the intensive cell infiltration and tissue ingrowth from/with host tissue [27, 28]. In particular, many achievements have shown that the pre-defined and uniform porosity distribution of the scaffolds manufactured through 3D bioprinting techniques could lead to remarkable tissue infiltration and regeneration, as compared with the scaffolds having non-uniform porosity distribution produced in the conventional approaches, such as salt-leaching and gas foaming [29, 30]. From the previous achievements, we herein speculate that the pre-defined regular micro-pores from the printed PCL-based structure would offer crucial spaces where the host subcutaneous tissue could be readily infiltrated into the produced spaces, probably resulting in the rapid initial engraftment of the PTs. On the other hand, in the group of PTs only composed of dense human tissue, the implanted PTs were out of the original located position. Because there are insufficient micro-pores provided from PTs only, thereby it would be difficult to properly infiltrate the host subcutaneous tissue into the implanted tissue. In conclusion, the porous structure produced in the 3D bioprinting system is thought to play a role in stabilizing the integration and enhancing the function of printed PTs. Histological evaluation confirmed that this structure induced the integration with the host tissue.
To meet the purpose of this study, the optimal source of tissue material would be healthy parathyroid glands. However, it is impossible to detach and acquire the healthy parathyroid glands from patients. Because both primary and secondary hyperparathyroidism require surgery, the tissues with either primary or secondary one could be utilized as a promising material source for tissue printing. Nevertheless, there are three reasons that the tissue from patients with secondary hyperparathyroidism was used in our study. The first reason is the material affordability on human tissue. Primary hyperparathyroidism is usually observed at one gland of four parathyroid glands and the minimum parathyroid gland is surgically removed. On the other hand, in the case of secondary hyperparathyroidism, significant proliferation is observed in all the four parathyroid glands and plenty of tissues are removed during surgery. The second reason is that the tissue is relatively soft and consistent in patients with secondary hyperparathyroidism. In patients with primary hyperparathyroidism, the hard and stiff tissues are observed together with debris. It is anticipated that such tissues would be difficult to be uniformly minced. In addition, the debris throughout the tissues might cause unexpected experimental errors. As a third reason, theoretically, the disease in patients with secondary hyperparathyroidism is less dangerous. In primary hyperparathyroidism, the tissue itself is problematic. However, the main cause of patients with secondary hyperparathyroidism is kidney failure rather than PT itself. In other words, the parathyroid gland itself has less abnormalities, indicating that the tissue from secondary hyperparathyroidism would be closer to normal parathyroid condition.
We investigated if engraftment and integration of these patches into the subcutaneous tissue resulted in enhanced secretion of PTH. Although in vitro experiments showed no differences in cell viability and PTH secretion between PTs-printed patches and PTs alone, in vivo experiments revealed that PTs-printed patches provide better tissue engraftment and secretion than PTs alone.
Despite advances in this technology, several drawbacks should be addressed in the future. Specifically, it is hard to establish a PT-absent animal model because tiny PT cannot be found and isolated from the rat model. The existing PT in the animal might maintain body homeostasis to keep the hormonal level consistent in the blood. Due to this limitation, the measured human PTH from the blood did not exhibit significant differences between PTs alone and 3D printed PTs patch (not shown). For the same reason, this study did not evaluate PTH-independent regulation of blood calcium concentration. Therefore, a further study is needed, with the establishment of a PT-nonexistent animal model, to examine PTH and calcium concentration in the blood through the transplanted patch. Moreover, although the immunological response was not observed after the transplantation, immunosuppressive drugs might be considered according to the sensitivity of immunological response to animal models. Finally, additional studies in larger animals are required to assess the feasibility of the method described in this study.
5. Conclusion
To overcome the concerns in the traditional method for parathyroid autotransplantation, we suggested a new biofabrication strategy through introduction of the novel concept 'tissue printing'. In vitro and in vivo observations demonstrated that PTs-printed patches could promote parathyroid engraftment and tissue integration into the subcutaneous layer. These enhancements could eventually increase the PTH secretion in vivo. We believe that the findings of this study present a new technological solution for better parathyroid engraftment and integration and enhance hormone secretion in clinic.
Acknowledgments
This work was supported by Grant No. 13-2017-014 from the Seoul National University Bundang Hospital Research Fund. This work was also supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (NRF- 2017M3A9C6032067, 2019R1A3A3005437, and 2020M3H4A1A02084827).
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.