Abstract
The aim of our research was to study the behaviour of adipose tissue-derived stem cells (ADSCs) and vascular smooth muscle cells (VSMCs) on variously modified poly(L-lactide) (PLLA) foils, namely on pristine PLLA, plasma-treated PLLA, PLLA grafted with polyethylene glycol (PEG), PLLA grafted with dextran (Dex), and the tissue culture polystyrene (PS) control. On these materials, the ADSCs were biochemically differentiated towards VSMCs by a medium supplemented with TGFβ1, BMP4 and ascorbic acid (i.e. differentiation medium). ADSCs cultured in a non-differentiation medium were used as a negative control. Mature VSMCs cultured in both types of medium were used as a positive control. The impact of the variously modified PLLA foils and/or differences in the composition of the medium were studied with reference to cell adhesion, growth and differentiation. We observed similar adhesion and growth of ADSCs on all PLLA samples when they were cultured in the non-differentiation medium. The differentiation medium supported the expression of specific early, mid-term and/or late markers of differentiation (i.e. type I collagen, αSMA, calponin, smoothelin, and smooth muscle myosin heavy chain) in ADSCs on all tested samples. Moreover, ADSCs cultured in the differentiation medium revealed significant differences in cell growth among the samples that were similar to the differences observed in the cultures of VSMCs. The round morphology of the VSMCs indicated worse adhesion to pristine PLLA, and this sample was also characterized by the lowest cell proliferation. Culturing VSMCs in the differentiation medium inhibited their metabolic activity and reduced the cell numbers. Both cell types formed the most stable monolayer on plasma-treated PLLA and on the PS control. The behaviour of ADSCs and VSMCs on the tested PLLA foils differed according to the specific cell type and culture conditions. The suitable biocompatibility of both cell types on the tested PLLA foils seems to be favourable for vascular tissue engineering purposes.
Export citation and abstract BibTeX RIS
This article was updated on 21 October 2021 to correct an error in the header of table 1.
1. Introduction
Cardiovascular diseases have a high incidence in many countries, and are responsible for almost one third of all deaths worldwide. Atherosclerosis is a very frequent degenerative disorder of the blood vessels, characterized by the impaired function of endothelial cells (ECs) leading to abundant storage of low-density lipoprotein particles. Non-invasive treatment of atherosclerosis usually involves adjusting nutrition habits and medication. However, when there is excessive vessel degeneration, surgical vascular replacement often becomes necessary.
Current vascular replacement options include biological and synthetic vascular grafts. Biological grafts are mainly represented by autologous grafts. Allografts and xenografts are less routinely used. Autologous grafts can be harvested from arteries (e.g. from arteria thoracica interna or arteria radialis) or from superficial veins (e.g. vena saphena). These grafts are widely used. They are the ideal choice for small-diameter vascular replacements (i.e. <5 mm), which are associated with a high risk of thrombosis (for a review, see Carrabba et al 2018). Although autologous grafts provide ideal properties, the opportunities for harvesting a vessel graft in good condition and of sufficient size are very limited, and the required surgery can increase patient morbidity. Unmodified allografts or xenografts can evoke an immunogenic response in the host organism. However, procedures for decellularizing these grafts seem to be an interesting approach to the use of a low-immunogenicity scaffold composed of natural extracellular matrix (ECM), which can be seeded with the patient's autologous cells. Nevertheless, the unavailability of suitable biological grafts continues to intensify the demand for novel synthetic vascular grafts.
For decades, synthetic scaffolds for clinically-used vascular grafts have been made of non-degradable polyethylene terephthalate or polytetrafluorethylene (for a review, see Chlupac et al 2009). These materials are non-toxic per se, but they are hydrophobic and bio-inert, with a limited bioactive capacity to somehow remodel or support the cell–material interaction. New biodegradable natural or synthetic compounds have therefore been studied with a view to improving the properties of the graft (i.e. by improving the mechanical properties of the graft, by supporting cell attachment and growth, by finding ways to avoid activating the blood coagulation cascade and thrombosis formation, or by improving the gradual controlled bio-degradation of the biomaterial) (Chlupac et al 2009). The list of promising compounds includes various forms of polyurethanes, polycaprolactone, poly(L-lactide) (PLLA), poly(glycolic acid), polyvinyl alcohol, alginate, chitosan, and some copolymers of these compounds, such as poly(DL-lactic acid-co-glycolic acid) (PLGA) (for a review, see Pashneh-Tala et al 2016, Hielscher et al 2018). These polymers can be used in various ways to create mono-, bi- or tri-layer scaffolds (for a review, see Goins et al 2019). In addition, the biomaterial itself can be improved with the use of various natural components and coatings (e.g. collagen, fibrin, fibronectin, laminin or dextran (Dex) coating), which can mimic the naturally present ECM and other molecules, and can support the adhesion, growth and differentiation of vascular cells (Goins et al 2019, Pashneh-Tala et al 2016, Filová et al 2014). Moreover, various growth factors (e.g. VEGF, FGF2, PDGF), biologically active molecules (e.g. heparin, Dex, sirolimus, simvastatin), and various amino acid sequences, which serve as ligands for cell adhesion receptors, e.g. RGD, can be incorporated into the coatings in order to ameliorate the bioactivity of the grafts (for a review, see Strobel et al 2018). Last but not least, scaffold-free approaches suggest the use of cell-sheets only (i.e. confluent layers of cells to create appropriate tissue-engineered vascular grafts (TEVGs) (Carrabba and Madeddu 2018).
PLLA is a semi-crystalline polyester with a wide range of biomedical applications thanks to its biocompatibility, its relatively slow biodegradability and high mechanical strength (for a review, see Gritsch et al 2019). PLLA can be fabricated into scaffolds of various shapes and with various properties (e.g. fibres, membranes, films, foils). However, the material is relatively highly hydrophobic. This property can restrict the biomedical applications of PLLA, due to the impaired cell–material interactions (Gritsch et al 2019).
Plasma treatment is generally used to modify the chemical and physical properties of polymers. Various gases, e.g. air, oxygen (O2), argon (Ar), nitrogen (N2), NH3, C3F8, can be applied as plasma sources to create reactive functional groups on an originally inert biomaterial. These functional groups can enhance the attachment of other biomolecules, such as peptide molecules or natural polymers. In addition, these changes reduce the water contact angle (CA), i.e. they increase the wettability of the material, which is known to affect the subsequent cell adhesion, morphology, viability or growth (Wan et al 2003, Nakagawa et al 2006).
Polyethylene glycol (PEG) is a hydrophilic polymer without or with low levels of toxicity and immunogenicity, which is advantageous for biomedical applications. Depending on its molecular weight, it can be used for surface modifications, as a component of particles for transporting various molecules, or as a hydrogel compound that can be further modified to achieve better bioactive properties (Zhu 2010). PEG can serve as an inhibitor of protein adsorption (Alcantar et al 2000) and cell attachment, which gives anti-adhesive and anti-bacterial characteristics to this material (Mas-Moruno et al 2019). PEG is also suitable for vascular engineering, due to its non-thrombogenic and highly elastic properties (Hahn et al 2007).
Dex is a hydrophilic polysaccharide that can be present in a wide range of molecular weights. It provides suitable biodegradable and biocompatible properties in biomedical applications, with multiple positive effects specifically in vascular applications, similarly to PEG. It has been reported that Dex provides an anti-thrombogenic effect by inhibiting the activation of blood coagulation (Alexandre et al 2015) and, similarly to PEG, it has also been used as a plasma expander that lowers the viscosity of the blood (Chatpun and Cabrales 2011).
Current vascular tissue engineering aims to create cellular TEVGs characterized by zero or at least low immunogenicity, by non-thrombogenicity or anti-thrombogenicity, and also by appropriate biodegradable and mechanical properties. Patient autologous cells are believed to potentially ameliorate the acceptance of vascular grafts. However, autologous mature cells, i.e. ECs and vascular smooth muscle cells (VSMCs), can be used for graft seeding only in limited quantities. New sources of immature pluripotent or multipotent stem cells have therefore been investigated.
VSMCs are the main component of tunica media (i.e. the middle layer of arteries), and they are responsible for the contractile function of blood vessels. The ability to contract is very important for maintaining appropriate blood pressure levels (Chang et al 2014).
Adipose tissue-derived stem cells (ADSCs) are adult multipotent stem cells of mesenchymal origin. Currently, adult mesenchymal stem cells seem to be one of the most promising sources of stem cells for vascular tissue engineering (Zhang et al 2017, Hielscher et al 2018). The main advantages of ADSCs are their relatively easy accessibility, the availability of sufficient quantities in almost all patients, a high proliferation rate, a low tendency to senescence and, last but not least, the potential to differentiate towards various cell types (for a review, see Trávníčková and Bačáková 2018, Bacakova et al 2018a). ADSCs have also been reported to differentiate towards VSMCs, depending on the composition of the medium, the properties of the biomaterial, and the dynamic culture conditions (Zhang et al 2017, Bacakova et al 2018b).
The influence of individual polymers and ECM components has been widely studied. However, our work presented here deals with more complex studies of combinations of modified polymers and composite media. Little is known about the interaction of ADSCs and VSMCs with PLLA modified by plasma treatment and subsequent grafting with PEG or Dex, i.e. molecules with positive effects in vascular applications. In particular, the influence of modifications to PLLA on the differentiation of ADSCs towards VSMCs, and the influence of modifications to PLLA together with a differentiation medium on its potential use in TEVG fabrication seems to be an uninvestigated topic.
Our previous study suggested favourable biocompatibility of plasma-treated PLLA foils in short-term interaction with ADSCs (Bacakova et al 2018a). The first aim of our present study was therefore to observe the interaction, biocompatibility, longer-term growth, and potential differentiation of ADSCs towards VSMCs, on the one hand, and the phenotypic maturation of VSMCs on pristine and variably modified PLLA foils, on the other. The second aim of our study was to investigate the cell behaviour on these same materials while culturing them in a differentiation medium.
2. Materials and methods
2.1. Biomaterial preparation
2.1.1. Material
Biopolymer poly(L-lactic acid) (PLLA), crystallinity (60%–70%), density 1.25 g · cm−3, thickness 50 µm (±20%) (purchased from Goodfellow, UK) was used for this experiment. Circular samples with a final diameter of 2 cm were cut from polymer sheets.
2.1.2. Modifications
2.1.2.1. Plasma treatment
The PLLA foils were treated by Ar+ plasma in Balzers SCD 050 under the following conditions: gas purity 99.997%, pressure 10 Pa, electrode distance 50 mm, power 3 W, treatment time 240 s, room temperature (RT), area of 48 cm2, chamber volume of ca 1 000 cm3 and plasma volume of 240 cm3.
2.1.2.2. Chemical grafting
Some of the plasma-treated polymer samples were chemically grafted by immersing them into a 2% aqueous solution of PEG (Mr = 20 000, Sigma Aldrich, USA) or into a 2% aqueous solution of Dex (Mr = 9 000–11 000, Sigma Aldrich, USA) for 20 h at RT. The non-bonded PEG or Dex was removed by immersing the samples into distilled water for 24 h. Subsequently the samples were dried for 12 h at RT.
2.1.2.3. Etching
Some of the plasma-treated polymers samples were immersed into deionized water for 20 h immediately after plasma treatment. These samples were used as a control to samples grafted with PEG or Dex.
In this work, we studied several types of polymeric substrates (samples): (i) pristine PLLA, (ii) PLLA plasma-treated for 240 s (PLLA240), (iii) plasma-treated PLLA etched in water, (iv) plasma-treated PLLA grafted with PEG and (v) plasma-treated PLLA grafted with Dex.
2.2. Biomaterial characterization
2.2.1. Surface characterization
Physico-chemical and morphological analyses of the pristine and modified PLLA samples were performed by a variety of measurement techniques. It is known that the water CA of plasma-modified PLLA depends on the period of time after modification. The ageing time was determined by goniometry. The other analyses were performed only on the aged samples (more than 30 d after plasma treatment).
2.2.2. Contact angle
The surface CA was measured using the static water drop CA method. The measurements and the evaluation were performed using the See System (Advex Instruments, CZ). The water drops for the CA measurements were created using drops of distilled water on three samples, at ten different positions on the surface of the material of each sample at RT. The CAs of all modified samples were measured over a period of 50 d after their final modification.
2.2.3. Surface morphology and roughness
The surface morphology and the roughness of the pristine and modified samples were determined using a Dimension ICON (Bruker Corp.), QNM mode in Air, silicon Tip on Nitride Lever SCANASYST-AIR, spring constant 0.4 N m−1 and a frequency of 70 kHz was used. NanoScope Analysis software was applied for scan evaluation. The mean roughness value (Ra) represents the arithmetic average of the deviation from the centre plane of the sample. Each sample was measured three times (regions of 10 × 10 µm2), independently.
2.2.4. Chemical composition
The concentrations of the C(1s), N(1s) and O(1s) atoms in the treated surface layer were measured by X-ray Photoelectron Spectroscopy (XPS). An Omicron Nanotechnology ESCAProbeP spectrometer was used to measure the spectra of the modified polymer surfaces. An area of 2 × 3 mm2 was analysed. The x-ray source provided monochromatic radiation of 1486.7 eV. The spectra were measured with a step size of 0.05 eV. The spectra were evaluated with the use of CasaXPS software. The concentration of the elements is given as the atomic percentage.
2.3. Cell isolation
2.3.1. ADSCs
The isolation of the cells was performed in compliance with the Declaration of Helsinki and under ethical approval from the Ethics Committee at Na Bulovce Hospital in Prague. Written informed consent approving experimental use of extracted adipose tissue was obtained from the healthy donor before the liposuction procedure was applied. The ADSCs were isolated using collagenase digestion of adipose tissue; for a detailed description of the isolation procedure, see our previous research (Przekora et al 2017, Travnickova et al 2020). In brief, the lipoaspirate was washed several times with phosphate buffer saline (PBS; Sigma Aldrich) and was digested using a solution of collagenase type I (Worthington Biochemical Corp.). After digesting and subsequent centrifuging, the mature adipocytes and the digested tissue were aspirated, and the remaining stromal vascular fraction containing the ADSCs was seeded into culture flasks. The ADSCs (passage 2) were characterized by flow cytometry (Accuri C6 Flow Cytometer) to confirm the presence of mesenchymal stem cells and the absence of other cell types. Phycoerythrin, FITC, Alexa488- or Alexa647-conjugated monoclonar antibodies against CD105, CD90, CD73, CD29, CD146, CD45, CD34 and CD31 were used. The percentage of positive cells for specific CD markers was as follows: CD105 (98.3%), CD90 (99.2%), CD73 (100%), CD29 (99.9%), CD146 (81.7%), CD45 (6.9%), CD34 (1.7%), CD31 (1.8%).
2.3.2. VSMCs
The cells were isolated from porcine aorta by the explantation method, according to a protocol previously described in Liskova et al (2017). In brief, the porcine aorta was washed with PBS and the outer tunica adventitia was gently removed. The aortic wall was longitudinally cut and the tunica intima, together with 2/3 of the tunica media, was gently separated and cut into small pieces. The cut pieces were digested using collagenase type III (Worthington Biochemical Corp.) and were subsequently seeded into culture flasks. The cells were cultured and were characterized by positive immunofluorescence staining for α-smooth muscle actin (αSMA), calponin, h-caldesmon, desmin and smooth muscle myosin heavy chain (SM-MHC), and were used as a positive control for ADSCs differentiating towards VSMC phenotype. Porcine VSMCs were chosen due to the relatively high similarity of the porcine and human cardiovascular systems, and due to the relatively high stability of the differentiated contractile phenotype in these cells (Christen et al 1999). The similarities in morphology and in physiology can be exploited for further preclinical in vivo studies of material biocompatibility.
2.4. Cell culturing
For the cell seeding experiments, pristine PLLA, PLLA plasma-treated for 240 s, plasma-treated PLLA grafted with PEG, and plasma-treated PLLA grafted with Dex samples were used. Prior to cell seeding, the samples (PLLA, PLLA240, PEG, and Dex) were sterilized in 70% ethanol for 1 h and were then rinsed with PBS. Subsequently, the samples were inserted in a 12-multiwell plate and were fixed by inert glass circles. Tissue culture polystyrene (PS), represented by the bottoms of the wells in a 12-multiwell plate, was used as the control.
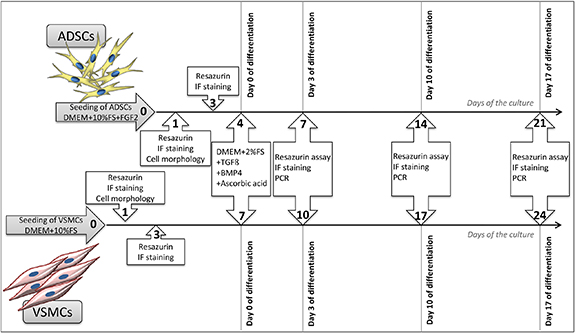
The ADSCs (passage 2) were seeded at a density of 8000 cells per cm2 in 2 ml per well of Dulbecco's Modified Eagle Medium (DMEM; Gibco, Thermo Fisher Scientific) supplemented with 10% (vol vol−1) foetal bovine serum (FBS; Gibco, Thermo Fisher Scientific), gentamicin (40 μg ml−1; Sandoz, Novartis) and basic fibroblast growth factor (10 ng mL−1, FGF2; GenScript). After 4 d of cultivation, the medium was changed to trigger differentiation of ADSCs towards VSMCs. The medium referred to as the 'differentiation medium' contained DMEM supplemented with 2% (vol vol−1) FBS, transforming growth factor beta 1 (2.5 ng mL−1, TGFβ1; Abcam), bone morphogenetic protein 4 (2.5 ng mL−1, BMP4; Sigma Aldrich) and ascorbic acid (150 µM, AA; Sigma Aldrich). The control medium referred to as the 'non-differentiation medium' contained DMEM supplemented with 10% (vol vol−1) FBS only. The ADSCs were cultured for 21 d in total; i.e. for 17 d with a differentiation medium. For the detailed design of the experiment, see figure 1. The ADSCs cultured in the non-differentiation medium were used as a negative control to ADSCs that were differentiated towards VSMCs.
Figure 1. A detailed scheme of the cell culture in the experiment. ADSCs and VSMCs were used for seeding on modified PLLA foils. The studied samples were as follows; pristine PLLA, plasma-treated PLLA for 240 s (PLLA240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and control tissue culture PS. After the initial growth of the cells in the non-differentiation medium, the differentiation medium was added on day 4 (in ADSCs) or on day 7 (in VSMCs). The adhesion, proliferation, and differentiation of the cells during the experiment was studied.
Download figure:
Standard image High-resolution imageThe VSMCs (passage 4) were seeded at a density of 14 000 cells per cm2 in DMEM supplemented with 10% (vol vol−1) FBS. From day 7 of the culture, the medium was changed similarly to ADSCs. The 'differentiation medium' contained DMEM supplemented with 2% (vol vol−1) FBS, TGFβ1 (2.5 ng mL−1), BMP4 (2.5 ng mL−1), and AA (150 µM). The control medium, referred to as 'non-differentiation', contained DMEM supplemented with 10% (vol vol−1) FBS only. Due to the slower initial adhesion and growth of the cells at the beginning of the experiment, the VSMCs were cultured for 24 d in total; however the time period with the differentiation medium was the same as for the ADSCs (i.e. 17 d). For the detailed design of the experiment, see figure 1. The VSMCs cultured in both types of media were used as a positive control for ADSCs differentiating towards the VSMC phenotype.
During cell culturing, the medium was changed twice a week, and both cell types were maintained in an incubator with a humidified atmosphere of 5% CO2 at a temperature of 37 °C.
2.5. Metabolic activity of the cells
Conversion of resazurin (Cat. No. R7017, Sigma-Aldrich) was used to measure the metabolic activity of the cells, which is considered as an indirect indicator of cell proliferation. The principle of this redox indicator assay is based on the colorimetric conversion of blue resazurin to pink resorufin, which can be quantified by fluorescence or absorbance measurements. This reduction is induced by the activity of the mitochondrial enzymes of the viable cells. In order to estimate the proliferation activity of the cells, the metabolic activity was measured on days 1, 3, 7, 14, and 21 (for ADSCs) and on days 1, 3, 7, 10, 17, and 24 (for VSMCs). In brief, the stock resazurin solution (4 mM) was added to DMEM without phenol red with 10% (vol vol−1) FBS to a final concentration of 40 μM. The samples with the cells were transferred to fresh 12-multiwell plates, were pre-washed with PBS, and 1.5 ml of the resazurin solution was added to the cells in each well. The time of incubation at 37 °C was the same on all days; i.e. 3 h and 15 min (for ADSCs) and 3 h and 45 min (for VSMCs). Subsequently, the fluorescence was measured (Ex/Em = 530/590 nm) on a Synergy™ HT Multi-Mode Microplate reader (BioTek, U.S.A.). The background control (resazurin solution without cells) was subtracted.
2.6. Immunofluorescence staining and microscopy techniques
Immunofluorescence staining was used to visualize the cells during the process of adhesion, growth and differentiation. Prior to all types of immunofluorescence staining, the cells were fixed with 4% paraformaldehyde (for 10 min), were pre-treated in PBS with 1% (vol vol−1) bovine serum albumin (BSA, Sigma-Aldrich) and 0.1% (vol vol−1) TritonX-100 (for 20 min), and were then incubated in PBS with 1% (vol vol−1) Tween 20 (for 20 min). Washing in a pure PBS solution was applied after each step.
On day 1, the cells were stained with Texas Red C2-maleimide (1.7 µg mL−1 in PBS; Invitrogen) and the cell nuclei were counterstained with Hoechst 33258 (10 μg mL−1 in PBS; B1155, Sigma-Aldrich) for 30 min in order to visualise the morphology of the cells.
On days 1 and 3, the cells were stained with monoclonal anti-vinculin primary antibody (clone hVIN-1, dilution of 1:200 in PBS; V 9131, Sigma-Aldrich) overnight at 4 °C, and then with phalloidin-TRITC (100 ng mL−1 in PBS; Sigma-Aldrich) for 1 h at RT in order to visualize the filamentous actin. The secondary antibody, i.e. anti-mouse IgG conjugated with Alexa Fluor 488 (dilution 1:400; A11017, Thermo Fisher Scientific), and Hoechst 33258 for staining the cell nuclei (10 μg mL−1 in PBS) were applied for 1 h at RT.
On days 7, 14, and 21 (for ADSCs) and on days 10, 17, and 24 (for VSMCs), the cells were stained with primary antibodies against α-smooth muscle actin (αSMA, clone 1A4, dilution 1:200 in PBS; A2547, Sigma Aldrich) or against myosin heavy chain 11 (SM-MHC, MYH11 (G-4), dilution of 1:200; sc-6956, Santa Cruz Biotechnology) overnight at 4 °C. Then anti-calponin (EP798Y, dilution 1:200 in PBS; ab46794, Abcam) or anti-type I collagen (dilution of 1:400 in PBS; LSL-LB-1197, CosmoBio) were applied for 3 h at room temperature. Subsequently, the secondary antibodies were applied; i.e. anti-mouse IgG conjugated with Alexa Fluor 546 (dilution 1:400; A11003, Thermo Fisher Scientific) and anti-rabbit IgG conjugated with Alexa Fluor 488 (dilution 1:400; A11070, Thermo Fisher Scientific) for 1 h at RT. The cell nuclei were counterstained with Hoechst 33258 (10 μg mL−1 in PBS). The Olympus epifluorescence microscope IX71 (DP71 digital camera, objective magnification of 10x, 20x or 40x) was used to take the images.
2.7. Image analysis
Hoechst counterstaining of the nuclei was used to count the cells on days 1, 3, 7, 14 and 21 (for ADSCs) and on days 1, 3, 10, 17 and 24 (for VSMCs). Microphotographs of 6–7 randomly selected microscopic fields were analysed for each sample type. The initial doubling time of ADSCs and VSMCs on the PLLA samples was calculated from the cell numbers between days 1 and 3 (i.e. 48 h of cell culture) according to the following equation: DT = t × ln(2)/(ln(N)–ln(N0)), where t represents the duration of the culture, N represents the number of cells on day 3, and N0 represents the number of cells on day 1.
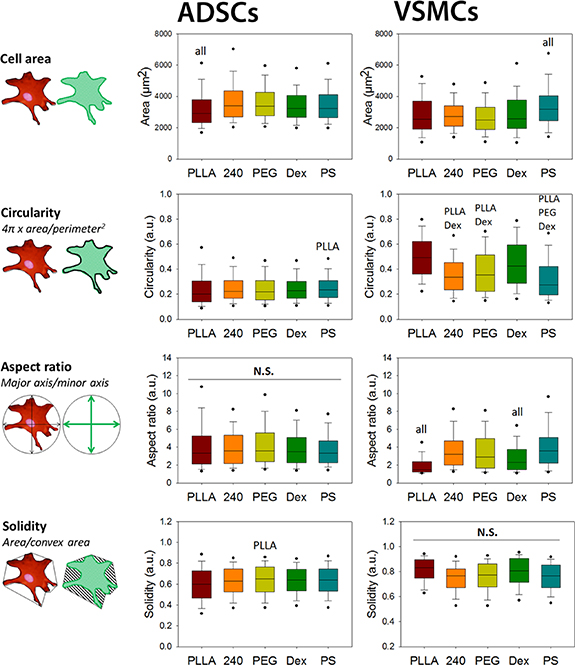
The spreading area, the circularity, the aspect ratio, and the solidity of the cells were measured in order to analyze the morphology of the ADSCs and VSMCs on day 1. The analyses of these parameters were performed in ImageJ software according to the following calculations; i.e. circularity: 4π x area/perimeter2, aspect ratio: major axis/minor axis, solidity: area/convex area. From 86 to 248 cells were analysed for each sample.
2.8. RNA isolation and RT-qPCR
Total RNA isolation was performed using a Total RNA Purification Plus Micro Kit (Norgen Biotek) according to the manufacturer's protocol. The RNA concentration and purity was evaluated from measurements of absorbance at 260 nm and 280 nm using a NanoDrop One Spectrophotometer (Thermo Fischer Scientific, USA). Reverse transcription was carried out using an Omniscript Reverse Transcription Kit (Qiagen, Germany) according to the attached instructions with the use of random hexamers (New England Biolabs, USA). The reaction mixture containing aliquots of 1 µg of isolated RNA in a final reaction volume of 20 µl was incubated at 37 °C for 60 min. The synthesized cDNA was then stored at −20 °C for further use.
In human ADSCs, the mRNA levels were measured with 5xHOT FIREPol Probe qPCR Mix Plus (ROX) (Solis BioDyne, Estonia) and TaqMan Gene Expression Assays (Life Technologies) containing hydrolysis probes labelled with FAM reporter dye specific to COL1A1 (Hs00164004_m1), CNN1 (Hs00154543_m1), SMTN (Hs01022259_m1), ACTA2 (Hs00909449_m1), MYH11 (Hs00975796_m1), and B2M (Hs00187842_m1) as a reference gene. In porcine VSMCs, the mRNA levels were quantified with hydrolysis probes specific to ACTA2 (Ss04245588_m1), CNN1 (Ss03392449_g1) and SMTN (Ss03373737_m1). B2M (Ss03391154_m1) was used as a reference gene. qPCR was performed in a 96-well optical reaction plate in a final reaction volume of 20 µl per well using the Viia 7 Real-time PCR System (Thermo Fischer Scientific, USA). The thermal profile consisted of pre-incubation for 2 min at 50 °C, enzyme activation for 10 min at 95 °C and 40 cycles of denaturation (15 s, 95 °C) and annealing/elongation (1 min, 60 °C). The relative gene expression levels were calculated as –ΔΔCt.
2.9. Statistical analysis
The parametric data are expressed as mean + SD, One way ANOVA, Student-Newman-Keuls test, p ≤ 0.05. The non-parametric data are expressed in box plots as median values, ANOVA on ranks, Dunn's Method, p ≤ 0.05. Three parallels from each sample type were used. The statistical comparison among the samples was made on cells cultured in the same medium type and on the same day of the culture. Statistical analyses were performed using SigmaStat 3.5 software and SigmaPlot 10.0 software (Systat Software Inc. USA).
3. Results
3.1. Material wettability
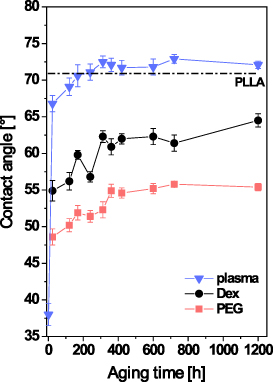
It is known that plasma treatment of polymer macromolecules results in their cleavage, ablation, and alterations to their chemical structure. Plasma treatment thus affects the physicochemical surface properties, e.g. the water CA and the surface wettability. Subsequent grafting with various substances (e.g. biomolecules, nanoparticles, etc) on these plasma-treated surfaces leads to further changes in CA. The change in the surface within the specific time interval from plasma modification is known as ageing (Slepička et al 2013, Slepičková Kasálková et al 2013). The dependence of CA on ageing time for modified PLLA is shown in figure 2. All modified substrates exhibit a similar trend. The lowest value of CA was detected immediately after modification. During ageing, the CA of all tested samples increased. The increment in the CA values is caused by a rearrangement of the oxygen-containing groups that emerged after exposing the plasma-treated polymer to the ambient atmosphere into a polymer volume or by a rearrangement of grafted substances (Slepička et al 2013, Slepičková Kasálková et al 2013). In the case of plasma-treated PLLA, 10 d after modification the value of CA is higher than the value of pristine PLLA. The CA values of the grafted substrates are lower than the pristine PLLA values. This may be caused by the fact that there are oxygen-containing substances grafted on to the plasma-activated surface. This usually causes an increase in wettability, because these substances are of hydrophilic character. The ageing time necessary for surface stabilization is 10 d for PLLA modified in plasma discharge, and 15 d for PLLA grafted with PEG or Dex.
Figure 2. Dependence of the CA on the ageing time for pristine PLLA and PLLA modified in plasma discharge for 240 s (plasma), PLLA modified in plasma discharge and subsequently grafted with PEG or with Dex. The value of the pristine PLLA is represented by a dotted line.
Download figure:
Standard image High-resolution image3.2. Surface morphology and roughness
Atomic force microscopy (AFM) was used to qualify and quantify the changes in surface morphology and in the roughness of the PLLA samples. The roughness of all tested samples, estimated by the Ra parameter, was in the nanoscale. AFM scans showed slightly higher roughness of the plasma-treated PLLA and Dex-modified PLLA samples than of the pristine PLLA (figure 3). The surface morphology of the pristine PLLA was almost flat, in contrast with the differentiated surface of the plasma-treated PLLA and the single-pillar surface of the Dex-modified PLLA. PEG-modified PLLA showed 5–6 times greater roughness, but the irregularities had a bulging appearance, i.e. they were more rounded and less sharp than in the other samples, particularly in the Dex-grafted samples.
Figure 3. AFM scans of pristine PLLA and PLLA modified in plasma discharge for 240 s (plasma), PLLA modified in plasma discharge and subsequently grafted with PEG or with Dex. The mean roughness value (Ra) represents the arithmetic average of the deviation from the center plane of the sample.
Download figure:
Standard image High-resolution image3.3. Surface chemistry
The results obtained by goniometry determination of CA are in good agreement with the results of the chemical analysis of the surfaces performed by the XPS method. The atomic concentration of selected elements is shown in table 1.
Table 1. Elemental composition of the surface layers of pristine PLLA and PLLA modified by plasma discharge, PLLA modified by plasma discharge and subsequently etched in water or grafted with PEG or Dex.
| Atomic concentration [at.%] | |||
|---|---|---|---|
| C(1s) | O(1s) | N(1s) | |
| PLLA | 63.6 | 36.4 | 0 |
| Plasma | 65.8 | 33.4 | 0.8 |
| Etching | 66.5 | 32.9 | 0.6 |
| PEG | 60.3 | 38.9 | 0.5 |
| Dex | 62.3 | 37.2 | 0.4 |
As has been mentioned above, plasma treatment causes the formation of radicals and double bonds. New functional groups (i.e. carbonyl, carboxyl and ester groups) therefore form after an 'activated' surface has been exposed to the ambient oxygen-containing atmosphere. At the same time, plasma modification leads to cleavage of the polymer chains and subsequent surface ablation of PLLA (Slepička et al 2013, Slepičková Kasálková et al 2013). As a result, the oxygen concentration in the surface layer of plasma-modified PLLA decreases. Etching plasma-activated samples removes a part of the newly-formed oxygen-containing groups, and also removes the attached atmospheric nitrogen. Grafting PEG or Dex onto the plasma-modified PLLA surface increases the oxygen concentration, since both compounds contain a large amount of oxygen in their molecules. These results therefore also confirm successful grafting of PEG and Dex on to the surface of PLLA.
3.4. Cell number and doubling time
The initial doubling time was counted from the cell numbers between days 1 and 3 after seeding. The cell numbers were counted from the Hoechst-stained cell nuclei on days 7, 14 and 21 for ADSCs, and on days 10, 17 and 24 for VSMCs, i.e. in time intervals when the cells were being cultured either in a non-differentiation medium or in a differentiation medium.
3.4.1. ADSCs
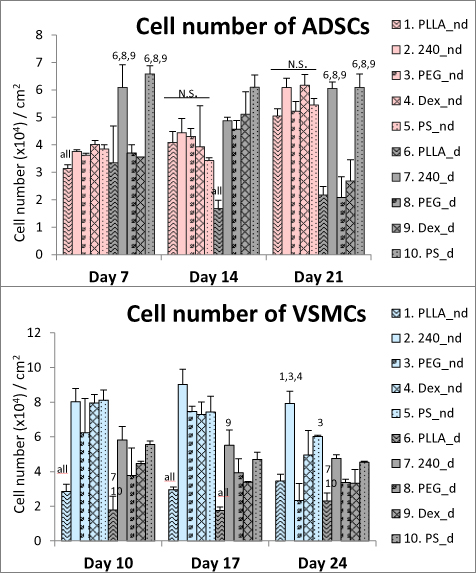
The initial doubling time of ADSCs on all PLLA samples was similar to the value obtained for the control PS (table 2). Except on day 7, the ADSCs cultured in the non-differentiation medium reached similar cell numbers on all tested samples on the corresponding day of the culture (figure 4). However, when cultured in the differentiation medium, the ADSCs reached higher values on the PLLA240 and PS samples than on the pristine PLLA, PEG and Dex samples (figure 4). On day 21, the cells were strongly attached and were well-spread on all tested samples when cultured in the non-differentiation medium; however, they started to detach on pristine PLLA, PEG, and Dex when cultured in the differentiation medium. The detachment of ADSCs had a negative influence on the results of nuclei counting.
Figure 4. The cell numbers counted from microscopic fields on days 7, 14, and 21 (for ADSCs) and on days 10, 17, and 24 (for VSMCs). The studied samples were as follows; pristine PLLA, plasma-treated PLLA (240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and control tissue culture PS. From day 4 (in ADSCs culture) or from day 7 (in VSMCs culture), the cells were cultivated either in the non-differentiation medium (nd) or in the differentiation medium (d). Mean + SD, one way ANOVA, Student-Newman-Keuls test. The statistical comparison among the samples was made on cells cultured in the same medium type and on the same day of the culture. Statistically significant differences (p ≤ 0.05) are marked above the columns by the numbers of tested groups of samples. All: statistically significant differences in comparison with all other groups of samples.
Download figure:
Standard image High-resolution imageTable 2. Initial doubling time of ADSCs and VSMCs on pristine PLLA, on PLLA plasma-treated for 240 s (PLLA240), on plasma-treated PLLA grafted with PEG, on plasma-treated PLLA grafted with Dex, and on the control PS counted between days 1 and 3 after seeding.
| Doubling time (hours) | ||
|---|---|---|
| ADSCs | VSMCs | |
| PLLA | 16.74 | 39.67 |
| PLLA240 | 15.00 | 19.92 |
| PEG | 16.86 | 18.82 |
| Dex | 15.12 | 13.33 |
| PS | 14.94 | 13.42 |
3.4.2. VSMCs
The initial doubling time of VSMCs on pristine PLLA was almost twice as long as on the other PLLA modifications or on the control PS (table 2). The cell number on pristine PLLA was lower than all the other tested samples on all days of the culture, either in the non-differentiation medium or in the differentiation medium (figure 4). The VSMCs cultured in the non-differentiation medium reached almost two times higher cell numbers than the VSMCs cultured in the differentiation medium.
Because the cells had already reached confluence by day 7, the cell numbers further increased only slowly or remained stable, in the case of ADSCs. In the case of VSMCs, the numbers decreased slowly.
3.5. Metabolic activity of cells
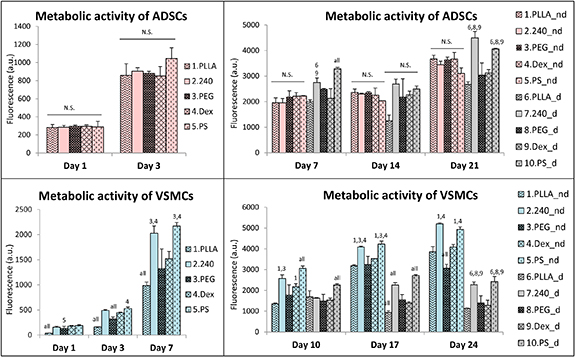
The metabolic activity of the ADSCs and VSMCs was estimated by the resazurin conversion assay. The ADSCs showed a similar metabolic activity on all samples on days 1 and 3 (i.e. before the differentiation medium was added) (figure 5). This trend continued on days 7, 14 and 21, when the ADSCs treated with the non-differentiation medium still showed similar activity on all samples. By contrast, the differences in the metabolic activity of ADSCs on the samples became significant after the differentiation medium had been added, when the PLLA240 and PS samples supported the highest metabolic activity of the cells (figure 5).
Figure 5. The metabolic activity of the ADSCs and VSMCs estimated by the resazurin conversion assay on days 1, 3, 7, 14 and 21 (for ADSCs) and on days 1, 3, 7, 10, 17 and 24 (for VSMCs). The studied samples were as follows; pristine PLLA, plasma-treated PLLA (240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and the control tissue culture PS. Initially, the cells were cultured only in the non-differentiation medium. From day 4 (in ADSCs culture) or from day 7 (in VSMCs culture), the cells were cultivated either in the non-differentiation medium (nd) or in the differentiation medium (d). Mean + SD, one way ANOVA, Student-Newman-Keuls test. The statistical comparison among the samples was made on cells cultured in the same medium type and on the same day of the culture. Statistically significant differences (p ≤ 0.05) are marked above the columns by the numbers of tested groups of samples. All: statistically significant differences in comparison with all other groups of samples.
Download figure:
Standard image High-resolution imageConcerning the metabolic activity of VSMCs, all the samples performed better than the pristine PLLA in the initial intervals, i.e. on days 1, 3 and 7 (figure 5). On days 10, 17 and 24, the VSMCs showed a similar trend in metabolic activity to that of the ADSCs grown in the differentiation medium. The highest cell metabolic activity among the samples was revealed on PLLA240 and on PS, both in the non-differentiation medium and in the differentiation medium (figure 5). However, the VSMCs cultured in the non-differentiation medium reached higher proliferation activity in each interval than the VSMCs cultured in the differentiation medium.
3.6. Initial adhesion, spreading and morphology of the cells
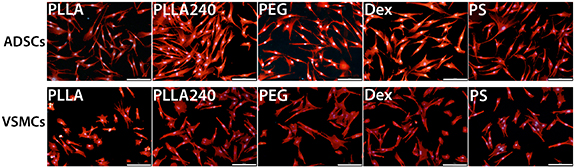
Immunofluorescence staining followed by Image Software analyses was used to characterize the morphology of the cells on the PLLA foils on day 1 after cell seeding. In ADSCs, the smallest cell spreading area was on pristine PLLA (figure 6). In VSMCs, the spreading area was almost the same on all tested PLLA samples; however, the spreading area was significantly lower than on the PS control sample (figure 6). The circularity, the aspect ratio, and the solidity of the ADSCs were similar on all modified PLLA and control PS samples (figure 6). These findings were also in accordance with visual observations, where the cells showed a similar morphology for all samples (figure 7). In contrast to the similar cell spreading area, the VSMCs showed significantly greater circularity and a lower aspect ratio on pristine PLLA and on Dex than on PLLA240, on PEG, and on the control PS sample (figure 6). On days 1 and 3, we also visually observed more rounded and less-spread cells on pristine PLLA than on the modified PLLA samples, where the cells were better spread and were more elongated (figure 7 and supplementary figure S1 (stacks.iop.org/BMM/16/025016/mmedia)).
Figure 6. The morphological characteristics (i.e. cell area, circularity, aspect ratio and solidity) of ADSCs and VSMCs on pristine PLLA, plasma-treated PLLA (240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and the control tissue culture PS on day 1 after seeding. Data are presented as box plots with a median line, the outer edges representing the 1st and 3rd quartile, the whiskers depicting the 10th and the 90th percentile, the dots representing the 5th and the 95th percentile. ANOVA on ranks, Dunn's method, p ≤ 0.05. All: statistically significant differences in comparison with all other groups of samples.
Download figure:
Standard image High-resolution imageFigure 7. The morphology of ADSCs and VSMCs on day 1 after seeding on pristine PLLA, on plasma-treated PLLA (PLLA240), on plasma-treated PLLA grafted with PEG, on plasma-treated PLLA grafted with Dex, and on the control tissue culture PS. The cells were visualized by Texas Red C2-maleimide. The cell nuclei were counterstained with Hoechst 33258. IX71 Olympus microscope, DP71 digital camera. Objective magnification x10, scale bar 200 μm.
Download figure:
Standard image High-resolution image3.7. Immunofluorescence staining
Immunofluorescence staining was performed to reveal the presence and the arrangement of various proteins in ADSCs and VSMCs; namely αSMA, calponin, SM-MHC and type I collagen.
3.7.1. ADSCs
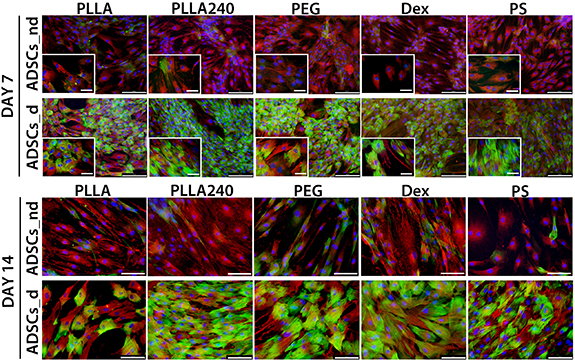
Initially, anti-αSMA staining revealed a diffuse signal in ADSCs; however, treatment with the differentiation medium supported the fibrillar structure of αSMA, mainly on days 14 (figure 8) and 21 (figure 9). Anti-calponin staining revealed only sporadic positive cells in ADSCs cultured in the non-differentiation medium, whereas the differentiation medium supported the early formation of variably developed calponin fibres in almost all cells on all tested materials (figure 8). The presence of contractile calponin protein was also stable in later intervals of differentiation (i.e. on days 14 (figure 8) and 21 (figure 9)). The αSMA and calponin fibres were co-localized in some cells, though some of the cells were positive only for one of these proteins (figure 9). The differentiation medium also supported the formation of type I collagen fibres (figures 9 and S2). Anti-SM-MHC staining revealed sporadically positive cells mainly on day 14 (figures 9 and S2). These SM-MHC-positive cells were observed in cultures treated with the differentiation medium, and were found on all tested samples. Within the ADSCs culture in the non-differentiation medium, the cells only sporadically produced extracellular type I collagen, and no SM-MHC positive cells were observed (supplementary figure S2). The quantity of cells positive for αSMA, calponin and SM-MHC did not visibly differ among the tested materials, and the main observed influence was dependent on the type of culture medium (i.e. non-differentiation medium vs. differentiation medium). On day 21, the ADSCs cultured in the differentiation medium started to detach from the tested materials.
Figure 8. Immunofluorescence staining of αSMA (red) and calponin (green) in ADSCs on day 7 of the culture (i.e. 3 d of differentiation) and on day 14 (i.e. 10 d of differentiation) on pristine PLLA, plasma-treated PLLA (PLLA240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and the control tissue culture PS. ADSCs_nd were cultured in the non-differentiation medium. ADSCs_d were cultured in the differentiation medium. The cell nuclei were counterstained with Hoechst 33258. IX71 Olympus microscope, DP71 digital camera. Objective magnification x10, scale bar 200 μm (day 7) and magnification x20, scale bar 100 μm (day 14). For each sample, a microphotograph under detailed objective magnification x40, scale bar 50 μm is included (day 7).
Download figure:
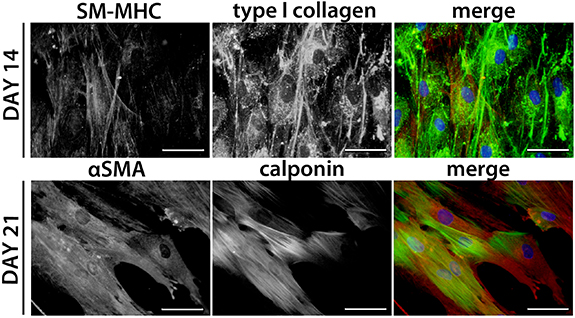
Standard image High-resolution imageFigure 9. Immunofluorescence staining of SM-MHC or αSMA (red) and of type I collagen or calponin (green) in ADSCs cultured with the differentiation medium. Day 14 of the culture (i.e. 10 d of differentiation) on plasma-treated PLLA grafted with Dex and on day 21 (i.e. 17 d of differentiation) on pristine PLLA. The cell nuclei were counterstained with Hoechst 33258. Representative images were chosen to show the detailed protein morphology. IX71 Olympus microscope, DP71 digital camera. Objective magnification x40, scale bar 50 μm.
Download figure:
Standard image High-resolution image3.7.2. VSMCs
The cells were positively stained for αSMA and calponin in all observed time intervals on all tested samples, either in the non-differentiation medium or in the differentiation medium. The αSMA and calponin fibres were mostly oriented in the same direction (supplementary figure S3). On days 17 and 24, the VSMCs treated with the non-differentiation medium started to detach from the materials (supplementary figure S3). By contrast, the VSCMs treated in the differentiation medium maintained their elongated morphology until day 24 (with the exception of the pristine PLLA sample, where the cells did not reach the confluence state) (supplementary figure S3).
3.8. PCR analysis
Real time qPCR was used to quantify and compare the gene expression of COL1A1, CNN1, SMTN, ACTA2, and MYH11 in cells on the tested samples in the non-differentiation and differentiation media. The measured values were normalized to the control values obtained in cells on PS treated with the non-differentiation medium for 7 d.
3.8.1. ADSCs
The gene expression of type I collagen (COL1A1) was supported by the presence of the differentiation medium (figure 10(a)). This increased expression of COL1A1 above the control values was almost stable in all time intervals and on all materials. Treatment with the non-differentiation medium led to a decreasing tendency toward COL1A1 expression in cells on all materials in comparison with the control values.
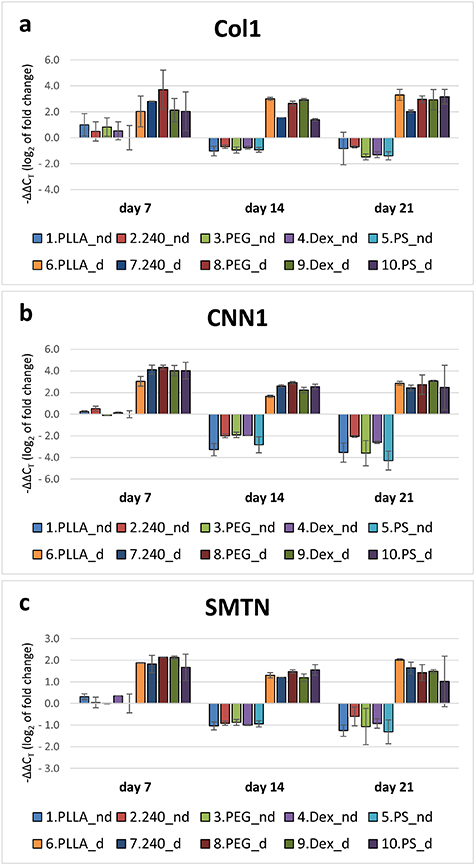
Figure 10. Gene expression of type I collagen (COL1) (a), calponin (CNN1) (b) and smoothelin (SMTN) (c) in ADSCs on days 7, 14, and 21. The studied samples were as follows; pristine PLLA, plasma-treated PLLA (240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and the control tissue culture PS. Initially, the cells were cultured only in the non-differentiation medium. From day 4, the cells were cultured either in the non-differentiation medium (nd) or in the differentiation medium (d). The values are normalized to PS_nd on day 7. Mean ± SD, ANOVA on ranks. The statistical comparison among the samples was made on cells cultured in the same medium type and on the same day of the culture. No significant difference was observed.
Download figure:
Standard image High-resolution imageSimilarly to type I collagen, the gene expression of calponin (CNN1) was strongly supported by the differentiation medium in cells on all tested samples in all time intervals (figure 10(b)). Moreover, this increased expression caused by the differentiation medium was almost stable throughout the cell culture period. No differences were observed among the tested materials. Treatment with the non-differentiation medium led to decreasing expression of CNN1 in comparison with the control values.
A similar trend was observed in the gene expression of smoothelin (SMTN). The expression of SMTN in ADSCs treated with the differentiation medium was increased throughout the cell culture period. By contrast, there was a decreasing tendency when ADSCs were cultured in the non-differentiation medium (figure 10(c).
The gene expression of αSMA (ACTA2) was similar in the non-differentiation media and in the differentiation media on all materials on day 7 of cultivation (figure 11(a)). Nevertheless, on days 14 and 21, the difference between the two types of culture media became apparent, when the gene expression increased in ADSCs treated with the differentiation medium (figure 11(a)).
Figure 11. The gene expression of αSMA (ACTA2) (a), and SM-MHC (MYH11) (b) in ADSCs on days 7, 14, and 21. The studied samples were as follows; pristine PLLA, plasma-treated PLLA (240), plasma-treated PLLA grafted with PEG, plasma-treated PLLA grafted with Dex, and control tissue culture PS. Initially, the cells were cultured only in the non-differentiation medium. From day 4, the cells were cultured either in the non-differentiation medium (nd) or in the differentiation medium (d). The values are normalized to PS_nd on day 7. Mean ± SD, ANOVA on ranks. The statistical comparison among the samples was made on cells cultured in the same medium type and on the same day of the culture. No significant difference was observed.
Download figure:
Standard image High-resolution imageThe gene expression of myosin heavy chain 11 (MYH11) in cells grown in the non-differentiation medium was lower in cells cultured on all tested PLLA samples in all time intervals than in the control cells (grown on PS for 7 d in the non-differentiation medium). In ADSCs treated with the differentiation medium, the expression of MYH11 increased, particularly in the cells on plasma-treated PLLA and on plasma-treated PLLA grafted with PEG or Dex on day 7, and also on plasma-treated and PEG-grafted PLLA on day 21, where the average expression of MYH11 became similar to or slightly higher than in the control cells (figure 11(b)).
3.8.2. VSMCs
Throughout the cell culture period, the gene expression of CNN1 and ACTA2 remained almost unchanged in the VSMCs that were treated with the non-differentiation medium (supplementary figures S4a and S4b). By contrast, treatment with the differentiation medium caused decreasing expression of these genes in cells on all tested materials. The expression of SMTN varied and did not show a specific trend during cell culture. However, the expression was generally lower on days 17 and 21 in cells grown in the differentiation medium than in cells grown in the non-differentiation medium (supplementary figure S4c).
4. Discussion
The set of experiments was performed to study the behaviour of ADSCs and VSMCs (a) on modified PLLA foils and (b) on the modified PLLA foils in combination with the differentiation medium. Our study provides evidence that PLLA foils supported the proliferation and differentiation of ADSCs and VSMCs, both in the non-differentiation culture conditions and in the differentiation culture conditions. These could be promising findings for vascular tissue engineering purposes.
According to many studies, PLLA in the form of nanofibres, microfibres or films is biocompatible with various cell types, including HSVECs, myoblasts, fibroblasts, keratinocytes and ADSCs (Sarasua et al 2011, Ballester-Beltrán et al 2014, Sabbatier et al 2015, Xavier et al 2016).
Our study showed similar adhesion, and also subsequent growth and metabolic activity of ADSCs on all tested materials when cultured in the non-differentiation medium. However, ADSCs cultured in the differentiation medium displayed higher cell numbers and higher metabolic activity on PLLA240 and on PS than on the other tested samples. Moreover, on pristine PLLA, PEG and Dex, the ADSCs cultured in the differentiation medium started to partially detach on days 14 and 21. Interestingly, similar cell behaviour as in the case of differentiated ADSCs was observed for VSMCs. The VSMCs showed worse adhesion, lower growth and lower metabolic activity, especially on pristine PLLA. When VSMCs were cultured in the non-differentiation medium, lower metabolic activity and slightly later detachment of the cells was also observed on the PEG and Dex samples than on PLLA240 and on PS.
Pristine PLLA and its modification by plasma treatment. Pristine PLLA can be burdened with a relatively high level of hydrophobicity, which can impair cell adhesion. It is generally known that, on hydrophobic surfaces, cell adhesion-mediating proteins, e.g. vitronectin and fibronectin, which are present in the serum supplement of cell culture media, are adsorbed in a rigid and denatured conformation, which hampers the accessibility of specific amino acid sequences in these proteins to cell adhesion receptors (for a review, see Bacakova et al 2011). The surface irregularities of PLLA can also influence protein adsorption from FBS, which is important for subsequent cell adhesion. Foldberg et al observed higher protein adsorption on PLLA films patterned with circular indentations than on flat pristine PLLA films (Foldberg et al 2012). In their study, they observed reduced cell adhesion on both PLLA films than on the control PS; however, the cells subsequently displayed the same growth rate on PLLA films and on the control PS materials. Moreover, cells cultured on PLLA substrates, especially on the patterned substrates, expressed higher mRNA levels of lineage-specific genes than the cells cultured on the control PS material (Foldberg et al 2012). The PLLA surfaces could therefore serve as a suitable microenvironment for ADSC growth and differentiation.
Protein adsorption can be further improved by plasma treatment, which is generally known to improve cell adhesion and proliferation (Yamaguchi et al 2004). The change in material surface characteristics induced by plasma treatment can better mimic the properties of ECM on the micro- or nanolevel scale (Bacakova et al 2011). Ar or O2 plasma treatment of PLLA membranes can increase the surface roughness and can tailor the hydrophobicity of pristine PLLA. This decrease in the CA can be controlled by the power or the length of time of plasma treatment (Correia et al 2016, Bacakova et al 2018a, Slepička et al 2018). According to a study by Argentati et al, it seems that O2 plasma treatment of PLLA films supported higher protein absorption mainly from 10% FBS and blood plasma rather than in the case of pristine PLLA films (Argentati et al 2018). Moreover, Argentati et al compared the morphology of various stem cell types, i.e. ADSCs, bone marrow stem cells (BM-MSCs) and Wharton's jelly stem cells (WJSCs), and they observed that the cell adhesion to the same PLLA sample seemed to be stem cell type-specific. Specifically, ADSCs cultured on pristine PLLA formed spheroid structures, while ADSCs cultured on plasma-treated PLLA had a fibroblast-like morphology. BM-MSCs maintained their typical fibroblast-like morphology both on pristine PLLA and on plasma-treated PLLA. WJSCs formed spheroid structures both on pristine PLLA and on plasma-treated PLLA (Argentati et al 2018).
Similarly, in our previous study, we observed different sizes of the cell spreading area and cell growth on pristine PLLA, on heat-treated carbon coated PLLA, and on the control PS samples, depending on the specific cell type (namely: MG-63, Saos-2, fibroblasts, CPAE, and VSMCs) (Lišková et al 2019). The cell type-specific interactions with pristine PLLA are in accordance with our current study, in which our pristine PLLA foils were favourable for the adhesion of non-differentiated ADSCs but, at the same time, unfavourable for the initial adhesion of VSMCs. These differences in adhesion could also be influenced by the presence of variably expressed surface adhesion molecules and cytoskeleton proteins in non-differentiated ADSCs (for a review, see Argentati et al 2019), in differentiated ADSCs, or in mature VSMCs (for a review, see Moiseeva 2001). The quiescent non-proliferative phenotype of VSMCs is characterized by high RNA expression and well-developed protein structures interacting in the cell contraction (i.e. αSMA, calponin, caldesmon and SM-MHC) (Bacakova et al 2018c). Logically, our study suggests that, on some PLLA samples, VSMCs could manifest worse adhesion and subsequent higher detachment because of their contractile properties and because of poorly-developed cell-material interactions. These findings are also in accordance with those observed in differentiated ADSCs in our experiment, where the rapid development of contractile proteins was accompanied by a later slight tendency to detach from the same samples, as in case of VSMCs. Stem cells in general are also susceptible to mechanosensing and mechanotransduction signalling, which is usually caused by ECM and/or biomaterial properties. These specific properties (e.g. stiffness, elasticity, tension, etc) can influence the expression of variable adhesion and cytoskeleton proteins, and can drive the stem cell differentiation towards specific cell lineages (Vining and Mooney 2017, Argentati et al 2019).
The differences in adhesion and growth of ADSCs and VSMCs on the studied materials could be potentially influenced by the different origin of the cells, i.e. human ADSCs and porcine VSMCs. The porcine cardiovascular system has been reported to have a relatively high similarity to the human cardiovascular system. Moreover, the porcine VSMCs showed a relatively high stability of the differentiated contractile phenotype which makes them suitable for in vitro experiments (Christen et al 1999). Nevertheless, some differences between porcine and human cells of various types have been reported. For example, porcine mesenchymal stem cells can differ from human mesenchymal stem cells in the cell size or in the presence of some specific CD surface markers such as CD73 or CD105 (Schweizer et al 2020). Interestingly, mature human chondrocytes were reported to have a lower proliferation rate and to show a lower expression of β1-integrins and of vinculin than animal-derived chondrocytes (Schulze-Tanzil et al 2009). Beta1-integrins and vinculin are, among others, involved in cell–material interactions, and the specific expression rate could therefore influence the process of cell adhesion in cells from different species. The same authors also reported visual differences in the F-actin cytoskeleton between human and animal-derived chondrocytes (Schulze-Tanzil et al 2009).
Interestingly, even the same cell type can display different behaviour on the same sample type, depending on the culture conditions. Wan et al (2003) studied the behaviour of mouse fibroblasts on pristine PLLA and on NH3 plasma-treated PLLA, in static culture conditions and also in dynamic culture conditions. The fibroblasts showed similar initial adhesion to each of the two compared PLLA materials under static conditions. However, the cells cultured on plasma-treated PLLA better withstood the shear stress conditions, while the cells on pristine PLLA detached immediately (Wan et al 2003). It seems that plasma treatment provides tighter adhesion cues for cells. This could be advantageous for the production of vascular grafts, because the cells, mainly the ECs, are continually exposed to dynamic shear stress conditions. Stronger adhesion of the cells (both ADSCs and VSMCs) to the plasma-treated samples than to the pristine PLLA samples was also observed in our study, mainly in later time intervals under static culture conditions.
PLLA grafting with PEG and Dex. PEG (also referred to as polyethylene oxide, PEO) is a hydrophilic and biocompatible polymer that is generally referred to as an 'antifouling' molecule, because of its negative impact on protein absorption and consequent cell adhesion (Bacakova et al 2011). However, it seems that the length of the PEG chain has an important influence on potential cell adhesion and growth. Thus, PEG can act as an anti-adhesive biomaterial coating or as a pro-adhesive biomaterial coating. Grafting the materials with PEG of high molecular weight (i.e. MPEG = 20 000) induced a lower CA, a more differentiated surface and better growth of VSMCs than grafting the materials with PEG with lower molecular weight (i.e. MPEG = 300 and MPEG = 6000) (Svorcík et al 2012). Thus, based on our previous results, MPEG = 20 000 was our choice for the current study to support suitable cell adhesion and growth.
Similarly, Dex grafting of PLLA foils was used in our study to ameliorate the cell adhesion and growth. Dex is a polysaccharide compound with favourable biocompatible and anti-thrombogenic properties (for a review, see Bacakova et al 2014). However, the influence of Dex on the growth of a specific cell type can be ambiguous. Dex derivatives can mimic some heparin effects in blood vessels. Specifically bound Dex copolymers can act as a pro-adhesive surface for ECs, and simultaneously as a low-adhesive surface for VSMCs (Derkaoui et al 2010). Nanofibres composed of Dex and pullulan can promote vascular phenotype and can provide a suitable environment for the growth of VSMCs and ECs (Shi et al 2012). In addition to the most widely studied polysaccharides in vascular tissue engineering (i.e. Dex and pullulan), cellulose as a similar polysaccharide compound seems to have favourable properties for the growth of VSMCs and/or ECs (Bačáková et al 2014).
Composition of culture media. The composition of the media used in our study (i.e. the non-differentiation medium and the differentiation medium) had a non-negligible influence on the behaviour of the ADSCs and VSMCs when cultured on PLLA foils. In general, the differentiation medium (containing BMP4, TGFβ1 and ascorbic acid) induced higher initial metabolic activity of ADSCs than the non-differentiation medium. It also triggered the differentiation of ADSCs towards VSMCs. This was successfully proved by RT-PCR and by immunofluorescence staining of specific early, mid-term and also some late markers of VSMC differentiation on all variably-modified PLLA samples. We also studied the influence of the same composition of the medium on mature VSMCs that were cultured on modified PLLA foils. The differentiation medium (containing BMP4, TGFβ1 and ascorbic acid) caused lower metabolic activity and lower cell numbers of VSMCs on the PLLA foils than the non-differentiation medium. Immunofluorescence staining revealed that all VSMCs were positive for their specific markers when cultured either in the non-differentiation medium or in the differentiation medium. However, we revealed a decrease in the mRNA expression of specific markers in time. Surprisingly, this decrease was greater when the VSMCs were cultured in the differentiation medium. The decrease in the mRNA levels of specific markers and/or the subsequent loss of contractile proteins could be caused by the static in vitro culture conditions, which are known to support a synthetic phenotype of VSMCs rather than a contractile phenotype of VSMCs (Chang et al 2014). These two phenotypes may have the ability to switch according to the culture conditions (Rensen et al 2007).
In addition, it is known that BMP4, TGFβ1 and ascorbic acid in various concentrations can act variably in cell proliferation and differentiation. A strongly positive effect of TGFβ1 and/or BMP4 on stem cell differentiation towards VSMCs has been proved in many studies (for a review, see Zhang et al 2017). However, in the case of mature VSMCs, it has been reported that TGFβ1 and BMP4 enhanced the proliferation of pulmonary artery smooth muscle cells (PASMCs) from donors suffering from primary pulmonary hypertension (Morrel et al 2001). At the same time, TGFβ1 and BMP4 inhibited the proliferation of PASMCs isolated from healthy control donors and from patients suffering from secondary pulmonary hypertension (Morrell et al 2001). Interestingly, the response of VSMCs to BMP4 can be site-specific. BMP4 inhibited the proliferation of PASMCs from proximal segments of the pulmonary artery, but increased the proliferation of PASMCs from peripheral segments of this artery (Yang et al 2005). In the same study, BMP4 also promoted the survival of peripheral PASMCs, but not of proximal PASMCs, when exposed to apoptosis-inducing agents (Yang et al 2005). The presence of ascorbic acid in the culture medium is important for the synthesis of collagen fibres, which are one of the basic components of vascular ECM. In our study, the differentiation medium supported the production of extracellular type I collagen, the main component of ECM in tunica media, which together with type III collagen is very important for imparting strength to the vascular wall (Wagenseil and Mecham 2009). However, it should be pointed out that overexpression of RNA and abundant formation of ECM proteins can be a sign of vascular fibrosis (Ponticos and Smith 2014).
5. Conclusion
ADSCs and VSMCs confirmed that variously modified PLLA foils in a three-week culture have high biocompatibility, comparable to the level of biocompatibility of the control PS. The most stable monolayer of cells was observed on plasma-treated PLLA and on the PS control, whereas pristine PLLA and PLLA modified with PEG and Dex were characterized by slightly later cell detachment. From this point of view, plasma-treated PLLA seem to be most suitable for obtaining a sufficient amount of cells for vascular wall reconstruction. However, all the PLLA materials supported the growth of ADSCs and their differentiation towards VSMCs. Although the cell behaviour on modified PLLA foils can be influenced by the specific cell type and by the composition of the medium, it seems that PLLA with all the modifications tested here is favourable for the purposes of vascular tissue engineering.
Acknowledgments
Dr Elena Filova (Institute of Physiology) is gratefully acknowledged for helping with isolating VSMCs. The authors would like to acknowledge Mr Robin Healey (Czech Technical University in Prague) for his language revision of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by the Grant Agency of Charles University (GAUK, Project No. 642217), by the Ministry of Education, Youth and Sports of the Czech Republic within LQ1604 National Sustainability Program II (BIOCEV-FAR Project) and by the project 'BIOCEV' (CZ.1.05/1.1.00/02.0109), and by the Grant Agency of the Czech Republic, Grant No. 17-00885S.