Abstract
Electrospinning technique converts polymeric solutions into nanoscale fibers using an electric field and can be used for various biomedical and clinical applications. Extracellular vesicles (EVs) are cell-derived small lipid vesicles enriched with biological cargo (proteins and nucleic acids) potential therapeutic applications. In this review, we discuss extending the scope of electrospinning by incorporating stem cell-derived EVs, particularly exosomes, into nanofibers for their effective delivery to target tissues. The parameters used during the electrospinning of biopolymers limit the stability and functional properties of cellular products. However, with careful consideration of process requirements, these can significantly improve stability, leading to longevity, effectiveness, and sustained and localized release. Electrospun nanofibers are known to encapsulate or surface-adsorb biological payloads such as therapeutic EVs, proteins, enzymes, and nucleic acids. Small EVs, specifically exosomes, have recently attracted the attention of researchers working on regeneration and tissue engineering because of their broad distribution and enormous potential as therapeutic agents. This review focuses on current developments in nanofibers for delivering therapeutic cargo molecules, with a special emphasis on exosomes. It also suggests prospective approaches that can be adapted to safely combine these two nanoscale systems and exponentially enhance their benefits in tissue engineering, medical device coating, and drug delivery applications.
Export citation and abstract BibTeX RIS
Abbreviations
| 3D | three-dimensional |
| AD | Alzheimer's disease |
| ADSC-CM | adipose-derived stem cells' conditioned media |
| ALI | Acute lung injury |
| ATMP | advanced therapy medicinal product |
| BMMSC | bone marrow-derived MSC |
| DCM | Dilated cardiomyopathy |
| EC | endothelial cell |
| ECM | extracellular matrix |
| EV | Extracellular vesicle |
| FBS | fetal bovine serum |
| LPS | lipopolysaccharide |
| mEV | mid-size EV |
| MSC | mesenchymal stem cell |
| MV | microvesicle |
| PBS | phosphate-buffered saline |
| PCL | Poly( -caprolactone) -caprolactone) |
| PLA | poly lactic acid |
| PMSC | placenta-derived MSC |
| UC | ultracentrifugation |
Vocabulary
Electrospinning: A technique wherein the electric force is used to produce charged fibers of polymer solutions or one-dimensional polymer melts of ⩽100 nm.
Nanofibers: Natural and synthetic cylindrical structures with an outer diameter ⩽100 nm and high aspect ratio (length-to-width ratio).
Stem cells: Undifferentiated cells that can divide into similar cells or differentiate into specialized cell types based on the biological milieu.
Extracellular vesicles: Bilayered lipid nanovesicles that are usually secreted by all cells for intercellular communication.
Exosomes: A nanosized subset of EVs (diameter ∼30−150 nm) that carry a variety of cargo molecules that act as cell modulators and contribute to various physiological and pathological processes.
1. Introduction
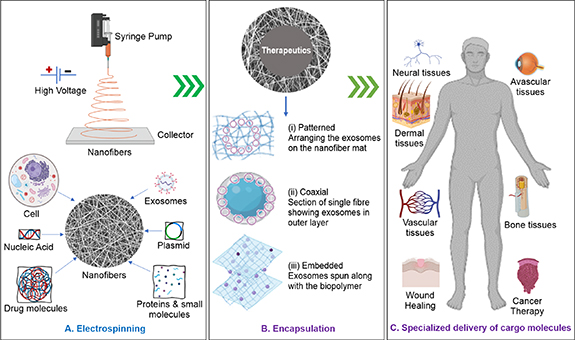
Over the years, electrospinning has developed significantly and exhibits tremendous potential in regenerative medicine and tissue engineering. Electrospinning is the most common technique used for generating fibers with diameters in the nano- to micrometer range. It has gained popularity owing to its capability of mass-producing continuous fibers, which can be easily molded into desired shapes, ranging from mats to three-dimensional (3D) structures [1]. The fibers produced are deposited on the collector in a nonwoven manner and have exceptional characteristics, such as a high surface-to-volume ratio that facilitates surface adsorption. The fibers form highly permeable networks with interconnected ultrafine pores, are lightweight, and have high mechanical strength and tortuosity [2]. The fibers are popular as matrices or substrates in tissue engineering owing to their easily tunable properties. The fibers can also be functionalized with biological molecules, enhancing their potential application manifold [3]. The process of electrospinning involves a polymer solution being subjected to a high electrostatic force that yields micro- or nanometer-thick fibers. The solution is loaded into a syringe connected to a syringe pump with a controlled flow and pushed out of a needle (spinneret) connected to a high-voltage source. This strong electrostatic force transforms the polymer droplet into a Taylor cone. The polymer at the tip of the cone accelerates toward the grounded collector, and in the process, the volatile solvent evaporates, leaving micron-to-nanoscale fibers at the collector [1, 2, 4]. Conventional electrospinning involves spinning together a solution of a single polymer and the therapeutic molecule of interest (figure 1(A)). The release rate of therapeutics from electrospun fibers can be controlled by modulating the properties of the polymer. Patterned or aligned nanofibers can be fabricated using diverse methods, including a rotating mandrel as a collector, employing a unidirectional patterned collector, or employing parallel electrodes to create various complex architectures [5]. Aligned topography with multiple layered scaffold can be achieved with patterened electrospinning to construct regenerative centers such cell specific extracellular matrix in nanofibers [6]. Coaxial electrospinning, in which the nanofibers are engineered into a core-shell structure, offers further control over the release of therapeutics. The initial spontaneous or burst release of the cargo from shell (release of large mass of cargoes before the release rate reaches a stable profile) is followed by a controlled release from the core [7]. The core-shell nanofibers produced by coaxial electrospinning typically have cargo (drug)-embedded polymeric core and inert protective shell as physical barrier to provide sustained release of the drug [8]. Tri-axial electrospinning is another subset that leads to three-layered nanofibers with better control over the initial spontaneous release [9]. Melt electrospinning, gas jet electrospinning, and emulsion electrospinning are other well-established techniques used in several controlled-release applications [10–12]. Embedding of active biomolecule in nanofibers by adding them in a suitable solution (e.g. polyvinylpyrrolidone) can improve their stability and biological activity [13] (figure 1(B)).
Figure 1. Schematic of potential applications of electrospinning technology in delivering different cargo molecules especially for exosomes for tissue regeneration, (A) fabrication of different cargo molecule-loaded electrospun nanofibers. (B) Methods for incorporating exosomes into nanofibers (example of cargo molecule) in (i) patterned, (ii) coaxial, or (iii) embedded arrangements in nanofibrous structures suitable for specific applications. (C) Delivery of exosomes to targeted tissues.
Download figure:
Standard image High-resolution imageElectrospun fibers, often referred to as nanofibers if one or two dimensions of fiber are in nanoscale; researchers have obtained fiber diameters as low as 1 nm [1, 3]. The characteristics of nanofibers, such as fiber diameter, alignment, surface area, porosity, and surface functionality, can be selectively fine-tuned to engineer smart scaffolds for the delivery and release of drugs, genes, nucleic acid, proteins and exosomes at target site [14–16] (figure 1(C)).
The potential of the electrospun nanofibers is not limited to controlled-release applications. Currently, there are nine nanofiber technologies approved for wound healing and regenerative medicine, eight of which are under clinical trials [17]. Nanofibers are also widely explored in biosensor design and diagnostics. Nanofiber scaffolds can form sensing membranes for biosensors owing to their high surface area. A high surface-to-volume ratio offers enhanced binding sites for the analyte, which further improves sensitivity [18]. Biosensors with nanofiber components can be used for timely cancer detection. To diagnose breast cancer, researchers have added epidermal growth factor receptor two antibodies to zinc oxide nanofibers [19]. The emergence of nanofiber-based in vitro 3D cancer models has added another dimension to cancer research. Because electrospun nanofibers mimic the structure and complexity of the extracellular matrix (ECM), these are used to recreate an extracellular environment for tumors. Poly( -caprolactone) (PCL)/gelatin nanofibers with perlecan (domain IV) peptides have been used to generate a prostate cancer model [20]. Additionally, nanofibers have also been explored as implant coatings for medical devices. They act as a bridge between the implant and host tissue to reduce the rejection rates of hard-tissue prostheses [21]. Although the potential applications of electrospun nanofibers are immense, their regenerative properties are enhanced when combined with therapeutic moieties such as extracellular vesicles (EVs), drug-encapsulated liposomes, proteins, enzymes, and genes [22]. Therapeutics are majorly loaded onto fibers either via immobilization or encapsulation. Encapsulation is a straightforward process and requires only a stable polymer-therapeutic mixture and electrospun using specialized techniques. However, immobilization involves either the electrostatic adsorption of the therapeutic to charged fibers or its linkage to the fibers using crosslinkers [17, 23]. There have been efforts to obtain a nanofibrous matrix encapsulating biological moieties (stem cells, EVs) directly from the solution phase to the final electrospun product. However, due to limited options in selecting suitable solution to preserve the functionality of the biological moieties very few have been reported so far [24–26]. However, immobilization of exosomes using hydrogel-nanofiber composite, biotin–Avidin grafting, PEG-phospholipid tagging and hydrogen bonding have been explored extensively for multiple purposes [27–30]. This review attempts to pinpoint the challenges and suggests measures to obtain therapeutic molecule-loaded electrospun nanofibers, with a special focus on the practicality associated with exosomes, the rising star in biotechnology. The review also discusses the generation of nanofibers using electrospinning technology and their integration into emerging exosome-based therapeutics. It attempts to provide comprehensive information on the therapeutic and regenerative properties of EVs and their delivery using electrospun nanofibers. Recent developments, challenges, and prospects of nanofiber-exosome systems are discussed in detail in the following sections.
-caprolactone) (PCL)/gelatin nanofibers with perlecan (domain IV) peptides have been used to generate a prostate cancer model [20]. Additionally, nanofibers have also been explored as implant coatings for medical devices. They act as a bridge between the implant and host tissue to reduce the rejection rates of hard-tissue prostheses [21]. Although the potential applications of electrospun nanofibers are immense, their regenerative properties are enhanced when combined with therapeutic moieties such as extracellular vesicles (EVs), drug-encapsulated liposomes, proteins, enzymes, and genes [22]. Therapeutics are majorly loaded onto fibers either via immobilization or encapsulation. Encapsulation is a straightforward process and requires only a stable polymer-therapeutic mixture and electrospun using specialized techniques. However, immobilization involves either the electrostatic adsorption of the therapeutic to charged fibers or its linkage to the fibers using crosslinkers [17, 23]. There have been efforts to obtain a nanofibrous matrix encapsulating biological moieties (stem cells, EVs) directly from the solution phase to the final electrospun product. However, due to limited options in selecting suitable solution to preserve the functionality of the biological moieties very few have been reported so far [24–26]. However, immobilization of exosomes using hydrogel-nanofiber composite, biotin–Avidin grafting, PEG-phospholipid tagging and hydrogen bonding have been explored extensively for multiple purposes [27–30]. This review attempts to pinpoint the challenges and suggests measures to obtain therapeutic molecule-loaded electrospun nanofibers, with a special focus on the practicality associated with exosomes, the rising star in biotechnology. The review also discusses the generation of nanofibers using electrospinning technology and their integration into emerging exosome-based therapeutics. It attempts to provide comprehensive information on the therapeutic and regenerative properties of EVs and their delivery using electrospun nanofibers. Recent developments, challenges, and prospects of nanofiber-exosome systems are discussed in detail in the following sections.
2. Biogenesis of EVs
EVs are cell releases that include bilayer lipid vesicles, such as exosomes (endosome-origin), ectosomes (plasma membrane-origin), microvesicles (MVs) (cell shedding), and apoptotic bodies (apoptotic cell-origin) [31]. EVs are produced by all cells (prokaryotes and eukaryotes) and act as natural delivery systems to transmit biological signals such as coding and non-coding RNAs, short DNA stands, cytokines, enzymes, membrane proteins, and lipids that contribute to cell-to-cell communication [32]. Endosomal origin small EVs of size approximately ∼30–150 nm, canonically termed exosomes, can communicate in physiological and pathological conditions [33]. Sequential infolding of the cell membrane eventually leads to the creation of multivesicular bodies. These bodies interact with cell organelles and other intracellular vesicles, a process that plays a crucial role in the selection and incorporation of diverse cargo constituents into exosomes [34]. The physiological necessity and purpose of producing exosomes by cells is still unsolved and needs further investigation. However, recent studies showcase selective, functional, pathway-centric aggregation of discrete cellular ingredients in exosomes, illustrating their role in cellular communication [34, 35]. Exosomes can infiltrate organs and the interstitial spaces of tumors, sustain their properties in an immune-compromised milieu, and have a long shelf life [36, 37]. Recent findings have revealed the importance of paracrine signaling in regenerative mechanisms associated with exosomes [38]. The unique therapeutic properties of mesenchymal stem cell (MSC)-derived exosomes, attributed to their molecular and functional similarity to their parental cells, could provide extraordinary opportunities for the development of cell-free therapeutics [39].
3. Isolation, purification and characterization of exosomes
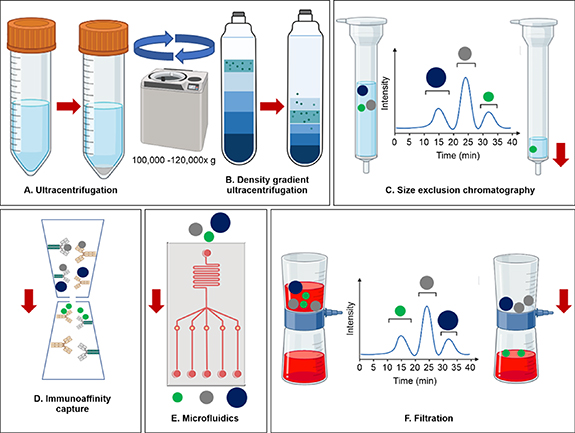
Several methods have been used to isolate and purify exosomes (figure 2), such as ultracentrifugation (UC) differential and density gradient-based, UC-tandem-configured and sequential, size-exclusion chromatography, precipitation (polymer-based), immunoaffinity capture (surface protein-based), and microfluidics (size-based), based on their size, density, and surface proteins. However, each method has advantages and disadvantages, producing different yields and purities of exosomes that might be contaminated with EV subtypes, cellular protein aggregates, or both [40, 41].
Figure 2. Classical and advanced methods of exosome isolation including (A) UC has been used to remove cellular debris by low centrifugation force (e.g. 700 × g, 2000 × g, 10000 × g) followed by high-speed centrifugation ⩾ 100 000 × g to sediment EVs, (B) Density or differential gradient UC uses a cushioning system (iodixanol or sucrose gradient) to separate subpopulation of EVs by differential acceleration rates (C) Size-exclusion chromatography separates molecules by differential elution based on their size with an inverse rate relation, i.e. smaller vesicles stay longer in the system (high elution time) compared to larger vesicles, (D) Immunoaffinity capture (surface protein-based) separates EVs from the biofluids by antigen- antibodies interaction where specific antibodies (magnetically labeled) are used to target exosomal surface proteins. (E) Microfluidics (size-based) uses nanoscale diameter channels and EV-specific antibodies to collect EVs from biofluids, and (F) Filtration (size-based) uses a specific pore-size filter to create an EV-rich filtrate.
Download figure:
Standard image High-resolution image3.1. Ultracentrifugation (differential and density gradient)
Ultracentrifugation is the most frequently used method for isolating different biological components based on size and is considered a classical method for isolating exosomes. It exploits the size difference between exosomes and other EVs to separate these via differential sedimentation at speeds ⩾ 100 000 × g. The major advantage of UC is that it yields highly enriched exosome fractions along with other large EV fractions (such as larger vesicles that sediment at a lower speed) and an EV-free supernatant, which is generated after a high-speed centrifugation step. A disadvantage of UC is its low throughput, which may affect the physical and biological integrity of exosomes and cause aggregation. Density gradient UC of biofluids and culture media offers advantages over classical UC; it maximizes recovery, preserves physical and biological functionality, and yields high-purity exosomes devoid of cellular proteins [42, 43].
3.2. Size exclusion chromatography
Size exclusion chromatography separates EVs based on size using biofluids (such as serum, conditioned media, and plasma) as the mobile phase and a pore-size-based gel as the stationary phase. The stationary phase or chromatography column, which allows differential elution, is packed with gel polymer beads such as crosslinked dextrans (Sephadex), agarose (Sepharose), polyacrylamide (Biogel P), or allyldextran (Sephacryl) containing multiple pores, for separation of EVs [40]. Exosomes are separated from EVs owing to the difference in their hydrodynamic radii; when the mobile phase passes through the column, the larger vesicles elute first, followed by comparatively smaller exosomes and the smallest protein moieties [44]. The major limitation of the technique is that it cannot differentiate between similar size vesicles [40]. Nevertheless, it effectively separates small vesicles or exosomes from soluble proteins and larger vesicles, providing a cost-effective exosome retrieval system that yields pure and intact exosomes.
3.3. Immunoaffinity capture-based techniques
This technique utilizes specific proteins, lipids, and polysaccharides that are ubiquitously found on the surface of exosomes and can be used to identify and separate exosomes based on the principle of antigen–antibody binding. Briefly, exosomes are labeled with magnetic beads, which are recognized by antibodies immobilized on the surface. However, owing to the specific requirement for a surface marker, this technique may inherently exclude some populations of EVs devoid of the same, thus affecting the yield. Nevertheless, the immunoaffinity capture method is twice as efficient with respect to purity and yield of exosomes as compared to UC [45]. Immobilizing exosomes specific surface markers (e.g. anti-human CD63, CD 81 or CD 9) on electrospun core-shell nanofibers is promising alternative for isolation of exosomes from body fluids. The core-shell nanofibers provide extended surface for immobilizing exosome capturing specific antibodies and size exclusion-like effect for isolation of exosome which later collected by a simple wash using phosphate buffer saline [46, 47].
3.4. Microfluidics-based isolation technique
Microfluidic isolation of exosomes is based on size-based separation using microchannels of different diameters. Microchannels can be designed according to specific requirements and are more efficient because these require lesser volumes of samples and reagents. However, this technique suffers from the technical limitations of channel blockages in case of EV size mismatch as well as that of a small sample limit per input, which may affect yield efficiency. However, an advanced equipment setup can improve the time required for isolation [41]. In addition to traditional methods for small-scale isolation of exosomes, several commercial kits are available that are suitable for one or two of the abovementioned methods. Most of these kits offer isolation reagents to precipitate exosomes by binding to specific surface markers or lipophilic moieties, following which the exosomes can be separated using standard centrifugation. Some of these kits include Total Exosome Isolation Reagent (Invitrogen), ExoQuick® exosome isolation (System Bioscience), Minute™ Hi-Efficiency Exosome Precipitation Reagent (Invent), Exosome Isolation Kit (CusaBio), ExoEasy Maxi kit (QIAGEN), Exosome Isolation Kit Pan (Miltenyi Biotec), and MagCapture™ Exosome Isolation Kit PS (Wako). These commercial kits have the advantages of short recovery time, high yield, and enhanced integrity for small samples; however, the isolation and purification of exosomes from mixed samples is difficult, tedious, and expensive. With the advancement in techniques, a ubiquitous method may be available to isolate exosomes from different sources [48].
4. Characterization of exosomes
Traditionally, EVs have been classified into multiple subtypes based on their origin (cellular or tissue), biogenic pathway, isolation method, size, and biological functions. The significant difference in the source and isolation processes for EVs requires a uniform and standard characterization parameter, as proposed by the International Society for EVs. The quantitative and qualitative characterization of nanoscale EVs or exosomes suffers from the technological limits of instruments; however, parameters mostly accepted include techniques for the characterization of single EVs for physical (size, shape, and structure) and molecular signatures of surface proteins (associated with the cell membrane or endosomes, or both, and cytosolic proteins) [49, 50]. The standard characterization of isolated exosomes involves three levels of validation: (a) physical characterization using nanoparticle tracking analysis to identify exosome size, peak, and distribution profile, and transmission electron microscopy to identify exosome architecture and morphology; (b) molecular characterization by immunoblotting, flow cytometry, and ELISA to identify and quantify surface proteins (e.g. CD9, CD63, CD81, TSG101, and Alix) and cargo proteins (e.g. hepatocyte growth factor, brain-derived neurotrophic factor, nerve growth factor); and (c) composition profiling by lipidomics, proteomics, and transcriptomics to identify lipid, protein, and mRNA/miRNA contents of exosomes [51–53].
5. Therapeutic potential of MSC-derived exosomes
The use of stem cells as advanced therapy medicinal products (ATMPs) has increased exponentially in the past decade with remarkable clinical results. However, the widespread use of any cell type in ATMPs requires rigorous regulatory evaluation for quality, safety, and efficacy [54]. EVs originating from autologous or allogenic MSCs can be beneficial in maintaining the efficacy, quality, and safety of ATMPs [55–58]. Exosomes derived from MSCs have tremendous regenerative capabilities, along with anti-inflammatory, anti-fibrosis, pro/anti-angiogenic, and wound-healing properties, which renders these suitable candidates for cell-free therapies and tissue engineering applications. Biological sources of MSCs include the umbilical cord, placental tissues, adipose tissues, and bone marrow, all of which regularly produce therapeutic exosomes (figure 3(A)). Exosomes derived from MSCs are known to promote regeneration because the cargo proteins are obtained from the producer cells. There are more than 100 ongoing clinical trials in phase 3 or 4 using MSCs, and more than 20 clinical trials at different stages targeting various tissues/organs using MSC-derived exosomes for their regenerative, anti-inflammatory, anti-fibrotic, and wound healing properties (figure 3(B)).
Figure 3. A schematic representing different sources of (A) mesenchymal stem cell (MSC)-derived exosomes and (B) their applications in the regeneration and repair of avascular and vascular tissues.
Download figure:
Standard image High-resolution imageMultiple approaches to deliver therapeutic exosomes to target tissues are being explored including inhalation of dry powder exosomes [59], direct injection [60], liposome-exosome hybridization [61], exosome-laden hydrogel [62–64]. The direct delivery of exosomes through injection has been shown short shelf life and rapid clearance from blood circulation (90–360 min) and accumulation in the liver, spleen, lung, and gastrointestinal tract administration [65]. However, a modified exosome with suitable surface can evade the immune system and have high residence time in circulation which increases the efficacy of their targeted content delivery [66]. Cationic lipid and peptide matrix increases the stability of exosomes, enhances the cellular uptake and stimulates the efficient cytosolic release of the exosomal contents at specific pH. However, incorporating exsomess into a stable solid polymeric matrix could preserve their stability and provide sustained targeted release, electrospun polyvinylpyrrolidone-based nanofibers has shown excellent storage capacity for exosomes for 12 weeks [25]. Further exosome loaded electrospun phosphoethanolamine phospholipid-grafted poly-L-lactic acid fibres in diabetic rat models showed improved retention and release profile and enchaned wound-healing process [29].
Table 1 summarizes the therapeutic and regenerative properties of MSC-derived EVs in vascular and avascular tissues and organs and describes their broad applications. The therapeutic properties of MSC-EVs highlight their suitability in combination with different approaches, such as nanofibers prepared by electrospinning, to enhance their utilization in tissue engineering applications. However, the two disciplines of electrospun nanofiber technology and MSC-EV production have inherent complexities and diversities that make their combinatorial use challenging, resulting in the information on nanofibers with embedded exosomes being extremely limited.
Table 1. Therapeutic and regenerative properties of mesenchymal stem cells (MSC)-derived extracellular vesicles (EVs).
| Target organ | EV source | Therapeutic potential | Study model/Key results |
|---|---|---|---|
| MSC-derived secretomes | Contribution in immune modulation, amelioration of injury, or reduction of fibrosis in the diseased liver [67, 68] |
|
| MSC-derived exosomes | An increase in hepatocyte proliferation and expression of proliferation proteins (PCNA and cyclin D1); inhibition of apoptosis in the induced hepatocytes in drug-induced liver injury models [69] |
| |
| MSC-derived exosomes | Reduction of fibrosis and attenuation of alveolar epithelium and vasculature in lung spheroids and animal models [70, 71] |
|
| Pluripotent stem cells and MSC-derived exosomes | Enhancement of proliferation, migration, cell cycle promotion, and inhibition of apoptosis of human corneal epithelial cells [72] |
|
| MSC-derived EVs | Protection of human corneal endothelial cells from apoptosis mediated by endoplasmic reticulum stress [73] |
| |
| Krüppel-like factor 2 (KLF-2)- overexpressing endothelial cells derived EVs | Regulation of cardiac inflammation in dilated cardiomyopathy [74] |
|
| MSC-derived EVs |
| |
| MSC-derived exosomes | Amelioration of Alzheimer's Disease pathology and improvement of cognitive deficit [77] |
| |
| Chondroprogenitor cell line (ATDC5)-derived exosomes with activated VEGF165 plasmid gene | Promotion of vascularized osteogenesis in situ and control of the differentiation of progenitor cells into osteoblasts [78] |
|
6. Electrospinning with biological cargoes
Nanofiber-based diagnostics have been widely explored, and the safe delivery of therapeutics holds a major share of nanofiber-based applications. The development of exosome therapeutics has widened the horizons of research on nanofibers. Recent findings suggest that exosomes play a crucial role in intracellular signaling, inflammation, antigen presentation, angiogenesis, and cellular apoptosis [79]. Individually, these two fields have seen immense advancement in diagnostics and therapeutics, but their combinatorial studies are still in their infancy. A recent study by Chen et al reported successfully loading the adipose-derived stem cells' conditioned media (ADSC-CM) onto micro-nano poly lactic acid (PLA) fibers via electrospinning. They showed that the controlled sustained-release of ADSC-CM from the fibers accelerated cell migration and inhibited the proliferation and differentiation of fibroblasts into myofibroblasts. Excessive production of ECM was also suppressed. Application of ADSC-CM nanofibers in vivo improved regeneration outcomes and accelerated wound closure. The superior pro-regenerative performance was evident from the in vivo studies [79]. Although cell culture media is a well-known source of exosomes, besides plasma, milk, saliva, urine, and other biological fluids used in this study, the authors did not define the nature of the therapeutic moieties present in the ADSC-CM [80–86].
7. Electrospinning with exosomes
Exosomes can be artificially engineered with precise control to target a specific application. These are usually administered by direct injection into the body, similar to biological ATMPs, which produces suboptimal results owing to rapid clearance, reducing their efficacy [58]. Recently, however, few research groups have attempted to use electrospun mats for the targeted delivery of exosomes (table 2). The core-shell nanofibers of PCL/Gelatin have shown good reactivity towards exosome isolation from body fluids. PCL imparts stability, and the CD63-specific antibody binding, enabled via gelatin's functionality, allows exosome attachment and isolation [47]. Kang et al synthesized an exosome-functionalized cell-free poly (D, L-lactide-co-glycolide)/Magnesium-gallate metal-organic framework or PLGA/Exo-Mg-GA MOF scaffold using electrospinning to construct unique nanostructural interfaces to enhance osteogenic, angiogenic, and anti-inflammatory capabilities. They demonstrated the effects of PLGA/Exo-Mg-GA MOF composite scaffolds on the osteogenic differentiation of human bone marrow-derived MSCs (BMMSCs), as well as angiogenic effects in human umbilical endothelial cells (ECs) in vitro. A rat calvaria defect model further confirmed the efficacy of PLGA/Exo-Mg-GA MOF scaffolds in bone formation and osseointegration [87]. Singh et al fabricated polyurethane-based nerve conduits via electrospinning. Surface adsorption enabled the loading of BMMSC-derived exosomes onto the scaffolds (nerve conduits and nerve guidance channels). These exosome-loaded scaffolds helped repair nerve injuries in diabetic peripheral neuropathy [88]. Hao et al isolated EVs from placenta-derived MSCs (PMSCs) and demonstrated that the PMSC-EVs possessed pro-angiogenic and anti-apoptotic capacity. They established an integrin-based binding technology for immobilizing PMSC-EVs onto electrospun ECM-mimicking scaffolds to mimic EV-ECM complexes. The PMSC-EV modified electrospun scaffolds promoted EC angiogenesis and prevented EC apoptosis in the ischemic environment [89]. Gandolfi et al showed the effect of mineral-doped PLA-based porous electrospun scaffolds enriched with exosomes on the osteogenic commitment of human adipose MSCs [90]. Chachques et al used the same strategies to entrap exosomes within the electrospun fibers of elastomeric PCL-based scaffolds for myocardial regeneration with enhanced wound-healing and anti-inflammatory proepties [91]. The biological activities including wound healing and anti-inlfammatory properties of exosome entrapped scaffolds were evaluated based on gene expression (MiR- 124, 130, 483, 877, 337, 546) and protein (collagen type I, vitronectine, VEGF) secrection of macrophages and MSCs seeded on the scaffolds.
Table 2. A comprehensive summary of the recent research in electrospun nanofiber-embedded exosome systems. The electrospinning parameters and the exosomes used in the process are summarized.
| Electrospun nanofibers | Exosomes | Technique | Electrospinning parameters | Application |
|---|---|---|---|---|
| PCL-Gelatin | Prostate cancer cell-derived | Anti-human CD63 antibody-coated nanofibers used to immobilize exosomes from culture media | Solvent: Trifluoroethanol | Efficient isolation of exosomes from body fluids using nanofibers [47] |
| Coaxial structure 290 nm (270 nm core and 10 nm shell) | Flow rate: 0.3 ml h −1 (gelatin) and 0.6 ml h −1 (PCL) | |||
| Voltage: 16 kV | ||||
| Silk fibroin (SF)-PCL nanofibers modified with polydopamine (pDA) 100–400 nm diameter | Chondroprogenitor cell line (ATDC5)-derived. Loaded with recombinant VEGF165 plasmid by electroporation | MC3T3-E1 progenitor cells differentiated into osteoblasts in vitro using SF-PCL nanofibers with gene-activated exosomes as a matrix and vascularized osteogenesis induced in rats with calvarial defects | Solvent: Hexafluoroisopropanol | Topical therapy for bone defect transplantation enables flexible and stable grafting of tailored exosomes onto substrate biomaterials for carefully controlled local release and treatment [78] |
| Flow rate: 0.3 mm min−1 (push speed) | ||||
| Voltage: 15 kV (+ve) and 1 kV (−ve) | ||||
| Exosome diameter: 111.4 nm | ||||
| Chitosan (CS)-PLA coaxial structure modified with biotin | ATDC5-derived. Loaded with recombinant VEGF165 plasmid | CS-PLA nanofibers modified with gene encapsulating exosomes and used for local delivery of VEGF and vascularized bone regeneration in rat cranial defect | Solvent: Trifluoroacetic acid, Glacial acetic acid and Hexafluoroisopropanol | Gene-activated matrix for VEGF gene delivery for vascularized osteogenesis in vivo [28] |
| Exosome diameter: 80–200 nm | ||||
| Flow rate: 0.1 mm min−1 (Chitosan) and 0.04 mm min−1 (PLA) | ||||
| Voltage: 15 kV (+ve) and 1.5 kV (−ve) | ||||
| Polyurethane-collagen | ADSC-exosomes | To accelerate heart repair, adipose-derived stem cell exosomes (ADSC-EXO) were added to an oxygen-releasing antioxidant nanofibrous bi-layered cardiac patch (PUAO-CPO-Collagen). | Solvent: Tetrahydrofuran and Dimethylformamide | Cardiac patch for promoting heart repair in myocardial infarction [100] |
| Voltage: 18 kV | ||||
| PCL | Human AT-MSC-derived | MCF-7 breast cancer cells cultured on the surface of electrospun sheets containing exosome encapsulated PCL nanofibers, and viability, proliferation, and expression of apoptotic and anti-apoptotic genes were studied. | Solvent: Chloroform/ Dimethylformamide | Using human AT-MSCs-derived exosomes for anticancer effect on breast cancer cells by induction of apoptosis [101] |
| Exosome diameter: 500–1000 nm | Flow rate: 0.53 ml h−1 | |||
| Voltage: 24 kV | ||||
| SF | Human BMMSCs' exosomes (HBM-MCS-Exo) | HBM-MCS-Exo mixed in alginate solution poured over the nanofibrous mat and allowed to gel | Solvent: Formic acid | Novel wound dressings with HBM-MCS-Exo added to SF-Alginate scaffolds [102] |
| Flow rate: 0.75 mm h−1 | ||||
| Exosome diameter: 24.8 nm | Voltage: 20 kV |
Su et al further designed scaffolds capable of inducing and guiding appropriate immune responses by immobilizing mesenchymal stromal exosomes onto electrospun fibrous polyester materials allowing cell-mediated delivery of membrane-bound vesicles [92]. Wang et al showed that adipose-derived stem cells' exosomes along with collagen/ poly (L-lactide-co-caprolactone) nanoyarn scaffold could help in regeneration in case of urethral defects [24]. In all these studies, exosomes were immobilized onto the nanofibrous scaffolds post-electrospinning. Because nanofiber generation via electrospinning occurs in an electrical field, the stability of exosomes at such a high voltage is an inherent challenge when attempting to combine the two approaches. Recently, a study demonstrated that exosome mixed in polyvinylpyrrolidone (PVP) solution were electrospun at a flow rate of 1 ml h−1, at an applied voltage of 8 kV did not affect potency and functionality of MSC-EVs [58]. However, with limited characterization of exosome loaded fibers, it is difficult to comment on membrane integrity of exosome during the electrospinning process. Wang et al have reported formation of electrospun nanoyarn using coaxial method with ADSC-derived exosomes in the core region protected with collagen poly (L-lactide-co-caprolactone) (Col/P(LLA-CL)). ADSC-derived exosomes were mixed in different v/v ratio (0%, 25%, 50%, 75%) in Col/P (LLA-CL) solution prepared in 2, 2, 2-trifluoroethanol as the core layer solution and Col/P(LLA-CL) dissolved in 2, 2, 2-trifluoroethanol as shell layer electrospun at 15 kV with 0.2 ml h−1 and 0.8 ml h−1 flow rate respectively [24]. Formation of core-shell structure was confirmed by transmission electron microsopy whereas mechanical and in-vitro studies showed ADSC-exos Col/P(LLA-CL) nanoyarn scaffold supported sufficient biocompatibility and mechanical properties to promote cell proliferation and tissue regeneration in urethral defects. Ping et al showed exosomes and TGF-β3 could be electrosoun together to form nanofibrous patch for acute myocardial infarction therapy in a rat model. The encapsulation of recombinant TGF-β3 and umbilical cord-derived mesenchymal stem cells (HUC-MSCs) derived exosomes in PCL/Col-1 was achieved by blending them together in an aqueous solution of PCL/Col-1 in acetic acid (90% v/v) and electrospun at 600 rpm for 5 h with 12.5 kV [93]. Recently, Kong et al reported application of meltelectrowriting (integration of electrospinning with 3D printing) in a composite patch made up of PCL and exosome loaded GelMA [30, 94]. Exosome used in this study were derived from electrospun coaxial microfibers encapsulated ADSCs using alginate-NaCl solution as shell stream which gels when mixed with CaCl2. The ADSC-exosome were mixed into GelMA solution before casting onto the electrostatic direct writing nanofiber membrane of PCL to obtain a 3D hydrogel mixed electrospun scaffold. This composite patch showed enhanced skin wound healing in rat model, a promising and effective strategy for clinical applications of therapeutic exosomes [30]. Recent studies supporting application of exosomes with electroconductive hydrogels highlight stable integration of exosome in nanofibrous structures without compromising hydrogel electrical activity and mechanical properties while maintaining therapeutic properties of the exosomes [63, 64, 95, 96]. Nanofibers have been explored for isolation, delivery, biosensing, bioimaging, preservation and spatial analysis of exosome [37, 97–99]. The interaction of these nanoscale materials (exosomes and nanofibers) has intrigued researchers to investigate their potential for clinical applications where electrospun nanofibers can be used for controlled release of exosomes.
The use of exosomes and electrospun nanofibers has demonstrated promising results in tissue engineering and regenerative medicine. However, the utility of exosomes in electrospun nanofiber research has not yet been fully realized. It is expected that solutions to the limitations hindering exosome research will be found, rendering the amalgamation of nanofibers and exosomes successful.
8. Suggested methods for incorporating exosomes in electrospun nanofibers
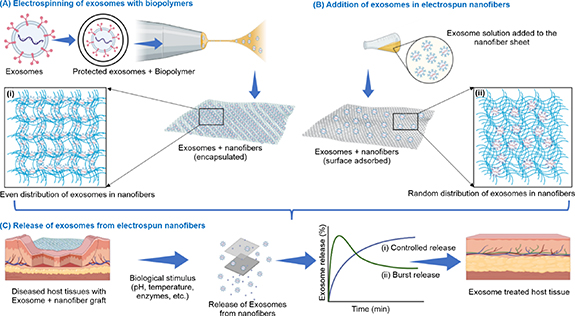
Electrospinning with exosomes is challenging but achievable. Recent studies have shown that nanofibers and exosomes can be amalgamated via two routes. The first involves mixing the exosomes with the polymer solution, followed by electrospinning, which requires a careful understanding of the electrospinning parameters that may interfere with the stability and functionality of exosomes [24, 93]. It also requires the safe integration of exosomes in the organic solvent mixture used for electrospinning to produce biologically functional and evenly distributed exosomes in the nanofibers (figure 4(A)). The second route involves surface-adsorption of the exosomes on the nanofibrous sheet after electrospinning. This method is relatively facile, and the exosomes are not exposed to a harsh electrospinning environment [29, 92, 103]. However, this strategy may lead to a random/non-uniform distribution of exosomes in the nanofibers (figure 4(B)). However, surface modified nanofibers could provide stronger attachment and controlled release profile of exosomes. Su et al showed conjugating PCL electrospun fibers with polyethylenimine (PEI) made them positively charged and enabled tethering of MSC derived exosomes [92]. Zha et al have prepared biotin conjugated exosome mimetic (EMs) by incubating ATDC5 cells in biotin functionalized 1,2-dioleoyl-sn-glycero-3-phos-phoethanolaminepoly (ethylene glycol)-2000 (DSPE-PEG-Biotin) media for 72 h. These biotin modified EMs were then conjuageted with biotin–avidin modified chitosan/poly-lactic acid (CS/PLA) electrospun core shell nanofibres. The biotin modification of CS/PLA nanofibers provided a better physical absorption of EMs and flexible covalent binding which promotes cellular uptake due to spatial elongation of EMs on nanofiber surface [28]. The attachment and distribution of exosomes in nanofibers would also affect their release profile in targeted tissues, where biological stimuli (changes in pH or temperature, systemic pressure, phagocytosis, or enzymatic degradation) would initiate the release of exosomes from nanofibers. The encapsulated and evenly distributed exosomes would show a more controlled release than the surface-adsorbed non-uniformly distributed exosomes in the nanofibers (figure 4(C)). It is important to ensure that exosomes maintain their structure and functionality when exposed to high voltage and solvent evaporation. The overall objective is to use the high surface area of the nanofibers to achieve a controlled and targeted release of exosomes. Because exosomes alone would be rapidly cleared from the body, the nanofiber scaffold is expected to protect these from rapid clearance and increase patient compliance. Thus, adopting the right strategy will ensure the successful merging of the nanofiber and exosome systems. Recent studies on both methods reported the initial findings as promising. Erfan et al reported silk fibroin/alginate structures with multifunctional exosomes for wound-dressing applications. The BMMSC-derived exosomes were mixed with an alginate solution, which was poured over the nanofibrous mat and allowed to gel. The exosome-containing nanofibrous sheets showed high cell viability and optimal swelling to absorb exudates and deliver exosomes to the wound site [102]. Trindade et al reported the electrospinning of PVP and exosomes isolated from fetal bovine serum (FBS) and MSCs. The exosome/phosphate-buffered saline (PBS) solution was suspended in PVP/trifluoroethane and electrospun at a flow rate of 1 ml h−1 and a voltage of 8 kV. The exosome structure and protein content were retained after electrospinning. Exosomes derived from both FBS and MSC were electrospun with no loss of potency. These findings are immensely promising because the encapsulation of exosomes in a polymer matrix enables their controlled release and offers greater therapeutic potential. The study also allowed exploration of the storage of exosomes in a solid medium that could be later reconstituted as per the desired application [58].
Figure 4. Schematic of amalgamation of exosomes with nanofibers and subsequent release; (A) electrospinning of exosomes (precoated/protected) with biopolymer using a suitable organic solvent at optimal voltage conditions would generate exosomes evenly distributed within nanofibers, (B) addition of exosomes to electrospun nanofibers by surface adsorption; incubating nanofibers in exosome solution would result in the random distribution of exosomes in nanofibers, and (C) release of exosomes, induced by biological stimulus, from electrospun nanofibers after grafting in diseased host tissues, either in (i) a controlled or (ii) burst release manner based on exosome attachment with nanofibers, to restore a healthy state of tissues.
Download figure:
Standard image High-resolution image9. Requisite process parameters
The parameters that play a major role in electrospinning include the concentration and viscosity of the solution, flow rate, voltage applied, type of solvent, humidity, and temperature. It is important to ensure that the exosome solution (prepared in PBS/water) and organic solvent are mixed in the appropriate ratio. This will ensure the integrity of the exosomes in a harsh organic environment. Trindade et al reported that FBS-and MSC-derived exosomes are stable in trifluoroethane [58]. The stability of exosomes in solvents such as ethyl acetate, dimethylformamide, tetrahydrofuran, methyl ethyl ketone, and 1,2-dichloroethane also needs to be determined because these comprise the extensively used solvent systems in electrospinning. Another important factor to consider is the applied voltage; a lower voltage (approximately 5 kV) is expected to allow the safe integration of exosomes into nanofibers. Further, a lower voltage would ensure that the morphology of the exosomes is intact in the solution that is transformed into nanofibers. Thus, spinning with exosomes is certainly possible and scalable, but it requires a systematic understanding of electrospinning concepts and intelligent execution to achieve a stable exosome-nanofiber combination product.
10. Characterization of exosome-loaded electrospun nanofibers
The characteristics of polymeric nanofibers are well-established and understood in detail. However, methods for understanding the stability of EVs in nanofibers are still being explored and developed. Recently, Németh et al encapsulated mid-size EVs (mEVs) in PVP-based nanofibers [25]. The presence of EVs in the nanofiber sheet and their stable interaction with PVP were confirmed via TEM, flow cytometry, and confocal laser scanning microscopy. According to their results, a PVP shell can be created to enclose medium-sized EVs. The presence of the PVP shell makes CD81 and phosphatidylserine less accessible to antibodies, and annexin V. Medium-sized EVs are more stable because of PVP and, as a result, less sensitive to Triton X-100 lysis. EVs were detected in the nanofibers using green fluorescent protein (GFP) fluorescence. Nanofibers with mEVs were stored at 4 °C for 12 weeks. Interestingly, GFP-positive mEVs were detected throughout the storage cycle at both temperatures. In another study by Rezaie et al, MSC-derived MVs were incorporated into PCL nanofibers, and the morphology and biocompatibility of the fabricated nanofibrous structures (PCL and PCL-MVs) were confirmed by scanning electron microscopy and MTT assay, respectively [101].
Conventionally, EVs are stored at −80 °C in lyophilized or non-lyophilized form for clinical application [104]. Electrospinning offers a viable alternative to increase the storage stability of vesicles. It is rapid, economical, scalable, and capable of forming intricate nanofibrous structures. Vesicles can be stored in the solid phase while maintaining their stability when embedded in nanofibers. Storage of EVs at different temperatures can open new research horizons and expand their therapeutic applications.
11. Commercial electrospun matrices and their suitability as exosome delivery systems
Electrospun nanofibers have garnered considerable attention in clinical research owing to their biocompatibility, adhesiveness, sterile nature, and diverse applications. Globally, nanofibers have been widely explored as scaffolds for wound dressing, drug delivery, filtration, and catalysts for reduction [23]. Commercially available products, using electrospun nanofibers for providing cell culture-related solutions in vitro, include 3D insert™ (3D Biotek, NJ, USA), Bio-Spun™ (BioSurfaces Inc., MA, USA), Cytoweb® Sheets (Espin Technologies, TN, USA), Mimetix® scaffold (AMS Biotechnology, Milton, UK), Nanofiber Solutions™ (Nanofiber Solutions, OH, USA), nanofibrous biomaterials (NanoSpun Technologies, CA, USA), Retissue™ (Medprin Biotech GmbH, Frankfurt, Germany), and tabular and disc scaffold (SKE Research Equipment, Milan, Italy). These products comprise a single biopolymer such as PLA, SF, PCL, Poly(L-lactide-co- -caprolactone), PLGA, or Polyacrylonitrile, or a combination of biopolymers electrospun onto nanofibrous mats suited to perform specific roles, such as acting as a culture substrate or insert for cell growth in aligned fibers or mimicking ECM in cell adhesion and proliferation Fadil et al [106]. Companies have also extended the use of electrospun matrices for clinical applications; Absorv™ and Aeos™ (Zeus Industrial Products, Inc., SC, USA, www.zeusinc.com/) are nonwoven nanofibers made up of bioresorbable polymers for use in medical devices and sutures whereas HealSmart™ (PolyRemedy), Neotherix scaffold (Neotherix), and SpinCare™ (Nanomedic Technologies) as wound dressings have shown improvement in healing outcomes as compared to regular dressing materials [105]. Other companies have developed implantable medical devices; AVflo™ vascular access graft (Nicast Ltd) made up of polycarbonate urethane nanofibers, and Medprin Biotech GmbH utilizes StypCel™ as an absorbable hemostat (Ferraresso, Bortolani and Amnon, 2016). Nanofibers have also found their way as synthetic dural substitutes in NeoDura™ and ReDura™ (Medprin Biotech GmbH), PK Papyrus® covered coronary stent (Biotronik), and ResQFoam™ (Arsenal Medical) for intracavity hemorrhage treatment [106]. All of these are polymeric nanofibers providing immediate mechanical support by acting as a barrier for wound closure on tissues or as a protective covering for devices. Researchers have attempted to develop nanocomposites utilizing the porous structure of nanofibers for the controlled release of immobilized or embedded anticancer drugs and have achieved varying levels of success in vitro and rat models [23]. This development can be exponentially enhanced by adding therapeutic value to the nanofiber matrices. A thorough understanding of cellular engineering and nanofiber technology is required to selectively modify nanofibers by introducing engineered exosomes. Exosomes are expected to enhance the regenerative and therapeutic potential of the technology, whereas the high surface area offered by nanofibers would contribute to efficient encapsulation and localized, sustained, and targeted delivery of exosomes with increased residence time at the required site. This would also reduce multiple invasions at the disease site as well as the chances of infection and thus increase the success rate of surgery or treatment.
-caprolactone), PLGA, or Polyacrylonitrile, or a combination of biopolymers electrospun onto nanofibrous mats suited to perform specific roles, such as acting as a culture substrate or insert for cell growth in aligned fibers or mimicking ECM in cell adhesion and proliferation Fadil et al [106]. Companies have also extended the use of electrospun matrices for clinical applications; Absorv™ and Aeos™ (Zeus Industrial Products, Inc., SC, USA, www.zeusinc.com/) are nonwoven nanofibers made up of bioresorbable polymers for use in medical devices and sutures whereas HealSmart™ (PolyRemedy), Neotherix scaffold (Neotherix), and SpinCare™ (Nanomedic Technologies) as wound dressings have shown improvement in healing outcomes as compared to regular dressing materials [105]. Other companies have developed implantable medical devices; AVflo™ vascular access graft (Nicast Ltd) made up of polycarbonate urethane nanofibers, and Medprin Biotech GmbH utilizes StypCel™ as an absorbable hemostat (Ferraresso, Bortolani and Amnon, 2016). Nanofibers have also found their way as synthetic dural substitutes in NeoDura™ and ReDura™ (Medprin Biotech GmbH), PK Papyrus® covered coronary stent (Biotronik), and ResQFoam™ (Arsenal Medical) for intracavity hemorrhage treatment [106]. All of these are polymeric nanofibers providing immediate mechanical support by acting as a barrier for wound closure on tissues or as a protective covering for devices. Researchers have attempted to develop nanocomposites utilizing the porous structure of nanofibers for the controlled release of immobilized or embedded anticancer drugs and have achieved varying levels of success in vitro and rat models [23]. This development can be exponentially enhanced by adding therapeutic value to the nanofiber matrices. A thorough understanding of cellular engineering and nanofiber technology is required to selectively modify nanofibers by introducing engineered exosomes. Exosomes are expected to enhance the regenerative and therapeutic potential of the technology, whereas the high surface area offered by nanofibers would contribute to efficient encapsulation and localized, sustained, and targeted delivery of exosomes with increased residence time at the required site. This would also reduce multiple invasions at the disease site as well as the chances of infection and thus increase the success rate of surgery or treatment.
12. Future prospects and conclusion
The unique potential of exosomes in tissue repair and regeneration is increasingly being recognized, opening an expansive horizon for clinical applications. The inherent regenerative capabilities of exosomes are anticipated to rise significantly in the future, provided they are integrated with suitable carrier systems such as nanofibers. Electrospinning presents a promising technique for creating nanoscale and microscale polymer-based fibers capable of carrying a wide array of biological cargos, including exosomes, antimicrobial drugs, anti-inflammatory cytokines, proteins, and anticancer therapeutics, without compromising their bioactivity. The loading and delivery of exosomes from nanofibers can be meticulously controlled by regulating inherent parameters (solution viscosity, fiber size, and alignment) and external factors (charge, temperature, and pH). Whereas, surface modification of electrospun nanofibers can be a useful method to generate highly absorptive centers for exosome attachments, the lipid bilayer of exosome can be conjugated using hydrophobic PEG-phospholipids or exploring biotin–stravidin interactions.
Evidence from cardiac and neural tissue engineering exemplifies the substantial potential of biomolecule-loaded nanofibrous patches, and similar success has been noted with biomolecule-loaded electrospun fibers used for implantation in animal models. MSCs derived exosome encaspulated in biocompatible ECM mimetics along with growth factors have shown immense potential as cardiac regeneration therapy in case of acute myocardial infarction. Similarly, human endometrial stem cell derived exosomes encapsulated in core−shell nanofibers have shown significantly enhanced nerve regeneration of axons and improved the functional recovery of rat sciatic nerve defects, confirming potential of exosome-nanofibre as neural auotgraft. Therapeutic exosome-loaded nanofibers, fabricated using hydrophobic polymers, can help achieve sustained delivery over long periods. This ability to maintain a supportive microenvironment for tissue regeneration highlights the potential of nanofiber-exosome combinations to enhance cell attachment, proliferation, and differentiation, all of which are imperative for successful tissue regeneration. Such exosomes loaded nanofibers can be used as membrane or vasculature as channels for microfluidic based experiments to simulate the physiological microenvironment and understand the tissue response.
Nanofiber-exosome combinations can improve cell attachment, proliferation, and differentiation, which are imperative for tissue regeneration. Electrospun nanofibers can act as biodegradable scaffolds to provide stability, prolong shelf life, and facilitate the sustained release of exosomes to target tissues. The potential to localize the therapeutic impact by creating exosome-laden electrospun fibers that exhibit regulated release is particularly noteworthy. This review underscores the potential for merging exosomes with electrospun nanofibers to add an innovative dimension to current tissue engineering strategies. Following the suggested approaches exosomes can be efficiently loaded in the electrospun nanofibers mainitniang their stability and enhancing targeting ability. Drawing on evidence from both in vitro and in vivo studies, it is clear that this approach holds significant promise, offering a pathway to enhance the regenerative and therapeutic characteristics integral to advanced tissue engineering and regenerative medicine. Future research in this dynamic field is encouraged to fully realize the transformational potential of this technology.
Acknowledgments
The figures contained in this article were partially created with BioRender.com.
Data availability statement
No new data were created or analysed in this study.
Author contributions
R R and P A have contributed equally. Exosome sections were written by R R and electrospinning sections were written by P A and U B. Concept and outline for the review were carried out by R R and A C. Literature search was carried out by R R, P A and U B. T B and A C contributed in study design and supervised the project. The manuscript was written by R R, P A and U B with feedback from T B and A C. All the authors have approved the final version of the manuscript.
Funding sources
This research did not receive any specific Grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no competing financial interest.