Abstract
Preclinical biomedical and pharmaceutical research on disease causes, drug targets, and side effects increasingly relies on in vitro models of human tissue. 3D printing offers unique opportunities for generating models of superior physiological accuracy, as well as for automating their fabrication. Towards these goals, we here describe a simple and scalable methodology for generating physiologically relevant models of skeletal muscle. Our approach relies on dual-material micro-extrusion of two types of gelatin hydrogel into patterned soft substrates with locally alternating stiffness. We identify minimally complex patterns capable of guiding the large-scale self-assembly of aligned, extended, and contractile human and murine skeletal myotubes. Interestingly, we find high-resolution patterning is not required, as even patterns with feature sizes of several hundred micrometers is sufficient. Consequently, the procedure is rapid and compatible with any low-cost extrusion-based 3D printer. The generated myotubes easily span several millimeters, and various myotube patterns can be generated in a predictable and reproducible manner. The compliant nature and adjustable thickness of the hydrogel substrates, serves to enable extended culture of contractile myotubes. The method is further readily compatible with standard cell-culturing platforms as well as commercially available electrodes for electrically induced exercise and monitoring of the myotubes.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Skeletal muscle is the most abundant tissue in the body and is essential to human health beyond motility, not least to the endocrine and immune systems [1]. The ability to generate and maintain physiologically accurate models of human muscle is thus of fundamental value for basic and applied biomedical and pharmaceutical research. The primary functional unit of skeletal muscle is the multinucleated and elongated myofibers that in vivo span the entire length of the muscle, and thus range from multiple millimeters to tens of centimeters [1, 2]. The myofibers originate from the self-organization and fusion of myoblast into myotubes and maturation of these into adult myofibers. Drawing from this native mechanism, most in vitro methodologies for engineering models of native muscle rely on guiding and stimulating the fusion of myoblast into elongated myotubes, and embedding these in an environment that supports long-term culture and maturation of contracting tissues [3–8]. With the emergence of automated 3D printing tools, a fundamental question arises on how simple procedures are efficient in achieving these core aims.
Multifarious approaches have been proposed for engineering mammalian skeletal muscle tissues from myoblasts in vitro. These methods not only vary drastically in complexity and scalability, but also in the length, alignment and maturity of the myotubes they generate. In general, when skeletal myoblasts are differentiated into myotubes in vitro, locally aligned myotube domains form spontaneously [3, 9, 10]. In conventional planar tissue culture dishes, these domains typically span hundreds of micrometers, which restricts the length and size of the myotubes [9]. However, we recently found that on ultra-compliant gelatin hydrogel substrates with stiffness below physiological range, the size of such domains can increase considerably, leading to longer myotubes of improved maturity [10]. Compliant substrates can further support longer culturing and maturation times by avoiding the rapid myotube delamination observed in conventional rigid dishes [6, 10, 11]. Beyond modulating the mechanics and biochemistry of the culturing substrate, engineered micrometer-scale line patterns of extracellular matrix (ECM) proteins or molded ridges in gels have been introduced to generate highly aligned and extended myotubes [6, 11–13]. Often, these have been made manually using stamps derived from clean room-based soft lithography [6, 12, 13]. For generating free-standing 3D models of skeletal muscle, the most common methodology is to seed myoblasts in low density hydrogels derived from natural ECM sources, which the cells over time compact into denser tissue. Such engineered tissues are often attached to external micro-fabricated pillars or beams, that anchor the constructs and generate an anisotropic load which induces myotube alignment and maturation [8, 14, 15]. For instance, Khodabukus et al recently generated human engineered tissues with myotubes of high maturity and average lengths of ∼500–600 µm through electrically induced exercise [5].
Within recent years, micro-extrusion—or direct-ink-writing—3D printing has emerged as a new standard tool within in tissue modelling and tissue engineering. It offers unique opportunities for automated, multi-material micro-structuring of bio-materials [16, 17], soft instrumented tissue modelling devices [18], and bio-printing of cell-laden inks [19–23]. 3D bio-printed skeletal muscles bundles have been reported by several groups [23–27]. Interestingly, filamentous shape in itself has been found to induce cellular alignment on filament surfaces [27]. However, compared to the low-density, cell-laden hydrogels employed in traditional, cast 3D engineered muscle tissue, hydrogel bio-inks must simultaneously enable reliable extrusion. This can impose constraints on ink formulation and solid content, which ultimately can have a negative impact on the length and maturity of the generated myotubes, not least in the filament core. To overcome such challenges, Choi et al applied embedded printing in a support bath to generate extended and highly fused human myotubes, with and without co-printed endothelial cells as vasculature precursors [25].
Aiming to provide an automated and scalable methodology, we here describe a simple 3D printing procedure for generating and maintaining skeletal myotubes, illustrated in figure 1. As we previously found that myotubes self-assembled from myoblasts on gelatin hydrogels of sub-physiological stiffness spontaneously show increased length [10], we hypothesized that minimally complex structures would be sufficient for guiding myotube formation on soft gel substrates. Specifically, following the optimization of several gelatin-derived inks, we identify simple printable hydrogel patterns capable of guiding large-scale self-assembly of murine and human skeletal myoblasts (hSkMs) into anisotropic and contractile myotubes that can span several millimeters. We find that variations in gelatin substrate concentration and resultant mechanics on the length-scale of several hundred micrometers, is sufficient for inducing large-scale organization into linear or non-linear patterns. The procedure is thus rapid, can be applied on a range of substrates, is compatible with most bio-printer on the market, and relies solely on low-cost, generally available materials.
Figure 1. Myotube alignment guided by simplistic patterning of 3D printed gelatin. (a) When cultured in planar environments, myotubes form spontaneously and align only on a local scale, limiting their size, length and physiological relevance. When developed on patterned gelatin hydrogel substrates, myotubes align on a global scale, increasing their size, length, maximum culture time and physiological relevance. (b) Fluorescent microscope images of immunostained actin filaments of C2C12 myotubes differentiated for 7 d in common tissue culture dishes (top) and on patterned gelatin hydrogel substrates (bottom). False-color images indicate local myotubes orientation angle, as calculated from actin immunostain image. Scale-bars: 500 µm. (c) Methodology for generating patterned gelatin hydrogel substrates: two different gelatin inks are 3D printed sequentially on a macroscopic scale.
Download figure:
Standard image High-resolution image2. Results
2.1. Printed gelatin line-patterns of alternating stiffness generates anisotropic myotubes
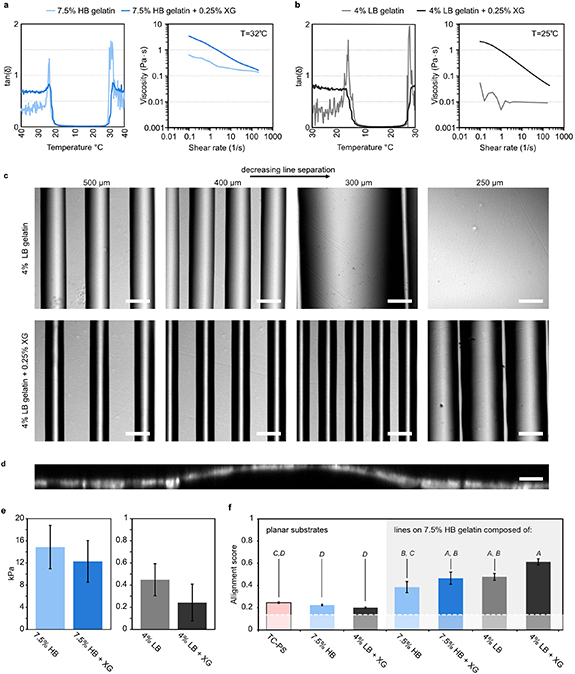
As basis for a controlled approach for directing the self-assembly of myotubes from myoblasts, we formulated two sets of gelatin inks, one of physiological and one of sub-physiological stiffness, see figures 2(a) and (b). For the first, we employed high-bloom (HB) gelatin at 7.5% (w/v) with and without 0.25% (w/v) xanthan gum (XG) as rheology modifier. These inks undergo sol-gel transition near room temperature at ∼24 °C, and so, to avoid clogging, we printed these using a slightly heated reservoir set at 32 °C, where they behave as shear-thinning liquids. For soft domains we employed, low-bloom (LB) gelatin at 4% (w/v) with and without 0.25% (w/v) XG. These inks must be cooled slightly to gel, and can therefore be extruded using room temperature reservoir. For the lower concentration, LB gelatin inks, XG prove highly beneficial for increasing viscosity and obtaining well-defined prints. Thus, when printing line-patterns of 4% LB gelatin onto 7.5% HB gelatin, the apparent line width decreases from ∼225 to ∼175 µm when adding 0.25% XG. Parallel lines can consequently be spaced more closely before they start to merge, see figure 2(c). Notably, the height of these LB gelatin lines are in the order of ∼20 µm, see figure 2(d). Hence, they do not constitute an impassable barrier. Following enzymatic crosslinking using microbial transglutaminase (mTG), the 7.5% HB gelatin gels had an apparent Young's modulus of ∼15 kPa, in the range of the physiological stiffness of skeletal muscle [11], while the stiffness of both 4% LB gelatin gels were well below 1 kPa, see figure 2(e).
Figure 2. Gelatin ink rheology defines print resolution and mechanical contrast induce myotube alignment. (a) Gelation curves and viscosity vs shear rate at 32 °C of 7.5% (w/v) HB gelatin with/without 0.25% (w/v) XG. (b) Gelation curves and viscosity vs shear rate at 25 °C of 4% (w/v) LB gelatin with/without 0.25% (w/v) XG. (c) Bright-field microscope images of 4% LB gelatin filaments printed onto 7.5% HB gelatin at gradually decreasing spacing using a 70 µm (inner diameter (ID)) nozzle. Without XG additives filaments merge at 300 µm spacing, while with XG additives more narrow filament are obtained, which start merging 250 µm spacing. Scale-bars: 200 µm. (d) Cross-sectional confocal image of C2C12 myotubes cultured on a 7.5% HB gelatin substrate with a 4% LB gelatin + 0.25% XG line pattern. Composite of immunostain of actin filaments and nuclei. Scale-bar: 20 µm. (e) Young's modulus of 7.5% HB gelatin and 4% LB gelatin with/without 0.25% XG, after crosslinking with 10 U ml−1 mTG. (f) C2C12 myotube alignment score (0–1) at differentiation day 7 on TC-PS, planar gel substrates, and 7.5% HB gelatin substrates with a top line-pattern spaced by 400 µm based on 7.5% HB gelatin or 4% LB gelatin with/without 0.25% XG. All error-bars indicate SEM (n = 3). Insert italic letters indicate outcome of a one-way ANOVA followed by pairwise Tukey's post hoc tests. Conditions that do not share a letter are statistically different (P < 0.05).
Download figure:
Standard image High-resolution imageWe next compared the degree of alignment of murine C2C12 myotubes generated from myoblasts on isotropic and patterned substrates. On uniform, planar substrates composed of either 7.5% HB gelatin or 4% LB gelatin + 0.25% XG, the degree of alignment matched that found in conventional tissue culture polystyrene (TC-PS) dishes. However, when introducing simple line patterns spaced by 400 µm onto printed 7.5% HB gelatin substrates, the degree of myotube alignment increased dramatically, see figure 2(f). Notably, while all line-patterned substrates displayed an increased alignment, we observed the highest numerical degree of alignment for line-patterns of 4% LB gelatin + 0.25% XG on 7.5% HB gelatin where the mechanical contrast between the regions is largest.
2.2. Defining tissue organization through minimally complex patterns
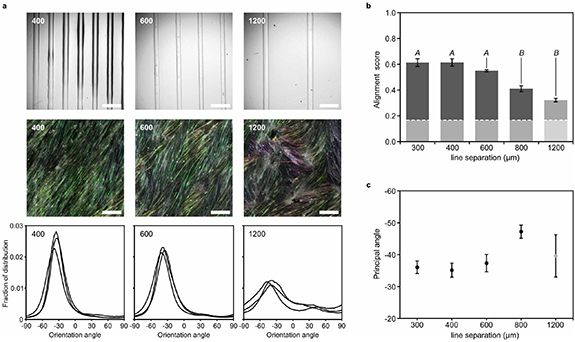
We next sought to identify the minimal spatial density of cues required for generating predictable myotubes architectures, when applying gelatin inks of contrasting stiffness. Using a conventional non-tapered 70 µm (ID) metal nozzle, we printed 4% LB gelatin + 0.25% XG lines at increasing spacing on printed 7.5% HB gelatin substrates and evaluated the degree of alignment of C2C12 myotubes developed on the substrates, see figure 3(a). For a spacing of 400 and 300 µm we observed a similarly high degree of alignment, which slightly decreased when the spacing was increased to 600 µm. When increasing the nominal line spacing to 800 and 1200 µm we observed a notable decrease in the degree of alignment, see figure 3(b). Interestingly, the primary angle of orientation appeared fixed at ∼36° from that of the printed lines for the aligned samples, and only changed, as global alignment was lost see figures 3(c) and 4(a). This angle of orientation was consistent across all samples, batches and all areas of the samples. To explore the consistency these largely self-organized architectures, we printed concentric circles of 4% LB gelatin + 0.25% XG on 7.5% HB gelatin, see figure 4(b). After myoblast seeding and maturation these pattern gave rise to a spiraling architecture of myotubes with almost perfect regularity, and locally consistent with a shift of ∼36° between the orientation of the LB gelatin lines and the myotubes orientation. This illustrates that within some design limitations, our method allows for creation of non-linear architectures with high degree of predictability.
Figure 3. Myotube alignment gradually decrease with gelatin line pattern spacing. (a) Top: bright-field microscope images of 7.5% HB gelatin substrates with 4% LB gelatin + 0.25% XG lines at spacing of 400, 600 and 1200 µm. Printed using a 70 µm (ID) nozzle. Middle: false-color images of C2C12 myotube orientation angle on each type of substrate. Derived from actin immunostain images at differentiation day 7. All scale-bars: 500 µm. Bottom: distribution of myotube angular orientations on each substrate (n = 3). (b) Alignment score (0–1) of C2C12 myotubes on 7% HB gelatin with 4% LB gelatin + 0.25% XG lines, as a function of line separation. All error-bars indicate SEM (n = 3). Insert italic letters indicate outcome of a one-way ANOVA followed by pairwise Tukey's post hoc tests. Conditions that do not share a letter are statistically different (P < 0.05). (c) Principal orientation angle of C2C12 myotubes on 7% HB gelatin with 4% LB gelatin + 0.25% XG lines on as a function of line separation.
Download figure:
Standard image High-resolution imageFigure 4. Non-linear myotube pattering. (a) Top: bright-field microscope images of 7.5% HB gelatin substrates with 4% LB gelatin + 0.25% XG linear patterns. Scale-bar: 500 µm. Bottom: false-color images of resultant C2C12 myotube orientation angle. Derived from actin immunostain images at differentiation day 7. Dotted lines indicate center position of line pattern. Angle between fiber and line orientation indicated. (b) Left: bright-field microscope images of 7% HB gelatin substrates with 4% LB gelatin + 0.25% XG in circular line patterns. Scale-bar: 500 µm. Right: false-color images of resultant spiraling C2C12 myotube orientation angle. Derived from actin immunostain images at differentiation day 7. Scale-bar: 1 mm.
Download figure:
Standard image High-resolution image2.3. Patterned gelatin substrates generate extended myotubes and enables long-term maturation
Achieving a high degree of myotube alignment is an important aspect of replicating the native in vivo architecture of skeletal muscle tissue. Still, it does not necessarily result in myotubes of improved physiological relevance, as reflected in the length, sarcomerogenesis and contractility. To investigate whether the increased organization observed on the printed substrates also had a positive influence on myotube length, we did an unbiased quantification of myotube length for C2C12 myotubes matured for 1 week on TC-PS, plain gelatin or gelatin line patterns composed of 4% LB gelatin + 0.25% XG on 7.5% HB gelatin, see figures 5(a) and (b). Doing so, we observed almost a doubling in mean myotube length from 476 ± 89 to 888 ± 164 µm, when comparing TC-PS to line-patterned gelatin, while on plain gelatin the mean myotube length was found to be 564 ± 68 µm, see figure 5(b). Similarly, while >30% of the myotubes on TC-PS, and >27.5% of the myotubes on plain gelatin had a length of 300 µm or less, only ∼10% of the myotubes developed on patterned gelatin substrates fell in this range, see figure 5(a). The average myotube width appeared smaller on both gel types as compared to TC-PS, though the differences was not statistically significant, see figure 5(c). The degree of myotube fusion as indicated by the fusion index and coverage of sarcomeric contractile proteins, were both the largest at ∼50% for the line-pattern substrates, though this could be artifact of incomplete staining and differences from plain gelatin and TC-PS were not statistically significant, see figures 5(d) and (e). In addition to an impact on myotube length, the compliant mechanics of gelatin hydrogel substrates enables longer culture times than rigid TC-PS, where we observed typically delamination after 1 week of differentiation. Increasing the maturation time to 3 weeks enables myotubes with a more mature contractile apparatus, as indicated by more clearly defined sarcomeres at a higher density, see figures 5(f) and (g). To verify the contractile function of the myotubes we applied a commercial electrical pacing system based on carbon electrode inserts. Electrical pacing can accelerate the maturation of myotube tissues, and the electrically induced contraction further serves as an indication of myotube fusion and sarcomerogenesis [5, 12, 28]. We found that pacing induced immediate contraction of the myotubes and verified that the myotubes could be paced for at least 1 week, see supplementary videos 1–3 (available online at stacks.iop.org/BMM/17/045013/mmedia). Lastly, to demonstrate that the printing procedure can be applied on a range of substrates we generated patterned gel coatings onto commercial multi-electrode systems for recording electrical signaling in tissue cultures. Doing so, we observed that the contraction-associated depolarization of myotubes was highly synchronized, further validating myotube fusion, see supplementary figure 1.
Figure 5. Printed gelatin pattern substrates increase myotube length and enables long term culture. Data describes C2C12 myotubes on differentiation day 7 unless otherwise noted. Pink: TC-PS substrates. Light grey: plain 7.5% HB gelatin. Dark grey: 7.5% HB gelatin with 4% LB gelatin + 0.25% XG linear patterns printed at 400 µm spacing. All error-bars indicate SEM. (a) Distribution of myotube lengths determined from phase-contrast microscope images. At least 180 myotubes measured from each condition in at least three separate seedings. (b) Average length of myotubes, separate seedings treated as independent n. (n ⩾ 3), insert italic letters indicate outcome of a one-way ANOVA followed by pairwise Tukey's post hoc tests. Conditions that do not share a letter are statistically different (P < 0.05). (c) Average width of myotubes, separate seedings treated as independent n. (n ⩾ 3) differences are not statistically significant. (d) Average fusion index, separate seedings treated as independent n. (n ⩾ 3) differences are not statistically significant. (e) Average percent of FOV stained positive for sarcomeric α-actinin, separate seedings treated as independent n. (n ⩾ 3) differences are not statistically significant. (f) Representative confocal images of C2C12 myotubes stained for sarcomeric α-actinin (white) and nuclear DNA (blue) at differentiation day 7. Left: on TC-PS. Middle: on plain 7.5% HB gelatin. Right: on 4% LB gelatin + 0.25% XG line pattern. (g) At differentiation day 21 on 7.5% HB gelatin with a 4% LB gelatin + 0.25% XG line pattern. Scale-bars: 50 µm.
Download figure:
Standard image High-resolution image2.4. Aligned and extended human myotubes generated on patterned gelatin substrates
While murine C2C12 myotubes can be useful as low-cost models, engineered human tissues naturally has more relevance for biomedical research. We therefore investigated whether the patterned gelatin would also affect the development of human myotubes. We thus seeded and matured primary human myoblasts into myotubes on three types of substrates: printed, line-patterned gelatin, isotropic gelatin and TC-PS substrates, see figure 6. Mirroring our findings for C2C12 myotubes, we observed a significantly larger degree of macroscopic orientation on the patterned substrates for human myotubes. We further wondered whether as similar self-organization would take place for other classes of engineered muscle tissues, not least cardiac. However, when seeding mouse primary neonatal ventricular myocytes at 150-, 200-, and 300.000 cells cm−2 on the patterned substrates with and without Matrigel coating, we did not observe any indications of macroscopic organization, see supplementary figure 3.
Figure 6. Printed gelatin pattern substrates generate extended and aligned primary human myotubes. Comparison of human myotubes derived from primary myoblasts developed on TC-PS, planar 7.5% HB gelatin substrates, and 7.5% HB gelatin substrates with 4% LB gelatin + 0.25% XG line patterns spaced by 400 µm. (a) Top: false-color images of myotube orientation angle derived from actin immunostain images at differentiation day 7. Scale-bars 500 µm. Bottom: distribution of myotube angular orientations on each substrate (n = 3). (b) Alignment score (0–1) of human myotube organizatoin on each substrate. All error-bars indicate SEM (n = 3). Insert italic letters indicate outcome of a one-way ANOVA followed by pairwise Tukey's post hoc tests. Conditions that do not share a letter are statistically different (P < 0.05).
Download figure:
Standard image High-resolution image3. Discussion
The ability to control the self-organization, alignment, and fusion of myoblasts into myotubes on a millimeter to centimeter scale is required for faithfully replicating the native architecture of skeletal muscle fibers. While it is well established that microscopic surface cues on a single cell level can direct the alignment and fusion of myoblasts into myotubes [6, 13], our results demonstrate that far cruder patterning of compliant gelatin substrates can lead to similar results. Thus, even on patterned substrates with the smallest feature size in the range of several hundred micrometers, a singular alignment spans uniformly across the full sample and multiple centimeters. As the vast majority of the myoblasts are exposed to a fully isotropic substrate, this alignment clearly depends on a cell-to-cell-induced self-organization. Further, the directed self-organization is induced by the local difference in substrate properties between the two types of gelatins, which only a subset of the myoblasts experience. Based on this, we propose that the small, but consistent and robust, angular shift seen between the myotube orientation and the direction of the printed patterns arises as compromise between initial preferential cellular elongation along the lines in the 7.5% HB region of the interphase, and across lines in the 4% LB gelatin region. While this mechanism is efficient for organizing skeletal myotube cultures, our studies herein indicate that similarly patterned substrates do not induce global alignment of cardiac cells. However, we cannot exclude that further tuning of the conditions for cardiac cell seeding indeed may unravel organization of heart cells on similarly patterned substrates as well in the future. Currently, we though propose either that the fusion of the myoblast is essential to achieving global organization from sparse cues, or that the myoblasts simply are more susceptible to minute cues in the surrounding, including from neighboring cells, than the primary neonatal mouse cardiomyocytes we studied here. Indeed, 3D printed micro-grooves with smaller feature sizes have previously been used to generate anisotropic cardiac tissues from primary rat- and human stem cell-derived cardiomyocytes. For instance, Lind et al 3D printed micro-grooved silicone substrates with features sizes of ∼50 µm to generate anisotropic cardiac tissues derived from human induced pluripotent stem cells, within instrumented microphysiological devices [18]. However, small features increase the print times and the requirements for 3D printing equipment, especially when applied for larger areas. As an alternative approach, Tijore et al 3D printed impenetrable barriers of gelatin onto gelatin-coated substrates [29] to create ∼200 µm wide micro-channels as means of locally aligning line-tissues of cardiomyocytes derived from human mesenchymal stem cells. Outside muscular tissues, prior work has also shown that printed topographical cues can also serve to align neurons [30]. Compared to these strategies, our approach simply relies on altering substrate mechanics on a large length scale of several hundred microns.
As a protocol, our findings are of interest as a simple way to obtain aligned and elongated human or mouse myotubes on deformable gelatin substrates. We have previously shown that extended culture on soft gelatin substrates improves contractile maturity of the myotubes as e.g. reflected in an increased expression of myosin heavy chain [10]. Our approach may thus serve as an alternative to bioprinted, cast or instrumented engineered muscle [4, 18, 23].
4. Conclusion
Our results demonstrate that macroscopically patterned gelatin hydrogel substrates generated by extrusion 3D printing are sufficient for directing the large-scale self-assembly of myoblasts into aligned and extended myotubes. The myotubes easily span several millimeters and can be matured into contractile fibers by extended culturing without delamination, due to the compliant nature of the substrates. Our work may thus serve as a simple, automated, and scalable method for engineering physiologically relevant models of murine and human skeletal muscle, for basic biomedical and pharmaceutical research. Of importance in this regard, the method requires no specialized or costly materials, can be implemented on diverse culturing platforms, and since high-resolution printing is not required, it is compatible with most low-cost micro-extrusion 3D printers.
5. Materials and methods
5.1. Ink preparation and 3D printing procedure
HB gelatin (G2500, Sigma-Aldrich), LB gelatin (48723, Sigma-Aldrich) and XG (G1253, Sigma-Aldrich) were used for ink preparation. All inks were prepared in sterile conditions. For a stock solution, 1% w/v XG was dissolved in PBS (D8537, Sigma-Aldrich) at 80 °C and stirred sporadically until the mixture is homogeneous. Stock solution was kept at 4 °C maximum 3 days before printing. Stock was diluted 1:3 with DMEM (D5796, Sigma-Aldrich) for a final 0.25% XG concentration before gelatin addition. All inks were prepared by freshly dissolving gelatin in DMEM (with/without XG) at 45 °C for 45–60 min. LB gelatin was dissolved at a 4% w/v ratio, HB gelatin was dissolved at a 7.5% w/v ratio. A cross-linking solution composed of 10 U ml−1 mTG (ACTIVA® TI transglutaminase, 100 U g−1, 1002) solution was prepared in DMEM. The mTG solution was sterile-filtered and kept cooled on ice until usage.
5.2. 3D printing procedure
A RegenHU 3D Discovery bioprinter was used for printing patterned gelatin substrates under a biosafety cabinet. Once the inks were dissolved at 45 °C, they were loaded on the cartridges and equilibrated approximately 1 h before the print. A 7.5% HB gelatin (with/without XG) was printed from a temperature-controlled cartridge set to 32 °C using volumetric dispensing. A 4% LB gelatin (with/without XG) was printed with a cartridge at RT (20 °C–23°C) using pneumatic dispensing. Unless otherwise noted, metal nozzles with a nominal inner diameter of 70 µm (Cellink Swe) were used. Six-well tissue culture plates were used as printing substrates and further cell culture. In general, an unstructured, homogeneous, layer of gelatin of ∼50 µm thickness was first printed using a meander-pattern with a line-spacing of 200 µm and a nominal nozzle height of 50 µm. On top of this flat layer, line-patterns of a second gelatin was printed at a nominal nozzle height of 70 µm. After printing, the plates were kept on ice for 15 min to ensure complete gelation. Chilled mTG cross-linking solution was added on the prints (1 ml well−1) and the plates were kept on ice for an additional 15 min before 1 h incubation at 37 °C. After incubation, the prints were washed with PBS. Plates were kept at 4 °C in PBS maximum 3 days until the cell seeding.
5.3. Rheological studies
The rheology of each ink was analyzed using a Discovery Hybrid Rheometer (TA instruments, DE, USA) equipped with a Peltier plate thermal controller and a plate geometry with a diameter of 40 mm and a fixed gap of 1 mm. All inks were freshly prepared in PBS before measuring. Amplitude sweep experiments were conducted to calculate the Young's modulus. The measurements for 4% LB gelatin ± 0.25% XG, and 7.5% HB gelatin ± 0.25% XG, were performed at 25 °C and 37 °C, respectively. For this, stock solutions of the inks with the following concentrations were prepared and mixed 1:1 with a 20 U ml−1 mTG solution in PBS: 8% LB gelatin, 8% LB gelatin + 0.5% XG, 15% HB gelatin, 15% HB gelatin + 0.5% XG. The inks were mixed with mTG solution by pipetting up and down three times and depositing the mixed gel on the peltier plate of the rheometer. The plate geometry was lowered to 1 mm and the ink kept at 15 °C for 10 min to allow for physical gelation of the gelatin ink. Afterwareds, the gel was heated to 37 °C to allow chemical cross-linking for 1 h. This procedure was repeated three times for each ink composition. The storage modulus G' and loss modulus G'' were recorded as a function of the oscillation strain (0.1%–200%) at a fixed frequency of 1 Hz at 25 °C and 37 °C. The Young's modulus E was calculated solely for the samples recorded at 37 °C as follows: E = 2G' (1 + ν).
With G' being the storage modulus in the linear viscoelastic regime and ν being the Poisson's ratio. Here, we assumed a perfectly incompressible material with a Poisson's ratio of 0.5. Flow sweep experiments were conducted at the respective printing temperature, 4% LB gelatin ± 0.25% XG at 25 °C, and 7.5% HB gelatin ± 0.25% XG at 32 °C. The inks were first equilibrated at the respective temperature for 30 s prior to measuring. The viscosity of the inks was recorded as a function of the shear rate from 0.1 to 200 s−1. Gelation curves were recorded from 45 °C to 15 °C and from 15 °C to 45 °C with a soak time of 30 s at a rate of 1 °C min−1. The strain and frequency were fixed to 1% and 1 Hz, respectively. G' and G'' were recorded as a function of temperature, the gelation point was determined by plotting tan(δ) as a function of temperature.
5.4. C2C12 murine myoblast culture and differentiation into myotubes
C2C12 myoblasts were cultured using standard sterile technique and incubator (37 °C, 100% humidity, 5% CO2). Prior to seeding on gel substrates, myoblasts were maintained in a tissue culture flask in C2C12 growth medium; DMEM (D5796, Sigma-Aldrich) with 10% fetal bovine serum (S1810, Biowest) and 1% Pen/Strep (P0718, Sigma-Aldrich). The culture was passaged at ∼80% confluence. The C2C12 cells were seeded on hydrogel prints at a density of 10 000 cells cm−2. The cultures were allowed to proliferate in C2C12 growth medium reaching ∼90% confluence before initiation of myotube formation. The culture was rinsed with PBS (D8537, Sigma-Aldrich) and C2C12 differentiation medium formulated as DMEM with 2% horse serum (H1270, Sigma-Aldrich) and 1% P/S was added. C2C12 differentiation medium was changed three times per week, unless cells were electrically stimulated.
5.5. Human myoblast culture and differentiation into myotubes
hSkMs (CC-2580, Lonza) were cultured following the manufacturer's instructions. Cells were maintained in skeletal muscle growth medium formulated as SkGM™-2 BulletKit™ Medium (CC-3245, Lonza). The culture was passaged upon reaching ∼60% confluence. The hSkMs were seeded on freshly prepared hydrogels at a density of 10 000 cells cm−2. The cultures were allowed to proliferate in skeletal muscle growth medium reaching 70% confluency before initiating myotube formation. Once sufficiently confluent, the culture was rinsed with PBS (D8537, Sigma-Aldrich) and switched to skeletal muscle differentiation medium formulated as DMEM:F12 (D6421, Sigma-Aldrich) with 2% horse serum (H1270, Sigma-Aldrich) and 1% P/S (P0718, Sigma-Aldrich). Differentiation medium was changed three times per week.
5.6. Electrical stimulation of myotube cultures
Following myotube formation and further maturation (differentiation day 14/pacing day 0), C2C12 cells were paced using a MyoPacer Field stimulator and a six-well C-dish carbon electrodes (IonOptix). The pulse generator was set for a constant 1 Hz frequency with 2 ms pulse duration with 11.5 V field potential. The cells were stimulated for 7 days (until differentiation day 21/pacing day 7). The medium was changed daily during the stimulation period. The pacing videos were recorded after daily medium change. Only for video recording purposes at pacing day 0 and pacing day 7 the frequency was set also with 0.5 and 2 Hz for shortly (max. 20 s) with the same pulse duration and field potential.
5.7. Immunostaining and microscopy
C2C12 myotubes were fixed in 4% paraformaldehyde in PBS, rinsed and permeabilized with 0.1% Triton-X in PBS. The fixed samples were incubated in 1% BSA in PBS blocking solution with 1:400 sarcomeric α-actinin monoclonal antibody (MA1-22863, Invitrogen) at room temperature for 4 h on a shaker. 1:200 Alexa Fluor Plus 488 conjugated goat anti-mouse IgG secondary antibody (A32723, Invitrogen) was applied together with 1:400 Alexa Fluor™ 647 conjugated phalloidin (A22287, Invitrogen) for F-actin staining in blocking solution overnight at 4 °C on a shaker. 4ʹ,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (62247, Thermo Scientific) was used as nuclear stain. Stained samples were then washed with PBS. The samples were imaged either in liquid mounting medium (50% Glycerol in PBS) or with ProLong™ Gold Antifade (P10144, Molecular Probes). Fluorescent images were acquired with Nikon Eclipse Ti2 microscope and NIS-Elements software. The bright field images were acquired with Zeiss Observer Z1 microscope with a mounted Zeiss AxioCam.
5.8. Myotube alignment quantification
Myotube alignment analysis were run on ImageJ plugin Orientation J [31] based on F-actin staining at differentiation day 7. The false color images were obtained by using the analysis tool color-survey where the hue and the saturation corresponds to the orientation angle and the coherency, respectively. The data obtained from the distribution tool was used to calculate the alignment score. Briefly, for each image, angular fraction of distribution was plotted. The angle with maximum fraction of distribution corresponds to the principal orientation angle. The total fraction of distribution within the frame of ±15° around the principal orientation angle corresponds to the alignment score.
5.9. Myotube length quantification
Myotube lengths were calculated similar to Jensen et al using bright field images [10]. In ImageJ, nine random counting windows (275 × 275 mm2 each) were drawn on each image in a fixed pattern (1775 mm separation in a 3 × 3 positioning, with the central counting window being in the center of the image). The length of all the myotubes passing through the random counting windows were measured manually.
5.10. Myotube width, contractile protein and fusion index quantification
A 0.4 mm2 of areas were imaged for alpha-actinin, phalloidin and DAPI staining. For each condition, three replicates were imaged at ten different areas in total. Among ten image per condition, three images were randomly selected for further analyses. Contractile protein expression: Actinin positive area was selected on alpha-actinin stained images by Huang's fuzzy thresholding method on ImageJ. Selected area over total area was calculated as actinin positive area percentage (n = 3 per condition). Myotube width: The average myotube width was calculated on phalloidin stained images (>20 myotubes per condition) on ImageJ by measuring membrane to membrane distance.
Fusion index: number of nuclei were counted in alpha-actinin and DAPI composite images (>1200 nuclei per condition). The fusion index was calculated as a percentage of the number of nuclei located in the alpha-actinin stained myotubes (with more than two nuclei per myotube) over the total number of nuclei in the field of view.
5.11. Statistical analysis
Statistical analysis was conducted using Origin Pro 2021. The data were analyzed for normality using Saphiro–Wilk test, and analyzed using one-way ANOVA followed by Tukey's post hoc tests for multiple comparison, p-values <0.05 were regarded as statistically different.
Acknowledgments
S D Cakal, C Radeke and J U Lind would like to gratefully acknowledge the European Commission MSCA-IF (798820), the Lundbeck Foundation (R250-2017-1425) and the Independent Research Fund Denmark (8048-00050) for their support. D G Ellman and D C Andersen gratefully acknowledges the Independent Research Fund Denmark (8045-00019B) and the Lundbeck Foundation (R313-2019-573).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).