Abstract
It is very desirable to have good antibacterial properties and mechanical properties at the same time for bone scaffolds. Graphene oxide (GO) can increase the mechanical properties and antibacterial performance, while forsterite (Mg2SiO4) as the matrix can increase forsterite/GO scaffolds' biological activity for bone tissue engineering. Interconnected porous forsterite scaffolds were developed by space holder processes for bone tissue engineering in this research. The forsterite/GO scaffolds had a porosity of 76%–78% with pore size of 300–450 μm. The mechanism of the mechanical strengthening, antibacterial activity, and cellular function of the forsterite/GO scaffold was evaluated. The findings show that the compressive strength of forsterite/1 wt.% GO scaffold (2.4 ± 0.1 MPa) was significantly increased, in comparison to forsterite scaffolds without GO (1.4 ± 0.1 MPa). Validation of the samples' bioactivity was attained by forming a hydroxyapatite layer on the forsterite/GO surface within in vitro immersion test. The results of cell viability demonstrated that synthesized forsterite scaffolds with low GO did not show cytotoxicity and enhanced cell proliferation. Antibacterial tests showed that the antibacterial influence of forsterite/GO scaffold was strongly correlated with GO concentration from 0.5 to 2 wt.%. The scaffold encapsulated with 2 wt.% GO had the great antibacterial performance with bacterial inhibition rate around 90%. As results show, the produced forsterite/1 wt.% GO can be an attractive option for bone tissue engineering.
Export citation and abstract BibTeX RIS
1. Introduction
Like calcium phosphate ceramics, bioceramics is a perfect option because of the chemical composition close to the bone. However, there are a lot of weaknesses that need to be addressed [1–3]. Regarding this, a class of active surface bioceramics consisting of bioactive silicates has been considered as proper candidates for hard tissue regeneration [4, 5]. In recent years, several researchers in multiple areas have regarded forsterite (FST; Mg2SiO4) as a silicate-based bioceramic due to the wide variety of uses [1, 6]. This bioceramic has strong cytocompatibility and mechanical characteristics that are suitable for tissue engineering applications [7]. Forsterite ceramics showed a dramatic increase in fracture toughness (KIC = 2.4 MPa m1/2) in comparison with hydroxyapatite (HAp) and bioactive glass (BG) for bone implant applications [8]. Silicate-based ceramics and BG possess osteoconductive and osteogenesis characteristics, however, HAp possess only osteoconductive characteristic [3, 9–11]. It was reported exhibited the existence of that Mg ions in forsterite present an essential role in bone reconstruction and skeletal growth. Likewise, Si was noticed to be engaged in new bone generation and reconstruction. It might stimulate osteogenic differentiation of MG63 cells through up-regulating the expression of collagen and extracellular matrix proteins [9–11]. Therefore, forsterite is an appropriate choice for both high load-bearing and hard tissue regeneration uses [9]. Moreover, forsterite, indicated as a compound that possesses great strength, is regarded as chemically stable, with a suitable thermal expansion rate [10]. Multiple studies have shown that employing nano-scale materials can dramatically boost their physicochemical characteristics throughout the past. Therefore, in contrast to micron-scale materials' properties, developed nanostructured materials also display a great combination of various biochemical, electrical, and magnetic characteristics [10–14]. The nanostructured materials are suitable for applications in various fields because of the mentioned remarkable and unique properties. The utilization of nanostructure FST might as well significantly develop its features, for example cell response and, biodegradation [5, 15]. Saqaei et al [7] revealed that encapsulation of forsterite into the BG enhances deposition of apatite on the surface of FST nanoparticles. Torkaman et al [15] exhibited that the generation of Mg ions from FST in simulated body fluid (SBF), resulting in the creation of adversely charged silanol (Si–OH) groups on the surface film, leading to nucleation of apatite on its surface. On the other hand, nanostructure bioactive ceramic-based scaffolds in bone repair are limited due to their poor mechanical properties, mostly low toughness, and weak antibacterial activity [6]. Therefore, in the manufacture of these biomaterials, mechanical strengthening of the bioactive scaffold has proven to be a key issue. Ceramic-based scaffolds fracture, as described in the Griffith fracture theory, derives from the micro-cracks within ceramics, rather than breaking atomic bonds [8, 16]. Micro-cracks throughout ceramic-based scaffolds are continuously widening and interacting during pressure with one another to induce final brittle fracture. Therefore, ceramic-based scaffolds' practical strengths are usually 2–3 times smaller than their theoretical values [17, 18]. Now, most researchers are mostly focused on controlling crack propagation conditions in bioactive ceramic-based scaffolds with the introduction of the second phase that further enhances the mechanical reinforcement of scaffolds [19, 20]. Moreover, because of their excellent mechanical properties (strength of approximately 130 GPa and modulus of around 1 TPa), two-dimensional nanosheets like graphene oxide (GO) as a main element of nanomaterials, are the most favorable second-phase materials for composites and also can facilitate the load transfer from ceramics to graphene nanosheets due to large specific surface area (approximately 2630 m2 g−1) that enables a high contact area with the matrix. These properties can improve the mechanical properties of graphene/ceramic composites [21–25]. Moreover, GO provides high antibacterial efficiency thanks to its membrane and oxidative stress with oxygen-containing functional groups at its edge and large surface area. As a matter of fact, GO attaches itself to bacterial cells with its small size and then disrupts and damages the cell membrane, leading to bacterial membrane degradation [18, 26–31]. Akhavan et al [31] found that when the bacterial cells were deposited on the surface of graphene materials, membrane stress occurred, which might destruct the membrane structure of cells and inactivating bacterial cells. Liu et al [12] demonstrated that once bacterial cells were attached to the sheet of graphene materials, membrane stress taken place, which might harm the cell membrane integrity. In several cases, the effect of forsterite on microstructure enhancement and the ceramic-based scaffold's mechanical properties are investigated. Although the impact of GO on the mechanical properties and antibacterial activity of forsterite scaffold has never been investigated so far [23, 29, 30]. In this regard, the addition of GO in forsterite scaffolds can lead to remarkable antibacterial properties against Gram-positive and Gram-negative bacteria [31–34]. In the present paper, dispersing GO nanofiller was constructed and introduced into forsterite scaffolds to increase antibacterial performance and mechanical properties. Three-dimensional porous forsterite/GO nanocomposite scaffolds were fabricated by a combination of semi powder metallurgy (SPM) and space holder methods. The morphology of GO in the forsterite matrix was investigated, and a possible mechanical strength and antibacterial mechanism of forsterite/GO scaffolds were proposed. Moreover, cytocompatibility consisting of cell adhesion, viability, and proliferation was analyzed.
2. Materials and methods
2.1. Materials and scaffolds preparation
Primary reagents were magnesium chloride hexahydrate (MgCl2.6H2O; 99.9% purity, Merck, Germany), sodium metasilicate nanohydrate (Na2SiO3.9H2O; 99.9% pure GR, Shantou Xilong Chemical Co, China) and sodium hydroxide (NaOH; 99.9% pure, Merck, Germany). These materials were mixed at a molar ratio of 2: 1: 2, respectively, and afterward poured into zirconia containers and exposed to high ball milling for a brief period (Retsch planetary ball mill type PM 400) (∼10 min). The obtained forsterite powders were then calcined at 1100 °C and milled under the parameters depicted in table S1 (presented in supporting information available online at stacks.iop.org/BMM/17/035011/mmedia). After that, GO additive with a thickness of 2–5 nm and a surface area of 1000–1300 m2 g−1 was prepared using the modified Hummer's method [33] through chemical oxidation without any further purifying and plunged into ethanol. Therefore, the appropriate weight of forsterite was dissolved in ethanol, and the blended solutions were homogenized for a further 1 h by the magnetic stirrer. Subsequently, the dispersed solutions were combined and sonicated for 30 min before ball milling. The composite was then dried for one day in a vacuum drying oven at 100 °C. After that, in order to create forsterite/GO scaffolds, the space holder method was used, during which a suitable weight ratio of forsterite/GO nanopowder to NaCl particles (as the spacer) with sizes of 450–650 μm was chosen. Therefore, the powder was pressed into a cylindrical shape under 110 MPa (10 mm diameter × 15 mm height) and then sintered for 4 h at 1100 °C at an effective heating rate of 5 °C min−1 as schematically shown in scheme 1. The composites selected for this paper were forsterite/GO composites with GO contents of 0.5, 1.0, and 2.0 wt%, respectively. To measure the total porosity of the scaffolds, the Archimedes principle has been used in accordance with the following equation
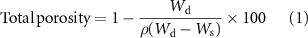
Scheme 1. A schematic representation of the preparation of forsterite/GO scaffolds.
Download figure:
Standard image High-resolution imagewhere, the weight of the specimen in the air is Wd, the weight of the specimen immersed in water is Ws, and the true density of the scaffold is ρ.
2.2. In-vitro bioactivity and antibacterial behavior
Each forsterite/GO scaffold was immersed in 100 ml of SBF with the chemical composition presented in supporting information (table S2) at 36.5 ± 1 °C for 28 days to investigate the bioactivity assessment. Scaffolds were withdrawn from the SBF containers after that time and cleaned with deionized water, and dried out in the ambient space. Ionic levels of deposited film were calculated in the SBF solution by the inductive coupled plasma. During processing, the solution's pH values were also recorded using a pH meter (PHS-3 C, Shanghai Lei Ci Device Works, China). The degradation rate of the scaffolds was determined from the change in samples' weight throughout soaking duration based on the equation (2) [14]
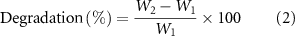
where W1 and W2 are scaffolds' initial mass and mass of scaffolds measured at a given time. The antibacterial performance of forsterite/GO specimen in disk diffusion antibiotic sensitivity was evaluated using S. aureus (S. aureus, ATCC 12 600) and Gram-negative E. coli (E. coli, ATCC 9637) bacteria. The positive control was antibiotic gentamicin (10 μg disc−1). The inhibition area (IA) evaluation was used to evaluate the antibacterial influence of specimens containing various amounts of GO.
2.3. In-vitro biocompatibility
The MG63 cell line from the National Cell Bank of Iran at the Pasteur Institute (NCBI, C555) was seeded on sterilized forsterite/GO specimens at a concentration of 2–104 cells ml−1 and cultured for three and seven days, whereupon the cell-scaffold constructs were stained with 4',6-diamidino-2-phenylindole (DAPI, blue fluorescence in live cells) and afterward exposed to fluorescence microscope. On the 3rd and 7th days, alkaline phosphatase (ALP) activity was measured to determine the effect of GO on the initial osteogenic differentiation of the MG63 cells. In a 24-well plate, cells with a concentration of 104 cells ml−1 were placed separately. The cells were remained to grow at 37 °C for different periods according to [34].
2.4. Measurements and characterizations
An Instron-5569 universal machine with a shear rate of 0.5 mm min−1 and a load of 10 kN at ambient temperature was applied to measure the compressive strength (CS) of cylindrical scaffolds. Scanning electron microscopy (SEM, JEOL JSM-6380LA) and transmission electron microscopy (TEM, Hitachi HT7700, Japan) were performed to evaluate the morphology of the scaffolds. The phase composition of forsterite powder was analyzed using x-ray diffraction analysis (Siemens D5000) using Cu-Kα radiation; in the angle range from 20° to 75° at a screening rate of 4° min−1. The crystallite size was also measured utilizing Williamson–Hall method [35], which is based on the equation (3)
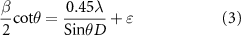
where β is the diffraction peak width at mid-height; λ is x-ray wavelength and D is an average crystallite size (nm); while,  is microstrain and θ is Bragg diffraction angle. The chemical compositions of forsterite/GO were analyzed by Fourier transform infrared (FTIR) analysis using a Thermo-Nicolet 5700 spectrometer. The spectra of samples were recorded at a resolution of 8 cm−1 and a spectral range of 400–4000 cm−1. The test outcomes were stated as mean ± standard deviation (SE), evaluated employing SigmaPlot software with p-value < 0.05 (*), p-value < 0.01 (**) and p-value < 0.001 (***) to display identified differences between all data.
is microstrain and θ is Bragg diffraction angle. The chemical compositions of forsterite/GO were analyzed by Fourier transform infrared (FTIR) analysis using a Thermo-Nicolet 5700 spectrometer. The spectra of samples were recorded at a resolution of 8 cm−1 and a spectral range of 400–4000 cm−1. The test outcomes were stated as mean ± standard deviation (SE), evaluated employing SigmaPlot software with p-value < 0.05 (*), p-value < 0.01 (**) and p-value < 0.001 (***) to display identified differences between all data.
3. Results and discussion
3.1. Microstructural characterization
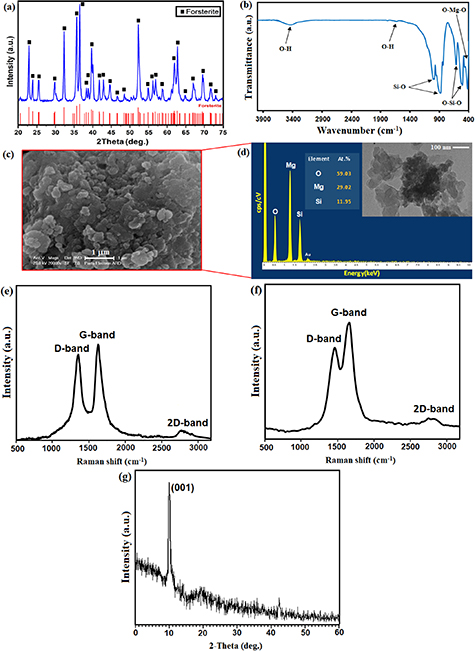
The x-ray diffractometer (XRD) spectrum of the calcination powder at 1100 °C for 2 h is shown in figure 1(a). At 1100 °C, forsterite (JCDP#34-0189) was synthesized, and no other peaks were observed apart from distinctive forsterite peaks. Additionally, the existence of all sharp peaks in the XRD pattern will indicate the existence of highly crystalline forsterite. Furthermore, the average crystallite size of the forsterite nanopowders obtained from the Williamson–Hall equation is about 71 ± 3 nm.
Figure 1. (a) XRD pattern, (b) FTIR absorption spectra (c) SEM image, (d) EDS analysis (in frame is TEM micrograph of forsterite nanopowders and (e) Raman spectra of GO powder, (f) FST/GO powder and (g) XRD pattern of GO powder.
Download figure:
Standard image High-resolution imageThe FTIR spectra exhibited the bands of 525 cm−1 and 682 cm−1 is assigned to Mg–O and Si–O bending modes, even though the band at 815–1000 cm−1 was assigned to Si–O bond (figure 1(b)). At 1075 cm−1, the strong band related to Si–O–Si stretching mode was observed [7]. Furthermore, the band of intense at around 3440–3450 cm−1 is attributed to water molecules, meanwhile the intense bands at about 1075 cm−1 is linked with hydroxyl groups was found at [1, 7]. The morphology of synthesized forsterite powders is presented in figure 1(c). As can be seen, the synthesized forsterite powders consist of almost alike particle size nanoparticles in the 70–100 nm range, which presented in agglomeration form, as presented by the field emission scanning electron microscope (FESEM) image.
The EDS analysis of the fabricated powder is aligned with the XRD outcome; the spectra contained peaks of oxygen, magnesium, and silicon and suggested values of their quantitative ratios that were partially similar to those of forsterite stoichiometry (i.e. 2:1:4 for Mg:Si:O as Mg2SiO4) as exhibited in figure 1(d). The particle size and shape of the fabricated FST powder via TEM image presented in the frame. The forsterite particle size distribution is in the 50–100 nm range with a mean particle size of less than 100 nm. Two dominant peaks were observed in the Raman spectra (figure 1(e)) of the neat GO, with D and G peaks being detected between 1355 and 1582 cm−1, which is aligned with the vibrational modes of the D–band caused by structural defects and the G–band of the graphic materials [36]. The D peak and G peak are attributed to the stretching of the in-plane C–C bond in GO. The attained ID/IG intensity ratio values for GO are about 0.88 [36, 37]. The existence of 2D–band at 2731 cm−1 verifies the presence of a few layers of graphene [25, 28]. As can be seen from figure 1(f), after GO was encapsulated with forsterite, ID/IG escalated slightly from 0.88 to 1.15. In general, an escalation in ID/IG value implying the presence of more defective sites in the structure [13, 28, 34]. The Raman results demonstrated the successful incorporation of graphene into forsterite scaffold during the synthesis process with no visible structural damage. As presented in figure 1(g), the XRD pattern of GO powder displays characteristic peaks [37] at 2θ = 10.03°, connected to the preferable orientation (001) of GO.
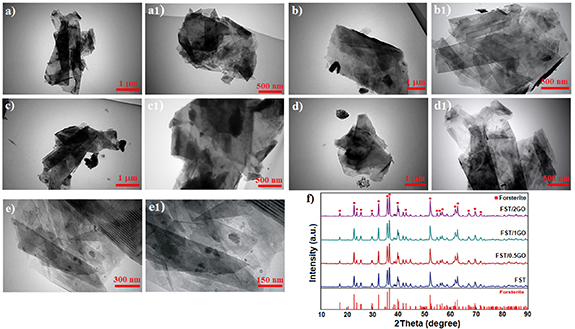
The TEM image showed that the forsterite/GO possesses a spherical shape, which merged and created agglomerate with a mean particle size of almost 80–100 nm (figures 2(a)–(e)). Although, GO offers a flake-like shape with a thickness of almost 3–5 nm, which is higher than that of neat graphene (0.355 nm) owing to the oxygenated groups in GO. Moreover, the images show the homogenous distribution of forsterite inside the GO sheets with great attachment. The homogeneous dispersion of nanofillers in the matrix, as described in the literature [38, 39], is an important requirement for preparing ceramic-based scaffolds nanocomposites with unique efficiency. As can be observed in a high magnification image, the forsterite particles were spherical with an agglomerate size of 200–250 nm. GO was very thin with a few wrinkles. Furthermore, almost all forsterite particles are attached on the GO sheet, and a very small number of forsterite particles are located outside the GO sheet (figure S1), which means that there is good interaction between forsterite and GO.
Figure 2. TEM images of (a), (a1) GO nanosheets and (b), (b1) FST-0.5GO, (c), (c1) FST-1GO, and (d), (e) FST-2GO and (f) XRD pattern FST-GO nanocomposite powders.
Download figure:
Standard image High-resolution imageThe XRD pattern of forsterite, and forsterite/GO scaffold is shown in figure 2(f). This pattern depicted the typical peaks of forsterite (JCDP#34-0189) [4]. The patterns were identical since there was no data from the second phase, indicating that GO and forsterite had no unusual reaction. Moreover, the XRD pattern of forsterite/GO shows that no other phases except forsterite phases have been identified in the scaffold because it is beyond the detection ability of XRD to identify GO due to its small amounts.
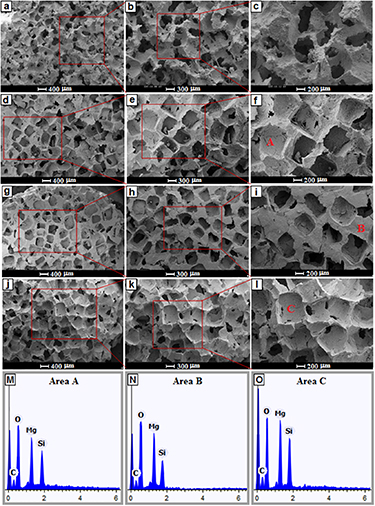
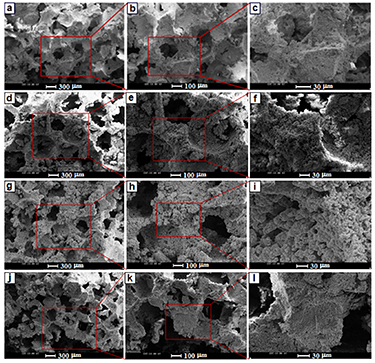
The SEM image of the forsterite and forsterite/GO scaffolds is presented in figures 3(a)–(i). Most pores in the framework of porous scaffolds maintained their open porosity on all sections for entire kinds of scaffolds. The SEM micrographs of four scaffold forms, achieving maximum porosity (∼76%–78%) of the scaffolds with pore size of around 300–400 μm. It is worth noting that, adding 0.5 to 2 wt.% GO in the forsterite scaffold has a less significant impact on the porous size of the scaffold. With the escalating GO amount, a similar pattern was observed concerning interconnected porosity and total porosity which implied that the amount of porosity did not correlate the composition of the scaffolds. Micropores on the macropores' walls of the forsterite-GO bioceramic have been shown to play a main role in osteoinduction. The 3D structure was already stated to enable the porosity, pore size, and interconnectivity of natural bone to be preferably imitated, escalating cell attachment and proliferation, creating room for new tissue growth and vascularization [40]. Highly porous scaffolds are required, but these high levels of porosities have great effects on the mechanical properties of scaffolds [41]. In this regard, it was suggested [8] that the minimum pore size needed for the growth of the surrounding bone with a blood supply is about 150–100 μm. Moreover, they indicated which the pores' minimum size that can fill the surrounding bones is so important. From the figure, it was obvious that the pore size and an interconnected pore structure are ideal and proper for the growth of osteoblasts, fast vascularization, and reconstruction of bones. To maintain the scaffold's mechanical stability, fast vascularization is required since it gradually degrades throughout bone reconstruction [42, 43]. To allow biomolecules to readily enter and leave the scaffold, an interconnected porous network was therefore required. The EDS analysis confirms the existence of Mg, Si, O and C in the forsterite/GO scaffolds as shown in figures 3(m)–(o). The spectra containing peaks of oxygen, magnesium, and silicon are related to the forsterite matrix while the presence of carbon Kα peaks at around 0.27 eV along with oxygen further confirms the incorporation of GO in the forsterite matrix.
Figure 3. SEM images of (a)–(c) FST and (d)–(f) FST-0.5GO, (g)–(i) FST-1GO, and (j)–(l) FST-2GOnanocomposite scaffolds, and (m)–(o) EDS analysis of area A, B, and C.
Download figure:
Standard image High-resolution image3.2. Mechanical properties of forsterite/GO scaffolds
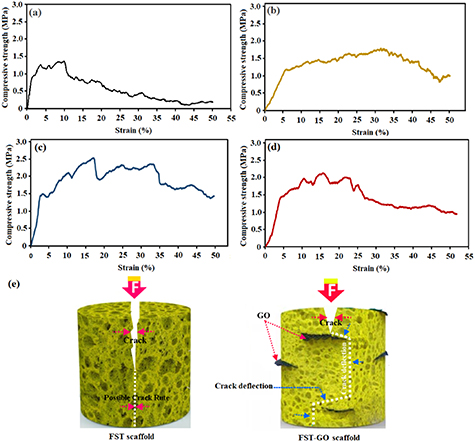
CS of the forsterite and forsterite/GO scaffolds is shown in figures 4(a)–(d). The CS of the forsterite, forsterite/0.5GO, and forsterite/1GO, and forsterite/2GO scaffolds was 1.4 ± 0.05, 1.7 ± 0.12, 2.4 ± 0.15 and 2.1 ± 0.12 MPa, respectively. This trend indicated which forsterite/1GO scaffolds showed more CS than other scaffolds. In comparison to neat forsterite, forsterite composites presented higher CS, which indicates a higher load-bearing capacity after using nanofillers, and a great impact of GO dispersion as a reinforcing phase in the forsterite scaffold matrix on the maintaining mechanical integrity of the scaffold [23]. Regarding this, GO might play a carrier's role and be willing to shift the stress from the scaffold to the GO sheets inside the matrix. Moreover, more CS is obtained by increasing the amount of GO due to its hard nature that increases the CS of the ceramic-based scaffold matrix. Although the mechanical performance can be increased by a further increase of the GO content (2 wt.%), which can be related to GO aggregation, even they are still increased compared to forsterite scaffolds without GO. The main reason for decreasing CS of the forsterite/2GO compared to the forsterite/1GO scaffold is attributed to the formation of large agglomerates of GO which leads to evolution of inner flaws inside the forsterite matrix. Thus, it can be concluded that adding GO can significantly enhance the mechanical properties of forsterite scaffolds. Additionally, the excessive addition of GO causes agglomeration, which may partly prevent the composites from densification, and a reduction in density can supply more space for grain size growth. So, the forsterite/1.0 wt.% GO scaffold has optimal CS due to better performance, higher density, and better GO distribution.
Figure 4. Compressive stress–strain curve of the (a) FST and (b) FST-0.5GO, (c) FST-1GO, and (d) FST-2GO nanocomposite scaffolds and (e) schematic diagram demonstrate crack deflection mechanisms by graphene in FST-GO nanocomposite scaffolds.
Download figure:
Standard image High-resolution imageIt was quite obvious which GO was at the border of the forsterite grains. The high surface area of GO can increase the contact surface with the forsterite matrix. Consequently, the CS of the scaffold is escalated as a result of the bonding strength among GO and the forsterite particles was greatly increased and more energy was required to pull-out the GO from the forsterite matrix [18], figure 4(e) shows the schematic view of enhancement mechanisms related to the morphological evidence, in order to study the toughening mechanisms in further detail. When a crack forms and expands in the forsterite matrix, the load is transferred from the forsterite matrix to the GO because of the elastic modulus difference. The texture of the GO wrinkled surface allows mechanical bonding and load transfer to the matrix. The mechanism of crack deflection is shown in the figure. Due to GO's extreme surface area, high stress is required to pass the crack; therefore, it causes crack deflection. In fact, GO acts as a barrier and prevents the crack from spreading on the scaffold surface of forsterite/1 wt.% GO scaffold. Scaffolds containing GO reinforcement need further energy to propagate cracks, which increases the toughness of the scaffold [39]. GO makes the preferential displacement between opposite crack surfaces difficult by reducing the stress required to propagate more cracks. In this respect, once a crack is propagated and clash with GO, the path is blocked, and then the crack path is deflected. This mechanism creates a complicated path for stress release that can increase toughness [18, 41–44].
In this perspective, Gao et al [39] discovered that the mechanisms of pull-out, crack bridging, crack deflection, and crack tip shielding are the main reason for the mechanical strengthening of the BG scaffold encapsulated with graphene. Likewise, the productive load transfer from the Mg-based matrix to reduced graphene oxide (RGO) resulted in an increase in CS and hardness [44]. Shuai et al [34] found that the existence of GO in polymer scaffolds led to cracks spreading along the GO surface throughout propagation, altering the propagation route and result in a crack deflection, implying GO might break and fragment the crack, and the crack spread was delayed by the generating of a crack branch. The group also reported that load might be moved from the Mg matrix to GO throughout loading. The high-stress region will be released and moved to other areas, improving the alloys' loading performance [40]. According to Gao et al [18], GO performs a crucial and successful role in preventing crack growth in the ceramic matrix. The fundamental explanation for enhancing the CS of FST-GO scaffolds in this study was a crack deflection and load transfer from the FST matrix to the GO sheets. The improvement of mechanical properties at total nanofiller amounts of 1 wt.% could be due to the accumulation of GO as a reinforcement agent at a great concentration (2 wt.%GO). Therefore, the desired mechanical characteristic was obtained when the optimum amount of 1 wt.% GO is encapsulated in the scaffold.
3.3. Bioactivity of forsterite/GO scaffolds
In vitro bioactivity was assessed for forsterite/GO scaffolds concerning apatite-forming potential by SBF tests. It was interesting that forsterite scaffolds showed a great increase in mechanical properties and kept optimal bioactivity after adding GO, as can be observed in figure 5. SEM image demonstrated that a variety of tiny mineral clusters precipitated over the surface of the scaffold during seven days of exposure to the SBF. As can be seen in the high-magnification image, these clusters are composed of crystallites with a spherical and flat appearance. The results obtained for forsterite/GO scaffolds were similar. According to figure 5, HAp formation appeared after seven days in SBF with normal cauliflower morphology. In addition, apatite film cracks are usually attributed to hydrated layer contraction throughout the drying procedure. Their capacity to trigger apatite generation has been well established in the SBF, mainly due to the strong forsterite reactivity. The origin of the process of this capacity can be described in the following stages: first, through the exchange of H+ in the solution, the Mg and Si ions are emitted into the surrounding fluid. The degradation of scaffolds leads to the release of Mg, and Si, which is biocompatible (figure 6(a)). Mg being released preferentially relative to Si ions, which causes a silanol-rich layer (Si–OH) creation. Secondly, Ca2+ ions in the SBF solution are electrostatically adsorbed on the newly formed layer that is negatively charged because of forming Si–OH [45]. The existence of a negative charge on the Si–OH group initially interacts with the Ca2+ ions (positively charged) in the physiological media. In interaction with the physiological media, it induces amorphous silicate on the overlayer [5]. This film continues to interact with physiological media until a film containing Ca2+ ions fully covers the scaffold surface [46]. After that, in the direction of the outer layer, the negatively charged CO3 2− or PO4 3− ions are attracted, and the apatite layer grows with increasing soaking time as the ions in the physiological media are absorbed. Consequently, the prediction of forsterite bioceramics' bioactive output appears to be based on silicon as a fundamental element [7, 47]. As the findings show, the addition of different GO amounts may not impede the bioactivity of forsterite scaffolds due to the formation of apatite on the scaffold surface.
Figure 5. SEM images of (a)–(c) FST and (d)–(f) FST-0.5GO, (g)–(i) FST-1GO, and (j)–(l) FST-2GO nanocomposite scaffolds after immersion in the SBF solution for 28 days.
Download figure:
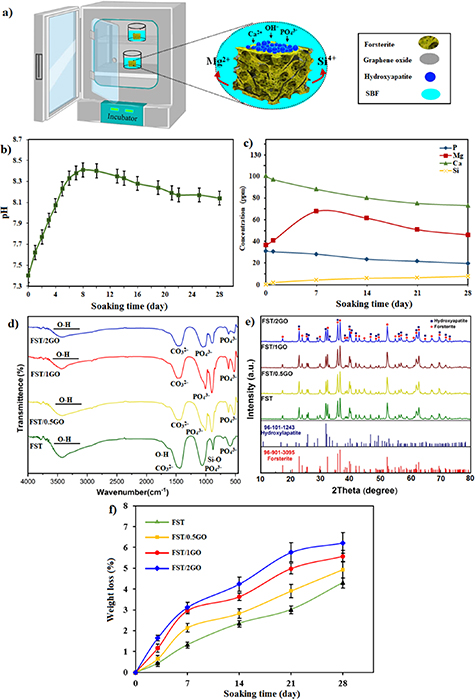
Standard image High-resolution imageFigure 6. (a) Schematic representation of the mechanism of apatite formation on the surface of FST-GO scaffolds in the SBF solution and (b) pH changes in the SBF solution, and (c) and ion concentrations changes, after soaking the FST-GO nanocomposite scaffolds and (d) FTIR absorption spectra and (e) XRD pattern after immersion in the SBF solution and (f) weight loss of FST-GO nanocomposite scaffolds after immersion for different times.
Download figure:
Standard image High-resolution imageFigure 6(b) exhibited the pH changes pattern of the SBF after soaking the forsterite/GO scaffold for various times until four weeks. In the forsterite/GO scaffold, a rapid escalation in the pH value to 8.45 was observed right after short soaking due to the interaction of Mg and Si ions with H+ in the SBF, leading to the creation of a silanol-rich layer on the scaffold surface. Accordingly, the Ca2+ transfer on the overlayer in the scaffold and SBF interface [1, 5], leading to diminishing the pH value. The pH value of the forsterite/GO scaffold decline to 8.15 after immersing it after the extension of the soaking period, and this change in pH value causes the apatite layer deposited on the scaffold surface. After immersing the forsterite/GO scaffold for different periods, the concentration change of the Ca, Mg, Si, and P ions in the SBF are shown in figure 6(c). The concentration of Ca and P ions reduced as the immersing time amplified, as seen in the pattern. In the case of Mg and Si ions, however, there was a reversal of the pattern. The apatite precipitation on the scaffold surface is associated with lessening the Ca and P ions with rising the immersing time [1, 5]. Conversely, raising the immersing time amplified the concentration of Mg and Si ions, which might be contributed to the forsterite/GO scaffold dissolution.
The FTIR spectrum of the forsterite/GO scaffold shows the creation of HAp-related typical peaks, i.e. the well-defined peak at 1070 cm−1 allocated to the P−O stretching mode and the double peak at 608 and 567 cm−1 of the P−O bending mode in HAp as shown in figure 6(d). In addition, the creation of HAp film accompanied by peaks at 1430 and 870 cm−1 was proved to be connected to the CO3 2−. Therefore, the existence of HAp on the surface of scaffolds with GO after immersion in SBF for 28 days showed which this nanofillers did not adversely affect the bioactivity of forsterite/GO. Peaks can be linked to Si−O stretching vibration modes at 832 and 965 cm−1 [19, 48–50]. Moreover, it was reported that a specific property of bioactive materials, like silicate-based ceramics, is dynamic and time-dependent changes in the surface that occur in contact with them. The bonding interface with bone and soft tissues is provided when a highly reactive HAp layer is formed.
Figure 6(e) illustrates the XRD spectra of the forsterite/GO scaffold containing various amounts of GO after exposure in SBF for 28 days. For entire scaffolds and alongside with the distinctive peaks of forsterite, the characteristic peaks of HAp at 2θ = 26.5°, 32° and, 47° can be seen, which show forming the HAp phase after 28 days of exposure in SBF [19, 50]. No phases containing magnesium were found on the scaffold surface, which may be due to the low concentration of magnesium in the deposited layer.
Figure 6(f) shows mass loss graphs for all forsterite-based scaffolds after 28 days of degradation in PBS. The mass loss of the scaffold escalated as the soaking duration extended, as shown in the graph. The mass loss of the scaffold in PBS became more noticeable as the GO content escalated. The mass loss for forsterite/GO scaffolds is more than that of forsterite scaffolds at the end of the degrading period. When the scaffold does not encapsulated with GO sheets, the mass loss is slower, and the final soaking for 28 days results in a smaller mass loss than other forsterite scaffolds with GO sheets. At 28 days, forsterite scaffolds lose 4.31% of their weight, while the mass loss of forsterite scaffolds encapsulated with 0.5GO, 1GO, and 2GO is 4.94, 5.57, and 6.22%, respectively. The findings revealed that GO sheets sped up the degradation rate of forsterite scaffolds. Indeed, as a result of the encapsulation of GO within the FST matrix, structural flaws in forsterite are become more severe. Furthermore, it was reported [14] that GO as a super hydrophilic nanomaterial has a considerable impact on the scaffold's wettability. In fact, after incorporating GO, the interaction of forsterite molecules with water molecules escalated, affecting the scaffolds' degradability rate. It was demonstrated that the hydrophilia nature GO with water contact angles of 23.7° was responsible for the improved wettability of the scaffolds [13]. It is worth noting that the forsterite/2GO scaffold presented a great degradation rate, owing to high hydrophilicity and structural defects in the scaffold encapsulated with high concentration of GO sheets [13, 23, 28, 34].
3.4. Cytocompatibility
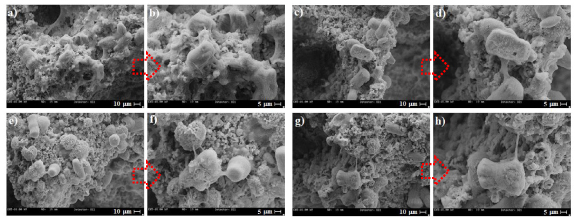
The SEM image presented the morphology of attached MG63 cells cultured on the forsterite and forsterite/GO scaffolds as shown in figure 7. It could be observed that MG63 cells strongly contacted and attached to the forsterite scaffold, implying no indications of cytotoxicity. This cell behavior is associated with the stimulatory results of the ionic products from the forsterite scaffold. Less significant alternation in the cell morphology may be noticed regarding the forsterite/GO scaffold compared to the forsterite scaffold. Moreover, the cells on forsterite/0.5GO and forsterite/1GO scaffolds spread greater, and even more cells were attached in comparison with the scaffold without GO. However, fewer cells attached to the forsterite scaffold were encapsulated with 2 wt.% GO, suggesting lower cytocompatibility. The results exhibited that incorporation of GO up to 1 wt.% has a favorable impact on the cell adhesion even though excess GO encapsulation presented an adverse effect. It is revealed that anionic functional groups (COO− and OH−) on GO sheets modified the surface charge and enhanced the wettability of the scaffold, which offered great possibilities for cell attachment [50]. In addition, the homogeneous distribution of GO sheets in the forsterite matrix presented more adhesion sites for cell attachment. Moreover, the ionic dissolution product of forsterite such as Mg and Si considerably escalates the interaction among different kinds of cells and elevates cell attachment. Taken together, forsterite and GO have a favorable impact on cell attachment, which elevates the growth and spreading of cells.
Figure 7. SEM images of MG63 cells cultured on (a), (b) forsterite scaffold and (c), (d) forsterite scaffolds with 0.5 wt.%, (e), (f) 1 wt.%, and (g), (h) 2 wt.% GO.
Download figure:
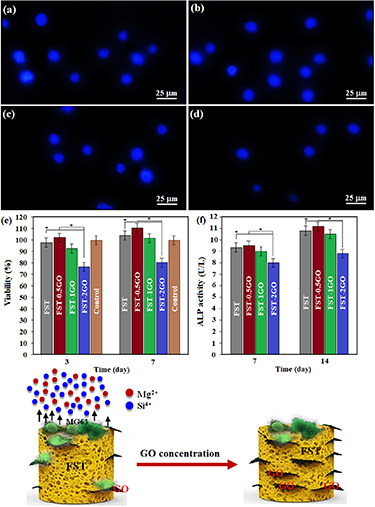
Standard image High-resolution imageAs cells can contribute effectively to apatite deposition and new bone tissue development, cell viability, and proliferation in the bone scaffold are required for bone reconstruction [51]. For various period intervals, MG63 cells were cultured on scaffolds and afterward evaluated by fluorescence staining, ALP staining, and DAPI staining in order to assess cell behavior in forsterite/GO scaffolds in the presence of different amounts of GO. The fluorescence image also shows the results of cell attachment on the forsterite/xGO scaffold after three days (figures 8(a)–(d)). The addition of 0.5 wt.% of GO sheets into the forsterite-based scaffolds leads to escalation of the cell viability, however, further addition of GO sheets to 2 wt.% into the forsterite-based scaffolds decreased cell viability. In fact, the cells attach and spread well all over the whole forsterite-based scaffold except scaffold with a high amount of GO showing that scaffolds with low GO amount have good cytocompatibility. It is worth noting that cells cultured in scaffolds incorporated with 2GO presented lower cell spread and propagated. It shows great cell number reduction in the forsterite/GO scaffold containing large amounts of GO indicating that extra GO amount could adversely affects the cell. According to the results, low amount of GO has an advantageous effect on cell attachment while excess GO may has a negative effect [23, 51, 52]. MTT method was used to evaluate cell viability, and the result showed that forsterite/GO scaffolds with low GO concentration have the least effect on cell viability (figure 8(e)). Regarding this, the toxicity of GO is reduced by its low incorporation in the scaffold, as it may prevent the direct connection of GO that results in cell destruction. Conversely, large amounts of GO (2 wt.%) in the scaffold causes low cytotoxicity of the scaffold implying that incorporating a high concentration of GO into the forsterite scaffold has an adverse impact on cell spreading. It might be obviously noticed that the viability of the cells on the forsterite/GO scaffold containing various concentrations of GO gradually amplified with the extension of culture time, articulating they were cytocompatible.
Figure 8. DAPI staining of MG63 cells cultured on a forsterite scaffold (a) and forsterite scaffolds with 0.5 wt.% (b), 1 wt.% (c), and 2 wt.% GO (d), and (e) cell viability, and (f) ALP activity of MG63 cells cultured for various times on forsterite/GO scaffolds (*p < 0.05) and (g) schematic demonstration of the interactions between the MG63 osteoblast cells and forsterite/GO scaffolds.
Download figure:
Standard image High-resolution imageALP is considered the first sign of osteoblast differentiation throughout bone growth owing to enhanced cell differentiation as long as bone cells are generated. ALP activity of forsterite/GO scaffolds after cell incubation is shown in figure 8(f) for various culture times. Typically, total scaffolds show higher ALP activity than forsterite/2GO over the time course of culture. ALP activity of forsterite and forsterite/GO scaffolds presented significant improvement after seven days of culture. Thus, forsterite with a low GO amount offers higher ALP activity in comparison to those with higher amounts and may ultimately better support osteoblast differentiation. As a matter of fact, ALP decreases significantly with increasing high GO concentration (2 wt.%) in forsterite-based scaffolds. The synergistic influence of GO on cell behavior may be attributable to oxygen-containing functional groups on their surface [46]. According to Munir et al [52], the hydroxyl groups in GO provide great sites for cell attachment and proliferation. Similarly, Gao et al [18] demonstrated that GO provides a forum for the creation of a biointerface for cell proliferation and adhesion.
Figure 8(g) shows schematic images to better understand the forsterite/GO scaffold's positive and negative effects on cell-surface interactions with the surface. To discuss the synergistic effect of carbon-based nanostructure on cell attachment and proliferation, protein absorption and better cell adsorption are related to wrinkles on the surface of graphene-based material. Gao et al [18] also reported similar results which their study confirms the positive effect of graphene-based material surfaces on protein absorption. In addition, the destructive effect of GO (at high doses) has been shown to be due to two reasons: mitochondrial dysfunction is related to the overproduction of reactive oxygen species (ROS). Lee et al [53] reported that the graphene-based materials could reduce the mitochondrial membrane potential. Thus the level of intracellular ROS is enhanced that activates the mitochondria-dependent apoptotic pathway. Moreover, ROS causes DNA damage, both results in reduced cell viability. Several previous studies [34, 52] have confirmed the toxicity of graphene nanomaterials in varying sizes in dispersed solutions. For example, nanosheet graphene is readily taken up and then accumulated in cells with lateral dimensions of 100 nm. Hegab et al [54] confirmed the edge-first insertion, penetration, and internalization of graphene microsheets (multilayered, 0.5–10 μm) into the three cell types' lipid bilayers by Brownian motion and lipid attraction. Very large macrosheets, such as 20–100 micrometers (larger than most cells), may pose a risk, for example, by using a similar 'wrapping/ encapsulation' of those bacteria, causing a nutrient deprivation. That being said, given the limited mobility of the sheet, owing to the covalent or electrostatic layers of the substrates, free-floating sheets are impossible [55–70]. Therefore, the risk of high-risk penetration and cell adsorption of nanoparticles or large film wrapping (e.g. bulk GO) of host cells should indeed be quite low [52, 54]. According to the mentioned results, the encapsulation of 1 wt.% GO to forsterite scaffolds could escalate biocompatibility base on the cell viability and attachment, ALP activity, and apatite precipitation, which can be very important for the clinical usage of forsterite/GO scaffolds.
3.5. Antibacterial properties
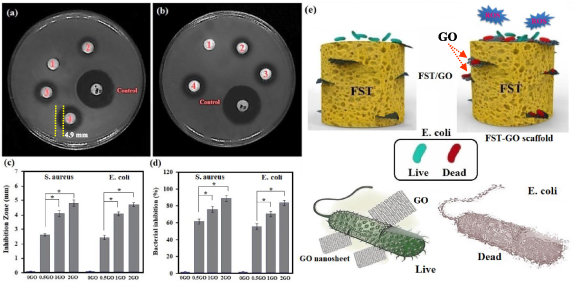
A qualitative assessment of the antibacterial efficiency of forsterite/GO scaffolds with IA was carried out for 24 h (figures 9(a) and (b)). Forsterite scaffolds do not show antibacterial performance due to the increase in the culture medium's pH because it has been shown that a change in pH is contrary to bacterial growth. As shown in the figure, IA was present around all forsterite/GO scaffolds, indicating the antibacterial performance of GO-containing forsterite scaffolds. Obviously, the forsterite/0.5GO scaffolds showed an IA diameter of 2.8 ± 0.2 mm; however, the forsterite/2GO scaffolds showed a diameter of 4.9 ± 0.3 mm against S. aureusbacteria. It was noteworthy which the forsterite/2GO scaffolds had a higher IA diameter than both forsterite/1GO scaffolds and forsterite/0.5GO scaffolds, showing the great impact of GO on the antibacterial performance of the forsterite-based scaffold (figure 9(c)). An increase of the GO amount from 0.5 to 2 wt% greatly increases the IA to 2.7 ± 0.2 mm and 4.7 ± 0.3 mm, because of the higher release content of GO against E.coli bacteria. Several papers [34, 52, 54–57] have shown that GO sheets might cut bacterial cell wall membranes to prevent them from proliferation and growing, creating oxidative stress that leads to disruption of the membrane. Direct interaction of bacteria with ultra-sharp GO edges contributes to the deterioration of the cell membrane and bacterial breakdown [34, 52]. In addition, the GO sheet represents an attractive bacterial attachment surface area, enabling actual interactions between the scaffold surface and the bacteria membrane. Therefore, the forsterite/GO scaffold kills E.coli bacteria caught on the surface of the scaffold.
Figure 9. Images of inhibition zones of the forsterite and forsterite/GO scaffolds after 24 h against (a) Gram-positive (S. aureus) and (b) Gram-negative (E. coli) and (c) values of growth inhibition zones and (d) and percentage of bacterial inhibition against S. aureus and E. coli bacteria (*p < 0.05) and (e) Schematic of the antimicrobial mechanism of forsterite scaffolds encapsulated with GO. Note: 1: FST; 2:FST/0.5GO; 3:FST/1GO; 4:FST/2GO.
Download figure:
Standard image High-resolution imageThe reduction percentage of S. aureus and E. coli for forsterite/GO scaffolds with different GO concentrations from 0.5 to 2 wt.% are shown in figure 9(d). The presented graph of forsterite scaffolds did not show any bacterial decrease while, forsterite/GO showed an incredible decrease of bacteria. There is a similar decrease trend for forsterite scaffolds containing 0.5 to 2 wt.% GO in which 65 ± 2–90% ± 2% of the bacteria are wiped. In general, forsterite/2GO has showed a significantly higher bacterial inhibition compared to other scaffolds, which indicates that incorporation of GO in the scaffold leads to a great impact on antibacterial reduction. Clearly, E. coli with additional cell walls composed of many peptidoglycan layers is more resistant to attack by GO sheet encapsulated forsterite scaffold. Figure 9(e) shows the antibacterial mechanism of forsterite scaffolds containing GO. The encapsulated GO as an antibacterial agent into the scaffolds can causes great antibacterial efficiency. The interaction of forsterite/GO scaffolds with bacteria causes the formation of GO sheets attached to the bacteria and disintegrates them. After that, GO sheets with sharp edges spread into the bacterial cytomembrane, damage the cells physically and inhibit cell growth, because of the action of oxidative stress on the bacteria. The antibacterial action of forsterite/GO scaffolds is at first related to the production of GO and escalated generation of ROS, that damage the bacterial respiratory system and also the penetration of sharp and thin edges of GO, leading to cell membrane integrity destruction. Three significant mechanisms, namely nanoknives, ROS generation and charge transfer, appeared to be functional in these situations. We intend to offer putative mechanistic ties for the formation of graphene interfaces through the use of these mechanisms.
4. Conclusions
The forsterite/GO scaffolds with various amounts of GO are effectively prepared by SEM coupled with space holder methods to achieve high strength, bioactivity, and strong antibacterial performance. Adding GO to the forsterite scaffold significantly improves the mechanical integrity of the forsterite-based scaffolds. A model was proposed to detect antibacterial activity and cellular interaction with forsterite/GO scaffolds. Forsterite/GO scaffolds with low GO concentrations provide a more suitable surface for adhesion, proliferation, and MG63 cell growth. Encapsulation of GO in forsterite scaffolds reduces S. aureus and E. coli bacterial growth, in which the percentage of bacterial reduction increases with increasing GO concentration from 0.5 to 2 wt.%. According to the obtained results, the performance of the incorporation 1 wt.% GO into forsterite scaffolds can be a potential strategy to increase mechanical performance and antibacterial properties.
Acknowledgments
The authors acknowledge the financial support from the Najafabad Branch, Islamic Azad University, University Teknologi Malaysia (UTM) and Norwegian University of Science and Technology for providing the facilities of this research to the present study.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Conflict of interest
The authors declare that they have no competing/financial conflict of interests.
Author contributions
H R Bakhsheshi-Rad, A Najafinezhad, A Saberi; Writing—original draft preparation, methodology, formal analysis
A F Ismail, S Sharif, S RamaKrishna, and F Berto: Conceptualization, supervision, review and editing A A Nourbakhsh, Y Dai and M Daroonparvar: Writing—review and editing