Abstract
In the field of medicine, we often brave the unknown like interstellar explorers, especially when confronting the formidable opponent of hepatocellular carcinoma (HCC). The global burden of HCC remains significant, with suboptimal treatment outcomes necessitating the urgent development of novel drugs and treatments. While various treatments for liver cancer, such as immunotherapy and targeted therapy, have emerged in recent years, improving their transport and therapeutic efficiency, controlling their targeting and release, and mitigating their adverse effects remains challenging. However, just as we grope through the darkness, a glimmer of light emerges—nanotechnology. Recently, nanotechnology has attracted attention because it can increase the local drug concentration in tumors, reduce systemic toxicity, and has the potential to enhance the effectiveness of precision therapy for HCC. However, there are also some challenges hindering the clinical translation of drug-loaded nanoparticles (NPs). Just as interstellar explorers must overcome interstellar dust, we too must overcome various obstacles. In future researches, the design and development of nanodelivery systems for novel drugs treating HCC should be the first attention. Moreover, researchers should focus on the active targeting design of various NPs. The combination of the interventional therapies and drug-loaded NPs will greatly advance the process of precision HCC therapy.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Primary liver cancers include hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, and other rare types [1]. According to the 2020 GLOBOCAN global cancer statistics [2], primary liver cancer is the sixth most commonly diagnosed cancer and the third leading cause of cancer death in the world. In 2020, about 906 000 patients worldwide were diagnosed with liver cancer, of which HCC accounted for 75%–85%, and 830 000 patients died.
The clinical management of HCC requires a multidisciplinary team to develop individualized treatment strategies based on the patient's tumor stage, liver function, and performance status [1]. Currently, the Barcelona Clinic of Liver Cancer (BCLC) is the most widely used liver cancer staging system. For patients with BCLC 0- or A-stage HCC, liver transplantation, hepatectomy, and ablation are possible curative options. However, only a small proportion of patients (<15%) are eligible for the above approaches, and the recurrence rate 5 years after hepatectomy could exceed 70% [3]. Transarterial chemoembolization (TACE) is the preferred regimen for patients with BCLC B-stage HCC [4], compared with untreated patients, TACE could increase median overall survival (OS) from 16 to 26 months [5]. With the development of molecularly targeted agents and immunotherapy, more options have emerged for the treatment of BCLC C-stage HCC. In a global randomized phase III trial (IMbrave 150 trial), the combination regimen of atezolizumab + bevacizumab achieved a median OS up to 19.2 months, significantly higher than the 13.4 months of sorafenib, and has been approved by the Food and Drug Administration (FDA) for first-line treatment of advanced HCC [6]. However, not all patients respond to these systemic therapies [7]. Targeted drugs represented by sorafenib have low efficiency, many adverse effects, and drug resistance [8]. And although PD-1 monoclonal antibody can effectively inhibit HCC, current clinical trials show that its objective remission rate in advanced HCC is still less than 20% [9, 10]. Overall, the global burden of HCC remains high and the treatment outcomes remain suboptimal. Novel drugs and treatments are urgently needed for HCC patients.
To solve the problem mentioned above, many researchers have turned to the field of nanotechnology. Drug-loaded nanosystem is a kind of drug delivery system (DDS) based on nanoparticles (NPs), which could load and deliver drugs or other active ingredients to the lesions. Nanoencapsulation of drugs could enhance their bioavailability, improve their biological activities and reduce their systemic toxicity [11]. In addition, some NPs could enhance the efficacy of certain drugs or therapeutic technologies due to their special physicochemical properties, and some NPs could also exert anti-disease effects themselves. Therefore, NPs have become a hot topic in the biomedical field, especially in the field of oncology [12].
In this review, we summarize the main types and characteristics of NPs and focus on the relevant studies of NPs-mediated HCC treatment. Furthermore, we also expound clinical application prospects for NPs to provide new insights into the treatment of HCC.
2. NPs in HCC management
2.1. Advantages of NPs in HCC management
As the most important detoxification organ and filtration system in the human body, liver could uptake 30%–99% of NPs from the blood [13], so NPs could serve as a suitable drug carrier for HCC administration.
Currently, various therapeutic options are applied for HCC, but each option has certain limitation. Gratifyingly, the rapid development of nanotechnology provides new prospects for solving the existing limitations in HCC treatment. For example, in the ablation treatment of HCC, there might be problems such as incomplete ablation and tumor recurrence. And some specific NPs could enhance the ablation effect through their own thermal effect, or eliminate residual tumors by targeted delivery of anti-tumor drugs [14]. NPs loaded with photosensitizers, when exposed to light of specific wavelengths, can generate heat for thermal ablation and produce reactive oxygen species (ROS), inducing cell death in cancerous cells, thereby achieving anticancer effects [15]. A limitation of TACE is the hypoxia-induced angiogenesis and tumor recurrence, which could be addressed by NPs loaded with antiangiogenic agents [16]. Delivering NPs loaded with VEGF-targeted drugs to tumor tissues via TACE can suppress the neovascularization of tumor tissues [17]. Systemic therapy in HCC is usually accompanied by systemic toxicity and severe side effects [18], and the application of NPs could remarkably increase local drug concentration, enhance the curative effects and reduce the side effects [19].
Gene therapy is an emerging therapeutic regime for the treatment of cancer that achieves anti-tumor effects by providing curative genes or genetic inhibition molecules, such as small interfering RNA (siRNA) or micro-RNA (miRNA). However, gene therapy agents have low stability and are rapidly degraded in intracellular and extracellular environments, which restricts their widespread application. Hopefully, gene therapy agents could be successfully loaded on the NPs surfaces and accurately delivered to tumor tissue, which avoids its degradation in the blood. NPs have contributed enormously to the development of gene therapy [20].
2.2. Tumor targeting of NPs
2.2.1. Passive tumor targeting
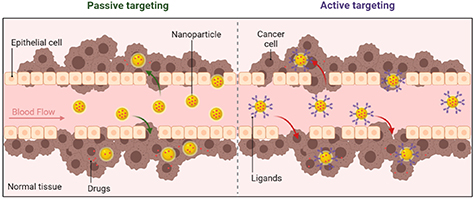
Unlike normal tissue, tumor tissue has abundant vessels and blood flow, large vascular endothelial gaps, poor structural integrity, and a lack of functional lymphatic system, which causes the passive accumulation of NPs in the tumor tissue. The above characteristics are known as the enhanced permeability and retention (EPR) effect, which is the functional basis for the passive tumor targeting of NPs (figure 1) [21]. The EPR effect is mainly based on tumor vessel leakiness with vascular endothelial gaps ranging from 100 to 780 nm, therefore, NPs with diameters <100 nm are considered the optimal option for passive tumor targeting [22]. A study conducted by Cabral et al demonstrated that NPs with diameters of 30, 50, 70, and 100 nm could penetrate hyperpermeable tumors in mice, but for hypopermeable tumors, only NPs with diameters of 30 nm could achieve tumor penetration [23].
Figure 1. The schematic illustration of passive and active tumor targeting of NPs.
Download figure:
Standard image High-resolution image2.2.2. Active tumor targeting
The passive targeting effect of NPs is still limited, the surfaces of NPs could be decorated with targeting ligands to achieve active tumor targeting. When the ligands attached on NPs surfaces bind to specific receptors on the surface of tumor cells, receptor-mediated endocytosis would induce cell uptake of drug-loaded NPs (figure 1). In this way, drugs can be selectively delivered to tumor tissue to increase drug accumulation in tumors while decreasing systemic toxicity [24], which improves the efficacy and reduces the adverse effects of systemic therapies such as targeted therapy and immunotherapy.
Ligands and receptors commonly used in active tumor targeting mainly include P-selectin [25], γ-aminobutyric acid type A receptor, epidermal growth factor receptor (EGFR), fibroblast growth factor receptor, somatostatin receptors, insulin receptor, prostaglandin E2, insulin-like growth factor [26], asialoglycoprotein receptor (ASGPR) [27], folate receptor (FR) [28], transferrin receptor [29], alpha-fetoprotein, Glypican-3 [30] and others [24].
3. Types of NPs used for HCC treatment
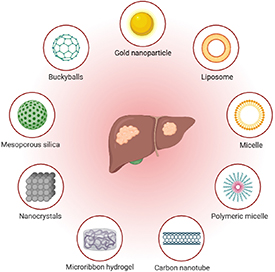
Currently, a variety of NPs for HCC management have been developed, which could be roughly divided into metal, carbon-based, calcium-based, silica and hybrid NPs (figure 2) [29]. In recent years, biomimetic NPs have also been gradually used in the biomedical domain. In addition, one crucial element of NP design influencing both pharmacokinetics and cell uptake is NP morphology (both size and shape) and NPs can be categorized as nanosphere, nanorod, nanobelt, nanowires, etc based on the morphology [31]. This review provides a detailed description of metal, carbon-based, calcium-based, silica and hybrid NPs.
Figure 2. Various nanoparticles applied in HCC management.
Download figure:
Standard image High-resolution image3.1. Metal NPs
The high surface area-to-volume ratio of metal NPs contributes to their high binding affinity with drugs and targeted ligands. In addition, due to the EPR effect of solid tumors, metal NPs could passively accumulate at the tumor site by crossing physiologic barriers [32]. The above characteristics allow metal NPs to become appropriate DDS for HCC management.
3.1.1. Gold NPs (GNPs)
Gold NPs (GNPs/AuNPs) is one of the most studied NPs. Due to their unique and tunable optical properties, high biocompatibility, and amenable to surface modifications and ligand attachments for targeted delivery, AuNPs have been widely used in the management of cancer [33]. Its unique and tunable optical properties make it ideal for applications such as photothermal therapy (PTT). Additionally, its high biocompatibility ensures minimal toxicity, while the versatility of surface modifications enables the loading of various drugs. As a result, GNPs are extensively employed in numerous fields, particularly in treatment, diagnosis, bio-labeling, drug delivery, biological sensing, imaging, photothermal/photodynamic therapy (PDT), and others. However, it is not easy to precisely control the size and shape of gold NPs. The heterogeneity and aggregation of gold NPs not only affect drug loading but can also lead to drug loss in the bloodstream circulation [34].
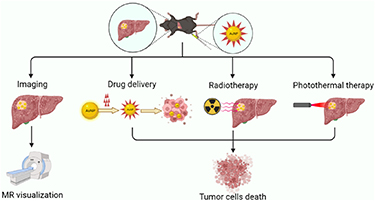
The most commonly used methods for the preparation of AuNPs include the Turkevich and Brust–Schiffrin methods. In the Turkevich method, tetrachloroauric acid (HAuCl4) and trisodium citrate are used as reducing and capping agents to stabilize AuNPs against aggregation. Mono-dispersed spherical AuNPs with diameters of 10–20 nm can be synthesized using the Turkevich method [35]. AuNPs could act as carriers for drug delivery to specific regions, and their surface plasmon effect allows them to enhance the effect of PTT or radiotherapy (figure 3) [36–38].
Figure 3. AuNPs in antitumor therapy: imaging, drug delivery, enhanced radiotherapy and enhanced photothermal therapy.
Download figure:
Standard image High-resolution imageAuNPs can be used as DDS for chemotherapy drugs. In the study by Tomuleasa et al, doxorubicin (DOX), cisplatin and capecitabine were non-covalently conjugated onto the hydrophilic assemblies of AuNPs-L-Aspartate nanostructure, and transmission electron microscopy showed that the uptake of AuNPs in HCC cells is dependent on the caveolin endocytosis, and then the conjugated drugs are released from endosomes/lysosomes, thereby inhibiting the proliferation of HCC cells [39]. In another study, Li et al fabricated DOX-loaded PEG polyethylene glycol-HAuNS hollow gold nanospheres and delivered the nanospheres directly to rat N1S1 liver tumors via the hepatic artery. The results show that the drug-loaded AuNPs could effectively accumulate in tumors, and generate heat when exposed to near-infrared (NIR) laser irradiation. Consequently, the temperature of tumor rapidly rises, and the elevated temperature could kill the tumor cells and trigger the release of DOX, thus exerting a sustained anti-tumor effect [40].
Aberrant expression of miRNA is involved in tumor initiation, proliferation, invasion and metastasis. MiR-375, a tumor suppressor gene downregulated in HCC, strongly inhibits HCC cell viability by reducing ATG7 expression in mouse models [41]. Xue et al designed and fabricated an AuNPs DDS for the delivery of miR-375, which exhibited high cellular uptake, significantly inhibited the proliferation and invasion of HCC cells, promoted their apoptosis, and further exerted anti-tumor effect in primary and xenograft mouse tumor models [42]. Another study conducted by Mo et al showed that miR-326-loaded AuNPs could exert an anti-HCC effect by targeting PDK1/AKT/c-myc axis [43].
PDT and PTT are noninvasive methods that showed remarkable effects in the treatment of multiple tumor types. They involve the tumor-localizing photosensitizer followed by local illumination with light of a specific wavelength to activate the photosensitizer. The excited photosensitizer then transfers its energy to molecular oxygen, thus generating cytotoxic ROS, which could oxidize key cellular macromolecules leading to tumor cell ablation [44]. However, photosensitizer is hydrophobic with poor penetration of tumor tissue, which might affect the bioavailability of photosensitizer and impede the activation of ROS, resulting in suboptimal PDT effectiveness. AuNPs can be used as the DDS for photosensitizer to enhance its tissue permeability and bioavailability, and the surface plasmon resonance of AuNPs could increase its surrounding light field, thereby enhancing the photosensitizer excitation efficiency. In addition, the AuNPs exposed to NIR light can generate heat for tumor ablation. Therefore, AuNPs can be used as a suitable carrier of photosensitizer for PDT and PTT. Salem et al prepared 5-fluorouracil (5-FU)-loaded AuNPs coated with chitosan, which showed a better inhibitory effect on HCC compared to 5-FU alone in the absence of laser irradiation, and the inhibitory effect is further enhanced in the presence of laser irradiation [45]. In the study of Zhang et al, AuNPs were used to load photosensitizer and prodrugs for the treatment of HCC, which achieved the aims of tumor micro-environment triggered programmable prodrug release, in-demand PDT and aggregation-induced photothermal ablation [46].
In addition to being used as a DDS for anticancer drugs and/or active ingredients, AuNPs also have antiangiogenic properties. Several studies have shown that AuNPs could inhibit the proliferation, cell motility and tube formation capabilities of human umbilical vein endothelial cells (HUVECs) [35, 47]. They exert an antiangiogenic effect by inhibiting the activation of the VEGF/VEGFR2 pathway and downregulating the AKT and ERK1/2 phosphorylation in endothelial cells [48, 49]. In addition, AuNPs could inhibit intracellular calcium release, reduce VEGF-165-mediated RhoA activity in HUVECs, and downregulate the expression levels of proangiogenic factors (Ang-1 and Ang-2) in tumor tissues [50, 51]. In the locoregional treatments of HCC, hypoxia-induced tumor angiogenesis is one of the most concerning issues, and the anti-angiogenic ability of AuNPs undoubtedly adds another compelling reason for their application in the treatment of HCC.
3.1.2. Silver/platinum NPs
Compared with AuNPs, silver/platinum NPs (Ag/PtNPs) exhibit higher biological toxicity [52]. Meanwhile, low surface plasmon resonant frequency limits the applications of Ag/PtNPs in biomedical fields. Despite certain shortcomings, Ag/PtNPs still have a certain potential value in the treatment of HCC.
Several studies showed that AgNPs could exhibit significant cytotoxicity to HCC cells, and this cytotoxic effect is mainly mediated by mechanisms such as the activation of ROS and the upregulation of proapoptotic Bcl-2 members [53, 54]. Unfortunately, the cytotoxic effects of AgNPs lack specificity, when they exert anti-tumor effects, they also affect normal cells. Mohammad Yousof et al found that oral administration of AgNPs caused severe liver structural and functional damage in rats, and this hepatotoxicity persisted even after discontinuation [55]. In addition, it remains controversial whether the activation of ROS serves as a promoting or inhibitory effect for HCC [56]. Therefore, AgNPs might not be suitable as a DDS in HCC treatment.
Platinum-based compounds are a kind of anticancer drugs commonly used in the clinic, represented by cisplatin, oxaliplatin and loplatin, etc which can bind to DNA, form adducts and inhibit DNA replication [57]. Due to the anti-tumor properties of platinum-based compounds, PtNPs are progressively attracting significant attention in the treatment of cancer. PtNPs are generally obtained from the reduction of Pt(II) or Pt(IV) ions, in the presence of a strong reducing agent, to obtain the chemical reduction to Pt(0) atoms that start the nucleation process. In a cellular environment with high oxidation potential, Pt(0) in Pt NPs could be oxidized to Pt(II) ions, which could exert a cytotoxic effect [58]. HCC cells have a higher oxidation potential than normal cells, so PtNPs could exhibit specific killing effects on HCC cells and are an ideal DDS for HCC [59]. Medhat et al prepared PtNPs by incubating hydrogen hexachloroplatinate with selected bacterial isolate, and the PtNPs showed anti-HCC effects superior to cisplatin in both in vitro and in vivo experiments [60]. To further improve the specificity of PtNPs for HCC cells, Shoshan et al prepared peptide-coated PtNPs, whose inhibitory effect was stronger on HCC cells and weaker on normal hepatocytes compared with cisplatin and sorafenib [61]. The highly specific anti-tumor properties of PtNPs against HCC make them a more promising DDS than AgNPs, which deserves further research and exploration.
3.1.3. Magnetic NPs
As early as the 70s of 20th century, Freeman et al pioneered the introduction of magnetism into medical research. Since then, biocompatible magnetic NPs have gradually developed and are widely used in biomedical research [62]. The major goal of the current research is to optimize the intrinsic properties of these magnetic NPs to reduce their early clearance in vivo, thereby minimizing the side effects, and improving the efficiency of cell internalization [63].
Superparamagnetic iron oxide nanoparticles (SPIONs) consist of a core made of iron oxide, typically maghemite (γ-Fe2O3) or magnetite (Fe3O4) with diameter of 10–100 nm. SPIONs are usually coated by a biocompatible organic or inorganic coating to improve stability and can be used for drug loading and delivery. Drug-loaded SPIONs can be guided by an external magnet to the target site. SPIONs are superparamagnetic, they can be magnetized under an external magnetic field, and after the magnetic field is removed, they no longer exhibit any residual magnetic interaction. As a result, they are less likely to accumulate in the body to avoid the risk of thrombosis or capillary occlusion [64]. When SPIONs are taken up by cells, the magnetic susceptibility difference between labeled cells and background tissue results in a hypointense region on T2* weighted magnetic resonance (MR) images, thereby enabling the visualization of SPIONs-labeled cells in vivo [65]. During the transarterial therapies of HCC, visualizing drug delivery is important for the operators, they can observe the real-time delivery of drugs in arteries and adjust the dose accordingly [66, 67]. Therefore, SPIONs are an ideal DDS for HCC, especially in the transarterial treatment of HCC.
Yuan et al designed and fabricated a DOX-loaded, polydopamine-coated single crystal hematite (α-Fe2O3) nanocubes, which exhibited good MR visualization, photothermal response, and pH-triggered DOX sustained release effects in vitro [68]. Sheu et al used SPIONs to label natural killer cells, which were successfully delivered via arterial route to the rat liver cancer model under MR visualization [69]. SPIONs are also good carriers for gene therapy agents, and researchers have loaded siRNA or miRNA into SPIONs and used them in preclinical HCC models, which have achieved remarkable anti-tumor efficacy [70, 71]. Preclinical studies have demonstrated that SPIONs serve as carriers for gene therapy agents in the treatment of HCC, offering advantages such as strong stability, targeted delivery, and non-invasive monitoring of therapeutic efficacy. Further research is warranted to optimize SPION-based gene delivery strategies, thereby improving treatment outcomes for HCC patients.
The management of HCC is a process that requires long-term dynamic monitoring, and the tumor response evaluation after TACE is crucial to the prognosis of HCC patients. Currently, the marketed microsphere products cannot be observed under both CT and MR scans, which makes it difficult to evaluate tumor response after DEB-TACE. Therefore, researchers are devoted to the study of visualization microspheres to address this problem [72, 73]. The microspheres incorporating SPIONs have been developed for the purpose of MR visualization. For example, Li et al designed and fabricated the polyvinyl alcohol microspheres encapsulated with in situ synthesized Fe3O4 NPs, Liu et al prepared the mesoporous organosilica microparticles decorating with Fe3O4 NPs, the above magnetic embolic microspheres are proved to be effective in MR visualization and vascular embolization in rabbit VX2 liver tumor models [74, 75]. In order to solve the problems of embolization-induced hypoxia and angiogenesis, some researchers used sorafenib-loaded microspheres incorporating SPIONs, which exhibited anti-tumor and anti-angiogenic activities in rabbit VX2 liver tumor models [76].
In addition to achieving the targeted precision delivery and MR visualization of drugs, magnetic NPs can also exert the role of magnetic hyperthermia under the alternating magnetic field [77]. Jeon et al treated the subcutaneous Hep3B hepatic tumor models in nude mice by intratumoral injection of DOX-loaded magnetic NPs, the results showed that DOX could be slowly and continuously released to exert an anti-tumor effect. In addition, the magnetic NPs within the tumor rapidly increase the tumor temperature under an alternating magnetic field, resulting in tumor apoptosis and necrosis [78]. The most remarkable advantage of magnetic NPs-mediated magnetic hyperthermia is its ability to penetrate deep tissues and selectively kill cancer cells without causing harm to the surrounding normal tissue [79]. In the study of Minbashi et al, microwave ablation with different input powers was used for the treatment of HCC injected and uninjected with magnetic NPs, when both groups achieved the same ablation effect, tumor tissues containing magnetic NPs required lower microwave input power. This implies that intratumoral magnetic NPs could reduce the input power of microwave ablation, thereby effectively avoiding the risk of damage to adjacent normal tissues caused by high power microwave [80].
In recent years, research on catalyzing tumor treatment by inducing the generation of ROS through the magnetoelectric (ME) response process under intervention of an alternating magnetic field (a non-invasive, controllable intensity, and externally applied physical stimulus without penetration depth limitations) has been widely reported. Strategies based on core–shell structured NPs for magnetic response modulation have achieved control over ROS generation in vivo and consequent tumor cell destruction [81]. Targeted drug release at tumor cells while sparing normal cells presents a significant challenge. Core–shell ME NPs have tackled this issue by leveraging shape-dependent magneto-electric attributes while enabling 'on-demand' drug release in vitro [82, 83].
The application of magnetic NPs also presents some challenges that need to be addressed. For instance, traditional SPIONs, used as MR T2 contrast agents, face difficulties in clinical use due to potential confusion with low signal areas and the need for improved image resolution. Additionally, as magnetic hyperthermia agents, their low magnetic heating efficiency has been a persistent obstacle in clinical targeted magnetic hyperthermia applications. It is comforting to know that with the continuous development of magnetic NP synthesis technology, new types of magnetic NPs are continually emerging, offering hope to address these issues.
3.1.4. Other metal NPs
The research on other metal NPs used for HCC treatment is relatively rare, but it is also of a reference value. Manganese (Mn) is a redox active metal that can activate ROS and disrupt cellular redox homeostasis, thereby killing cancer cells [84]. Abdel-Aziz N biosynthesized MnNPs using Lactobacillus helveticus, which presented a dose-dependent cytotoxic effect against HCC cells. Besides that, MnNPs administered via oral route could induce tumor apoptosis and necrosis in the Diethylnitrosamine (DEN)-induced rat liver cancer model, and improve the hepatic damage caused by DEN, while controlling redox state balance, reducing inflammatory and angiogenic factors [85].
Cerium oxide (CeO2) NPs are a kind of antioxidant and anti-inflammatory NPs with superoxide dismutase activity, catalase activity, and peroxidase activity [86]. Fernández-Varo et al administered CeO2NPs via intraperitoneal route to treat the DEN-induced rat liver cancer model, CeO2NPs significantly induced HCC cell apoptosis, reduced macrophage infiltration and inflammatory gene expression in liver tissue, ultimately prolonged the survival of tumor-bearing rats [87]. Similar to CeO2NPs, copper oxide (CuO) NPs could also induce oxidative stress, cytotoxicity and DNA damage, thereby suppressing proliferation and promoting apoptosis of HCC cells [88].
Copper sulfide (CuS) NPs are good photothermal conversion materials, when they are used as DDS, they can also exert a photothermal effect [89]. In the study of Cai et al, CuSNPs were used to load anti-TGF-β antibody and ataxia telangiectasia mutated inhibitor to act on the mice subcutaneous H22 hepatic tumor models via intravenous route, realizing the integrated effect of sustained release and photothermal [90].
3.2. Carbon-based NPs
Carbon-based NPs are highly biocompatible and biodegradable, and are often used only as a DDS without performing functions themselves. Although they do not possess therapeutic properties, they have great potential as a DDS to load anti-tumor drugs for the management of HCC.
3.2.1. Polymeric NPs
3.2.1.1. Polyethylene glycol (PEG)
Generally, bare NPs could be degraded and eliminated by the reticuloendothelial system (RES) in different cellular contexts. At this time, some biocompatible compounds or polymers could be used to modify the surfaces of bare NPs to avoid degradation and elimination [91]. At present, the compounds used for modification mainly include citrate, amine, nucleic acid, peptide, antibody and lipid, etc, and polymers include polysaccharide, polyacrylamide, poly(vinyl alcohol), PEG and PEG-copolymers. Of all the polymers used to improve the solubility and biocompatibility of NPs, PEG and PEG-copolymers are the most widely used and have proven to be the most effective in screening the surface charge of NPs [92].
3.2.1.2. Poly (lactic-co-glycolic acid) (PLGA)
PLGA is a copolymer of glycolic acid and lactic acid with biocompatibility and biodegradable properties. In aqueous conditions, PLGA undergoes hydrolysis of its ester bonds, the hydrolysates could be easily metabolized through the tricarboxylic acid cycle without systemic toxicity. PLGA is an ideal multifunctional DDS due to its properties of small size, high structural integrity, good stability, adjustability, controllable release capability and surface functionalization. For this, it got approval from FDA for biomedical applications [93]. Song et al used PLGA NPs to load vincristine and verapamil for the treatment of HCC, and confirmed the satisfactory drug encapsulation and delivery capacity of PLGA NPs [94].
3.2.1.3. PEG-PLGA copolymer
There are many limitations of PLGA NPs such as poor drug loading capacity, rapid drug release, and short circulation time in vivo [95]. To address these limitations, PLGA NPs are often modified or combined with other NPs to form new hybrid NPs, the most common form of which is the PEG-PLGA copolymer [96]. Devulapally et al applied PEG-PLGA NPs loaded with antisense-miRNA-21 and gemcitabine to HCC cells, they found that PEG-PLGA NPs could be well taken up by HCC cells, and exert a stronger pro-apoptosis effect than free drugs [97]. Long-term exposure to sorafenib for HCC cells induces sorafenib resistance through the upregulation of SDF-1/CXCR4 axis. Zheng et al prepared PEG-PLGA NPs loaded with mifepristone (a CXCR4-targeted drug) and sorafenib, mifepristone was precisely delivered into the HCC cells to overcome the sorafenib resistance [98].
PEG-PLGA NPs can also be used to load photosensitizer to achieve PTT for HCC. Heat shock proteins (HSPs) are upregulated when cells are exposed to elevated temperatures, which might trigger the self-protection mechanism of tumor cells, leading to insufficient apoptosis, increased cell viability and tumor recurrence. Therefore, HSP inhibitors are often used in combination with PTT for the treatment of malignancies [99]. Tang et al utilized a NIR dye SQ890 as both an iron-chelating and a photothermal converter agent, which was encapsulated with a GSH-sensitive polymer to attain the biocompatible SQ890@Fe NPs. After the above NPs were taken up by HCC cells with a high concentration of GSH, Fe3+ was reduced to Fe2+, which induced ferrozoosis. Subsequently, the expression of HSPs was inhibited, and the effect of PTT was enhanced [100].
In DEB-TACE treatment for HCC, embolic microspheres are generally not biodegradable, such as the commercially available DC Beads® and Hepasphere™ microspheres [101]. However, biodegradable microspheres show higher drug loading efficacy and better drug release properties than the above-mentioned microspheres. Because of its biodegradability, PLGA is more applicable than conventional microspheres in loading poorly water-soluble, effective and high-cost drugs (e.g. sorafenib, cisplatin, paclitaxel, etc). Drug-loaded PLGA microspheres for TACE have been reported in several studies. Choi et al prepared DOX-loaded PLGA microspheres, which exhibited great drug loading and drug releasing capacities, especially under acidic conditions. In an orthotopic HCC model, the intra-arterial administration of DOX-loaded PLGA microspheres exhibited sufficient embolic effect and lower systemic drug exposure than DOX perfusion alone [102]. Chen et al developed PLGA microspheres loaded with sorafenib and encapsulated with iron-oxide NPs, which were used for TACE treatment in rabbit VX2 liver tumor model [76]. These PLGA microspheres offer the potential to increase the efficacy of molecularly targeted MKI therapies while reducing systemic exposures via selective catheter-directed delivery to HCC. Li et al used PLGA microspheres encapsulated with sorafenib and catalase for TACE treatment in rabbit VX2 liver tumor model, on the one hand, catalase can neutralize the overexpressed H2O2 in tumor tissues and produce oxygen to improve the hypoxic environment after tumor embolization, on the other hand, sorafenib could inhibit hypoxia-induced angiogenesis by downregulating the expression of VEGF [103].
3.2.1.4. Polysaccharide-based NPs
Polysaccharide-based NPs have attracted much attention because of their rich properties, low cost, good biodegradability, good biocompatibility, low systemic toxicity and low immunogenicity [104]. The surface of HCC cells contains overexpressed ASGPRs, which can be specifically recognized by galactose, N-acetylgalactosamine, and glucose. Thus, polysaccharide-based NPs can be used as an ideal carrier for drug delivery to HCCs [105]. Zhang et al fabricated DOX-loaded Angelica sinensis polysaccharide (ASP) NPs, and in vitro cellular uptake revealed that DOX-loaded ASP NPs were internalized into HCC cells through ASGPR-mediated endocytosis, resulting in a higher anti-proliferation effect than free DOX [106].
Chitosan is one of the most abundant biomaterials prepared by the deacetylation of chitin, and chitosan-based NPs have been widely used as DDS targeting liver disease [107]. However, chitosan is poorly soluble in the neutral medium, resulting in a lower loading capacity of the non-ionic hydrophobic drugs. To address this question, amphiphilic modification is usually performed by attaching segments of galactosylated chitosan to the chitosan backbone of the hydrophobic and hydrophilic chain [108]. Ning et al fabricated galactosylated-chitosan NPs loaded with 5-FU and miRNA-122, which could effectively target HCC cells, inhibit cell proliferation, and further induce apoptosis while downregulating the expression of ADAM17 and Bcl-2 [109]. FRs were found to be overexpressed in various cancer cells, and folic acid (FA) is a more commonly used targeting ligand than galactose, Xiang et al introduced FA into galactosylated-chitosan NPs loaded with 5-FU, which had ASGPR and FR dual-targeting properties, and enhanced the HCC targeting efficiency and reduced nonspecific uptake by normal hepatocytes [21].
3.2.2. Liposome
Liposomes are vesicles comprised of phospholipids form a bilayer membrane that surrounds an inner aqueous core, this structure makes them able to load not only hydrophobic but hydrophilic drugs. Liposomes have distinct characteristics such as biodegradability, biocompatibility, low immunogenicity and long half-life, and a variety of liposomes have been approved by the FDA for cancer treatment, including Onivyde™, Marqibo®, Doxil®, Visudyne®, and Depocyt®, and others [110].
DOX-loaded liposomes are most commonly studied for the treatment of HCC, several studies have shown that DOX-loaded liposomes exhibit stronger anti-HCC cytotoxicity and better in vivo therapeutic efficacy than free DOX [111, 112]. In terms of nanodelivery of antiangiogenic drugs, Yao et al used liposomes to load VEGF-siRNA and sorafenib for the treatment of HCC [113]. To solve the problem of sorafenib resistance, Wang et al modified the CXCR4 inhibitor on the surface of sorafenib-loaded liposomes for HCC treatment [114]. Besides that, liposomes can also be used to carry photosensitizer for PTT. Yu et al prepared photo-activatable liposomes integrated with both photosensitizer Ce6 and chemotherapeutic drug triptolide, which accumulated at the tumor site due to the EPR effect. Then liposomes were destroyed in the intracellular environment to release Ce6 and triptolide. Under laser irradiation, Ce6 generated ROS and further oxidized the unsaturated phospholipids, exerted synergistically potentiated anticancer effects together with triptolide [115].
3.3. Calcium-based NPs
Calcium phosphate (CaP) degrades rapidly in acidic tumor microenvironment, releasing its loaded drugs or genes into the cytoplasm to play an anticancer role, so CaP NPs are appropriate drug delivery carriers for HCC therapy [116]. In the study of Wu et al, CaP NPs were used to deliver fingolimod and siRNA, siRNA could reduce autophagy of HCCs and thus increase their sensitivity to fingolimod. In the nude mice subcutaneous SMMC-7721 hepatic tumor models, fingolimod and siRNA accumulated well in the tumor and exerted a good tumor inhibitory effect [117]. In order to enhance the gene delivery capacity and immune adjuvant properties, tumor-targeted lipid-dendrimer-calcium-phosphate (TT-LDCP) NPs with thymine-functionalized dendrimers were engineered by Huang et al HCC cells were treated with siRNA against PD-L1 and IL-2-encoding plasmid DNA delivered by TT-LDCP NPs, which enhanced the tumor infiltration, CD8+ T cell activation and immunotherapy efficacy, and suppressed HCC progression [118].
Like CaP, calcium carbonate (CC) also exhibits pH-responsive drug release property. Kim et al developed Lipid-calcium-carbonate (LCC) NPs, which could play strong proton sponge effect at the low pH of the tumor microenvironment, resulting in endosomal lysis and drug release into the cytoplasm [119]. Zhao et al treated HCC using LCC NPs loaded with miR-375 and sorafenib, which exhibited pH-dependent drugs release and potent cytotoxicity in vitro, and increase the uptake of drugs in tumors and exert good tumor suppressive effects in vivo [120].
Wang et al synthesized polyvinyl pyrrolidone-coated calcium peroxide (CaO2) NPs, which were used to load DOX and delivered to rabbit VX2 liver tumors via tumor-feeding arteries. In tumors, CaO2 NPs could react with water and produce abundant O2, OH−, H2O2 and Ca2+, thereby relieving tumor hypoxia, neutralizing acid, and overloading Ca2+ to mediate antitumor effects [121].
3.4. Silica NPs
The silicon is an indirect bandgap semiconductor and recently widely used in drug delivery, mesoporous silica NPs (MSNs) are the most commonly used silica NPs in biomedicine. MSNs have strong drug-loading capacity due to their tunable pore pores (2–30 nm) capable of capturing and carrying small molecule drugs [122]. In addition, their abundant chemistry functionality and acceptable biocompatibility have attracted attention in biomedical applications in HCC [123]. Liao et al synthesized novel microspheres (MSN@Alg) composed of MSNs and organic alginate by air-dynamic atomization, DOX was loaded in the pores of MSNs, and K4YRGD peptide was functionalized onto the surface of the MSN@Alg for targeting HCC cells, which greatly improved the efficiency of intracellular DOX delivery and reduced systemic toxicity [124]. Xue et al loaded DOX and miR-375 into the pores of lipid-coated hollow MSNs HCC cells for synergistic anti-tumor effects [125]. The activation of autophagy represents a mechanism underlying resistance to DOX treatment in HCC, and isoginkgetin (ISO) could inhibit DOX resistance by modulating autophagy [126]. In the study of Wang et al, hyaluronic acid-conjugated and manganese-doped MSNs were designed to load ISO and DOX, which solved the problem of DOX resistance and exhibited superior anti-tumor efficiency [127].
3.5. Biomimetic NPs
NPs are exogenous materials, the early recognition by immune system and renal and hepatic clearance severely limit their clinical application. Although functionalization and modification has been widely used in various NPs to effectively avoid monocyte/macrophage phagocytosis and realize long circulation in vivo. Usually, these materials used for surface functionalization and modification of NPs are also exogenous and inevitably recognized and cleared by the immune system. Biomaterials such as natural cell membranes, extracellular vesicles or viruses might be more suitable for functionalization and modification of NPs than conventional materials. To date, red blood cell, platelet, stem cell, and cancer cell membranes have been used to establish functionalized biomimetic NPs [128–131].
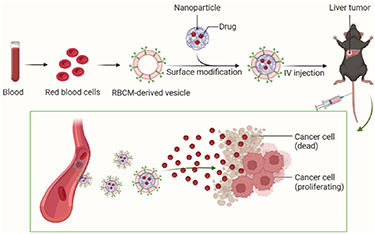
There are 5 billion red blood cells per milliliter of human blood, providing rich red blood cell membrane (RBCM) coating materials for the biomimetic surface modification of NPs. There are several advantages of biomimetic NPs modified by RBCM (figure 4) [132]: (1) escape from immune system surveillance and achieve long-term circulation in the body; (2) better biocompatibility and degradability; (3) low toxicity; (4) long service life of 120 d; (5) improve the drug-carrying capacity of NPs; (6) improve the stability of NPs and prolong the in vitro storage time. Lian et al developed RBCM-camouflaged arsenic trioxide (ATO)-loaded sodium alginate NPs. These NPs could avoid uptake by macrophages in in vitro experiments, increase HCC uptake, and showed significantly cytotoxicity against the HCC cells. In vivo results further showed that ATO could cause mild lesions of main organs while ATO-loaded biomimetic NPs could reduce the toxicity and improve the anti-tumor effects [133].
Figure 4. The advantages of biomimetic NPs modified by RBCM.
Download figure:
Standard image High-resolution imageNeutrophils are the most abundant leukocytes and play an important role in acute inflammation and tumor microenvironments. Neutrophil adhesion and infiltration to the vasculature are key processes in acute inflammation, so neutrophil membranes can be used for surface modification of NPs, allowing NPs to actively cross the vascular barrier for inflammation and tumor targeting [134]. Zhang et al prepared the Hypocrellins (HB)-loaded and neutrophil membrane-coated PLGA NPs (NM-HB NPs), and the results showed that NM-HB NPs could target tumor sites well and evade immune surveillance in vivo and in vitro. In mouse HCC models, NM-HB NPs showed significant tumor suppressive effects through combined application with PTT [135].
Generally, tumor targeting agents such as aptamers, peptides, and antibodies can target tumor site through ligand-receptor interactions, but cancer cells themselves have intrinsic homomeric adhesion properties to tumor tissue. Cancer cells can express surface adhesion molecules with homomeric adhesion domains (e.g. N-cadherin, galactoglutinin 3, epithelial cell adhesion molecules) to achieve multicellular aggregation, thereby achieving targeting of tumor tissues. Therefore, cancer cell membranes can also be used for the surface modification of NPs to achieve precise delivery of drug-loaded NPs through their homologous targeting [131]. Liu et al designed HepG2 cell membrane-coated PLGA NPs (HepM-PLGA NPs) and effectively encapsulated DOX in them. The results showed that HepM-PLGA NPs had excellent tumor cell targeting ability, exerted good anti-tumor effects and reduced systemic toxicity [136].
4. Conclusions and perspectives
HCC is frequently diagnosed at an advanced stage, and due to late detection and rapid tumor progression, the prognosis and survival of HCC patients is still poor despite the emergence of different preventive and treatment modalities. In addition, local and systemic treatment-related side effects severely reduce the patients' quality of life. At this time, NPs loaded with anti-tumor drugs might play an important role. By actively or passively targeting tumor tissues, drug-loaded NPs can increase the local drug concentration in tumors, thereby reducing systemic toxicity. This not only improves treatment efficacy but also decreases the occurrence of adverse reactions, thus enhancing patient quality of life and potentially prolonging OS. Therefore, in clinical practice, the development of novel technologies and drugs is important, and NPs for precision drug delivery are also worth paying attention to.
The design of drug-loaded NPs in HCC is a complex process, which requires not only optimizing their physicochemical properties to improve their biocompatibility and HCC targeting, but also requires a thorough understanding of the cirrhosis environment and the interaction between NPs and tumor microenvironments. These challenges hinder the clinical translation of drug-loaded NPs. At present, many NPs have been applied to HCC, and they are most commonly used to deliver conventional chemotherapy drugs (DOX, paclitaxel, etc), molecular targeted drugs (sorafenib, regorafenib, etc), gene therapeutic drugs (miRNA, siRNA, etc) and photosensitizers for PTT. In recent years, due to the success of HCC immunotherapy, NPs used to load immunotherapeutic agents or multiple drugs might become a hot spot for future research.
In summary, nanotechnology will occupy a important place in the management of HCC, and a lot of researches are still needed to lay the foundation of this promising technology. In future researches, the design and development of nanodelivery systems for novel drugs treating HCC should be the first attention. Moreover, researchers should focus on the active targeting design of various NPs, in addition to the most common FR and ASGPR modifications, pH and other stimulus-response elements guided tumor targeting are also attracting attention gradually. The combined application of NPs and PTT has provided new ideas for HCC treatment, and it is still worth exploring the photosensitive NPs and photosensitizers. Finally, interventional therapies such as arterial therapy and ablation can directly target drugs to HCC through tumor-feeding arteries or intratumoral puncture. The combination of the above interventional therapies and drug-loaded NPs will greatly advance the process of precision HCC therapy, which should attract extensive attention from researchers.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81873919).
Data availability statement
No new data were created or analyzed in this study.
CRediT author statement
Xiaoming Liu, Yaowei Bai and Binqian Zhou: Conceptualization; Methodology; Writing—original draft. Wei Yao: Methodology. Songlin Song, Jiacheng Liu and Chuansheng Zheng: Conceptualization, Writing—review & editing.
Ethics approval
The study protocol was approved by the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology.
Conflict of interest
The authors have no conflicts of interest to declare.